Abstract
The Amt proteins are ammonium transporters that are conserved throughout all domains of life, being found in bacteria, archaea and eukarya. In bacteria and archaea, the Amt structural genes (amtB) are invariably linked to glnK, which encodes a member of the PII signal transduction protein family, proteins that regulate enzyme activity and gene expression in response to the intracellular nitrogen status. We have now shown that in Escherichia coli and Azotobacter vinelandii, GlnK binds to the membrane in an AmtB-dependent manner and that GlnK acts as a negative regulator of the transport activity of AmtB. Membrane binding is dependent on the uridylylation state of GlnK and is modulated according to the cellular nitrogen status such that it is maximal in nitrogen-sufficient situations. The membrane sequestration of GlnK by AmtB represents a novel form of signal transduction in which an integral membrane transport protein functions to link the extracellular ammonium concentration to the intracellular responses to nitrogen status. The results also offer new insights into the evolution of PII proteins and a rationale for their trigonal symmetry.
Keywords: ammonium transport/membrane sequestration/nitrogen regulation/PII regulatory proteins/signal transduction
Introduction
The ammonium transport (Amt) family of proteins comprises a unique and ubiquitous group of integral membrane proteins found in all domains of life. They are present in bacteria, archaea, fungi, plants and animals, including humans, where they are represented by the Rhesus proteins (Saier et al., 1999; Howitt and Udvardi, 2000; Marini et al., 2000a; Thomas et al., 2000b). The Amt proteins are high-affinity ammonium transporters that probably function to scavenge ammonium and to recapture ammonium lost from cells by diffusion across the cell membrane. In Saccharomyces cerevisiae and Rhodobacter capsulatus, Amt proteins have also been implicated in sensing ammonium in the external medium (Lorenz and Heitman, 1998; Yakunin and Hallenbeck, 2000).
Analysis of the predicted primary amino acid sequences of Amt proteins suggests that all members of the family adopt a similar topology within the cell membrane. A combination of in silico, genetic and biochemical analyses suggests that most Amt proteins are likely to have 11 transmembrane (TM) helices, with the N-terminus being extracytosolic and the C-terminus being located in the cytoplasm (Marini and Andre, 2000; Thomas et al., 2000b). Some bacteria, including Escherichia coli, appear to have an AmtB protein with a twelfth TM at the N-terminus such that in these organisms the N-terminus would also be cytosolic (Thomas et al., 2000b). The C-terminal region of the Amt proteins is very variable in length, comprising a minimum of ∼30 residues but in some cases extending to >150 residues. As such, this region is predicted to comprise by far the largest part of the protein exposed to the cytosol.
Almost all bacteria and archaea encode at least one Amt protein, and with very few exceptions the gene encoding the transporter (amtB) is found in an operon together with a second gene (glnK) encoding a small signal transduction protein (Thomas et al., 2000a; Arcondéguy et al., 2001). GlnK is a member of the PII protein family, which act as sensors of the cellular nitrogen status in prokaryotes and which have also been identified in plants (Hsieh et al., 1998; Arcondéguy et al., 2001). The PII proteins have been studied in most detail in the Proteobacteria, especially in E.coli, which encodes two PII proteins, GlnK and GlnB. GlnK is homologous to GlnB at the primary sequence level and this is reflected in the very similar tertiary and quaternary structures adopted by both proteins (Carr et al., 1996; Xu et al., 1998). They are trimers and take the form of a squat barrel ∼50 Å in diameter and 30 Å high, above the surface of which protrude three loops (the T-loops). In response to nitrogen deprivation, the proteins are covalently modified by uridylylation of residue Tyr51 at the apex of the T-loop, and this process is reversed in nitrogen sufficiency (Jaggi et al., 1996; Atkinson and Ninfa, 1999). PII proteins regulate the activities of other proteins by protein–protein interaction, as exemplified by the role of GlnB in modulating the activity of the transcriptional activator NtrC by interaction with the histidine protein kinase NtrB (Pioszak et al., 2000). Whilst E.coli GlnK can substitute to some degree for GlnB, the primary function of GlnK has yet to be determined (van Heeswijk et al., 1996; Atkinson and Ninfa, 1998).
The conservation of genetic linkage in prokaryotes is characteristic of operons that encode either essential cellular components or proteins that physically interact (Dandekar et al., 1998). Neither glnK nor amtB is essential for growth in E.coli (Atkinson and Ninfa, 1998; Soupene et al., 1998), and this has led us to propose that their conserved association reflects a physical interaction and a related function between GlnK and AmtB (Thomas et al., 2000a). We now report that in E.coli and Azotobacter vinelandii this is indeed the case. We show that GlnK is sequestered to the cell membrane in response to nitrogen shock in an AmtB-dependent manner and that this interaction is dependent on the C-terminal cytoplasmic domain of AmtB. We discuss the potential role of this interaction and the implications of its conservation throughout bacteria and archaea.
Results
GlnK and GlnB associate with the membrane in an AmtB-dependent manner
The highly conserved genetic linkage between the glnK and amtB genes in both bacteria and archaea led us to hypothesize that GlnK was functionally associated with AmtB and that the two proteins might physically interact (Thomas et al., 2000a). We therefore used a variety of wild-type and mutant strains of E.coli to assess whole-cell extracts, cytoplasmic fractions and membrane fractions for the presence of PII proteins (GlnK or GlnB) using SDS–PAGE and western blotting. The cells were grown in nitrogen-limiting media with glutamine as the nitrogen source (M9Gln medium), conditions under which the glnK–amtB operon is highly expressed (Atkinson and Ninfa, 1998). In initial experiments, the membrane fractions isolated by ultracentrifugation were subjected to repeated washes with 50 mM sodium phosphate buffer.
Cellular fractions prepared from wild-type cells (strain ET8000) indicated that PII protein was present in both the cytoplasmic and the membrane fractions, and control experiments with strain FT8000 (ΔglnB,ΔglnK) confirm that these signals are PII specific (Figure 1). The data confirm that some PII protein is membrane associated under these growth conditions but do not distinguish between GlnB and GlnK. To clarify the nature of PII association with the membrane and the role of AmtB in this association, three isogenic mutant strains were employed (ΔglnK, GT1002; ΔglnB, PT8000; and ΔamtB, GT1001). The ΔglnK mutation is an in-frame deletion within glnK that is not polar on amtB (Arcondéguy et al., 1999).
Fig. 1. GlnK and GlnB associate with the membrane in an AmtB-dependent manner. Whole-cell extracts (W), cytoplasmic (C) and membrane (M) fractions were subjected to SDS–PAGE followed by western blotting using an anti-PII antibody. Extracts and fractions were prepared and loaded as described in Materials and methods. The strains used are indicated below each lane: wild type (ET8000), ΔglnB,ΔglnK (FT8000), ΔglnB (PT8000), ΔglnK (GT1002), ΔamtB (GT1001). The control lane contained 0.3 µg of purified E.coli GlnK.
Analysis of the ΔglnB strain gave an identical pattern to that seen with the wild type, demonstrating that GlnK does indeed associate with the membrane (Figure 1). In the ΔglnK strain, the pattern was somewhat different and GlnB was only detected in the whole-cell lysate and the membrane fraction. Consequently, GlnB also appears to associate with the membrane, at least in the absence of GlnK; however, the signal was considerably weaker compared with that seen in the ΔglnB strain, suggesting that under these growth conditions there is considerably less GlnB than GlnK in the cell. Control experiments indicate that the anti-PII antibody used in these experiments is significantly less active against GlnK than GlnB (R.Little and T.Arcondéguy, personal communication), hence the GlnK:GlnB ratio is actually underestimated. When the localization of the PII proteins was examined in extracts prepared from the ΔamtB strain GT1001, there was a striking absence of signal from the membrane fraction, while the proteins were just as abundant in the cytoplasmic fraction as in the wild type (Figure 1). Hence AmtB is essential for the association of the PII proteins with the membrane, most probably through a direct physical interaction between PII and AmtB.
The membrane association of PII is affected by the cellular nitrogen status
PII proteins typically function to regulate the activity of those proteins with which they interact in response to the nitrogen status of the cell (Arcondéguy et al., 2001). We therefore examined what effect, if any, a change in the cellular nitrogen status would have on the observed association between PII (in particular GlnK) and AmtB. Previous studies of this nature have investigated the role of GlnB in regulating adenylyltransferase, and thereby the adenylylation of glutamine synthetase, in response to a rapid addition of ammonium to a nitrogen-limited cul ture (termed ‘ammonia shock’) (Bender et al., 1977; van Heeswijk et al., 1996). We therefore used the same ammonia shock conditions to investigate effects on GlnK–AmtB association.
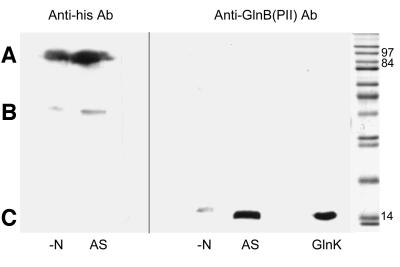
For these experiments, we used strain GT1000 (pMM280), which has chromosomal deletions of both glnK and amtB and contains glnK and amtB in trans with a modified version of amtB that encodes a C-terminally His-tagged version of the protein (D.Blakey, A.Leach, G.H.Thomas, G.Coutts, K.Findlay and M.Merrick, in preparation). The modified AmtB protein retains methylammonium transport activity at a level comparable to the wild-type protein (D.Blakey, A.Leach, G.H.Thomas, G.Coutts, K.Findlay and M.Merrick, in preparation). The cells were grown in M9Gln medium and then subjected to ammonia shock with 30 mM NH4Cl. Samples were taken immediately prior to addition of ammonium and 15 min later, and then fractionated as before. We monitored the membrane fractions for the presence of both PII and AmtB, the latter being detected using an anti-His tag antibody (Qiagen) to detect the C-terminal His tag on the modified version of AmtB. The levels of AmtB in the membrane fraction remained constant both pre- and post-ammonia shock (Figure 2). In contrast, there was a very significant increase in the amount of PII protein associated with the membrane after ammonia shock, indicating that membrane association is modulated by the nitrogen status (Figure 2). The levels of PII (GlnB + GlnK) in the cytoplasmic fractions showed a significant reduction after ammonia shock (data not shown).
Fig. 2. The membrane association of PII is affected by the cellular nitrogen status. Membrane fractions of strain GT1000 (pMM280) [ΔglnK amtB (glnK amtB3)] were subjected to SDS–PAGE followed by western blotting using either an anti-His tag antibody to detect AmtB-His6 (A; B is a non-specific cross-reaction) or an anti-PII antibody to detect PII (C). Extracts were prepared from cells grown in nitrogen-limiting conditions (–N) and 15 min after ammonia shock (AS). The control lane contained 0.3 µg of purified E.coli GlnK. Molecular weight markers (Sigma, wide molecular weight standards M6539) run on the same 12.5% SDS–polyacrylamide gel are shown for calibration.
Membrane association of GlnK reflects the uridylylation state of the protein
Given that GlnK is a trimer, the protein can exist in four states in the cell depending upon the number of subunits that are modified by uridylylation. Ammonia shock is known to cause rapid deuridylylation of both GlnB and GlnK (van Heeswijk et al., 1996; Jiang et al., 1998) and the uridylylation states of the PII trimers can be assessed by separating the different forms on native polyacrylamide gels (Forchhammer et al., 1999; van Heeswijk et al., 2000). Under these conditions, the non-uridylylated form of the protein has the lowest mobility and the fully uridylylated form the highest (Jaggi et al., 1996). For these experiments, we used a ΔglnB strain (PT8000) and a ΔglnB,ΔamtB strain (AT8000) in order to assess specifically the membrane association of GlnK. We also subjected the membrane fractions to a more stringent wash using 50 mM sodium phosphate supplemented with 600 mM NaCl to ensure that only those proteins that were tightly associated with the membrane were detected.
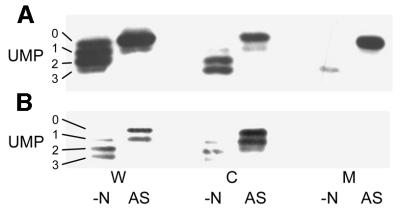
In whole-cell lysates from ΔglnB cells grown in M9Gln medium, four bands were detected corresponding to the uridylylated forms of GlnK (GlnK-UMP1, GlnK-UMP2 and GlnK-UMP3) and the unmodified protein (Figure 3). Following ammonia shock, this profile changes to reveal only two bands, the major one being the non-uridylylated form of GlnK and the minor species being GlnK-UMP1. The cytoplasmic fraction showed a very similar pattern, although GlnK-UMP1 was apparently less abundant than GlnK-UMP2 and GlnK-UMP3 pre-ammonia shock. Most strikingly, analysis of the membrane fractions gave a relatively weak signal pre-ammonia shock, with only GlnK-UMP3 being revealed, and a very much stronger signal post-ammonia shock, which was almost exclusively the non-uridylylated form of GlnK (Figure 3A). Identical amounts of membrane material were loaded in each lane and, as shown earlier, the amount of AmtB present pre- and post-ammonia shock did not vary significantly. Parallel analyses of the ΔglnB,ΔamtB strain produced very similar profiles for the whole-cell lysate and for the cytoplasmic fraction, but there was no membrane-associated GlnK either pre- or post-ammonia shock (Figure 3B). In similar experiments using the less stringent salt wash, we detected membrane association of other uridylylated species of GlnK in the pre-ammonia shock samples, suggesting that whilst there is association of GlnK in nitrogen-limited conditions, most of this protein is only weakly bound (data not shown).
Fig. 3. The membrane association of GlnK reflects the uridylylation state of the protein. Whole-cell extracts (W), and cytoplasmic (C) and membrane (M) fractions were prepared from strains (A) ΔglnB (PT8000) and (B) ΔglnB,ΔamtB (AT8000) both before (–N) and after (AS) ammonia shock. Extracts and fractions were prepared as described in Materials and methods and subjected to native PAGE followed by western blotting using an anti-PII antibody. Under these conditions, all four possible forms of GlnK (UMP 0–3) can be identified.
We carried out experiments comparable to those described above using the ΔglnK strain (GT1002) to examine the behaviour of GlnB in the absence of GlnK. Our initial experiments on nitrogen-limited cells had indicated that in the absence of GlnK essentially all GlnB was membrane associated (Figure 1). Nevertheless, we saw a very marked apparent increase in the amount of GlnB in the membrane fraction after ammonia shock (data not shown). We interpret this to reflect an increased affinity of GlnB for AmtB in response to ammonia shock so that membrane-associated GlnB that was lost from pre-ammonia shock samples during the salt wash was retained in post-ammonia shock samples.
Finally, we repeated the experiment with wild-type cells, but in this case interpretation of the results is complicated due to the potential presence of heterotrimers between GlnB and GlnK whereby at least 16 different species of PII protein can occur in principle (van Heeswijk et al., 2000). Using native gel electrophoresis, we did indeed observe more bands in western blots of whole-cell extracts, cytoplasmic and membrane fractions both pre- and post-ammonia shock than we detected in either the ΔglnB or ΔglnK strains (data not shown). Assignment of these bands was assisted by the previously reported differential migration of the homotrimeric GlnK and GlnB proteins (van Heeswijk et al., 2000). The membrane fractions from post-ammonia shock samples contained three major species of which the dominant form had a mobility consistent with that of the non-uridylylated GlnK homotrimer, the other species most probaby being heterotrimers containing predominantly GlnK subunits. Hence it would appear that although GlnB can associate with AmtB in the absence of GlnK, it does not compete significantly with GlnK in wild-type cells.
The C-terminal domain of AmtB is required for GlnK association
The C-terminal 32 residues of E.coli AmtB are predicted to be located on the cytoplasmic side of the membrane and to constitute the most extensive part of the protein exposed to the cytoplasm (Thomas et al., 2000b). The region contains a number of residues that are highly conserved amongst members of the Amt family and is therefore likely to be either functionally and/or structurally important. We reasoned that this region constitutes a potential interaction domain for GlnK and we therefore examined the consequences of deleting the C-terminus of the transporter.
The amtB gene was truncated (amtB4) so that 25 residues were removed from the C-terminus and the encoded protein terminated at residue 403. The activity of the truncated protein was compared with that of wild-type AmtB by introducing the wild-type glnK–amtB operon (on pGC2) and the derivative operon glnK–amtB4 (on pGC4) into the ΔglnK,amtB strain GT1000. The truncated transporter had 28% of the wild-type AmtB activity as measured by [14C]methylamine transport. We used a polyclonal antibody raised against purified E.coli AmtB (D.Blakey, A.Leach, G.H.Thomas, G.Coutts, K.Findlay and M.Merrick, in preparation) to assess the presence of the truncated AmtB in the cell membrane and found levels comparable to the wild-type protein both pre- and post-ammonia shock (data not shown). Hence the reduced [14C]methylamine transport activity of the truncated protein apparently is not due to reduced levels of the protein in the membrane, but rather to decreased activity of the protein.
Using strain GT1000 (pGC4), which synthesizes only the C-terminally truncated form of AmtB, we analysed the location of PII in cell fractions both pre- and post-ammonia shock using western blots after denaturing SDS–PAGE. The protein was detected in whole-cell lysates and cytoplasmic fractions at levels comparable to those observed previously, but there was almost no signal in the membrane fractions (Figure 4). Although this strain synthesizes both GlnK and GlnB, a combination of the differential expression of glnB and glnK, together with the multicopy effect of the plasmid-borne glnK gene, means that nearly all of the PII in these cells is expected to be GlnK. These data strongly suggest that the C-terminal domain of AmtB either constitutes the site of interaction between GlnK and AmtB or is required to allow AmtB to adopt a conformation required for GlnK association.
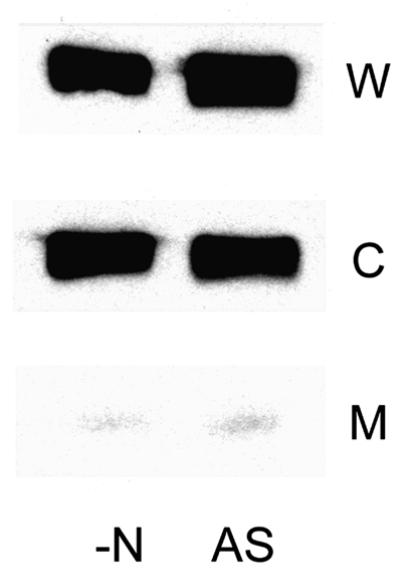
Fig. 4. The C-terminal domain of AmtB is required for GlnK association. Whole-cell extracts (W), and cytoplasmic (C) and membrane (M) fractions were prepared from strain GT1000 (pGC4) [ΔglnK amtB (glnK amtB4)] both before (–N) and after (AS) ammonia shock. Extracts and fractions were prepared as described in Materials and methods and were subjected to SDS–PAGE followed by western blotting using an anti-PII antibody.
GlnK–AmtB association is found in other bacteria
We have proposed previously that the conservation of linkage between the glnK and amtB genes in both bacteria and archaea reflects a highly conserved interaction between the two proteins (Thomas et al., 2000a). Having demonstrated evidence for such an association in E.coli, we sought to support the concept that this is a general phenomenon by carrying out a similar analysis in another species, namely the γ-proteobacterium A.vinelandii. Azotobacter vinelandii contains a single glnK–amtB operon and, unlike E.coli, is proposed to encode only a single PII protein, namely GlnK (Meletzus et al., 1998). We used a wild-type strain (UW136) and an amtB mutant (MV560) that has been shown to lack [14C]methylamine transport activity (Meletzus et al., 1998). Cells were grown in a nitrogen-limited medium and then subjected to ammonia shock in a similar manner to that used for E.coli. Membrane fractions were isolated pre- and post-ammonia shock, subjected to stringent washing and then run on native polyacrylamide gels prior to western blotting with antibodies against E.coli GlnB and AmtB. As in E.coli, GlnK was found to be associated with the membrane in an AmtB-dependent manner and the level of membrane-associated GlnK increased post-ammonia shock whilst the level in the cytoplasm was reduced (Figure 5). The AmtB antibody confirmed that the levels of AmtB present in the membrane do not vary pre- and post-ammonia shock and that membranes from the amtB mutant showed no cross-reacting material (data not shown). Hence the GlnK and AmtB proteins of A.vinelandii show very similar behaviour to that seen in E.coli in response to a rapid change in the cellular nitrogen status.
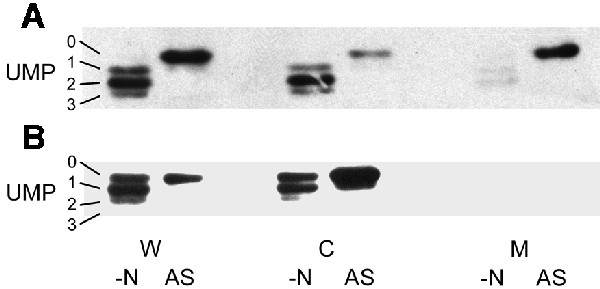
Fig. 5. GlnK–AmtB association is found in other bacteria. Whole-cell extracts (W), and cytoplasmic (C) and membrane (M) fractions were prepared from A.vinelandii strains (A) UW136 (wild type) and (B) MV560 (ΔamtB1::KIXX) both before (–N) and after (AS) ammonia shock. Extracts and fractions were prepared as described in Materials and methods and subjected to native PAGE followed by western blotting using an anti-PII antibody. Under these conditions, all four possible forms of GlnK (UMP 0–3) can be identified.
The GlnK protein negatively regulates the transport activity of AmtB
Having found evidence for an interaction between PII proteins and the ammonium transporter AmtB, we considered what functions such an interaction might serve. One possibility is that the interaction serves to regulate the activity of the transporter and, with this in mind, we carried out a series of experiments comparing the transport activity of AmtB in the wild type with that in strains either lacking or overexpressing glnK. The substrate in these assays is [14C]methylammonium, an analogue of ammonium, and as transport of methylammonium by AmtB is competitively inhibited by micromolar concentrations of ammonium it is not possible to assess the effect of GlnK under those conditions where its interaction with AmtB is maximal, i.e. after ammonia shock. Therefore, all these experiments were carried out using cells grown in nitrogen limitation, a condition where any effects of GlnK on AmtB activity were expected to be relatively small.
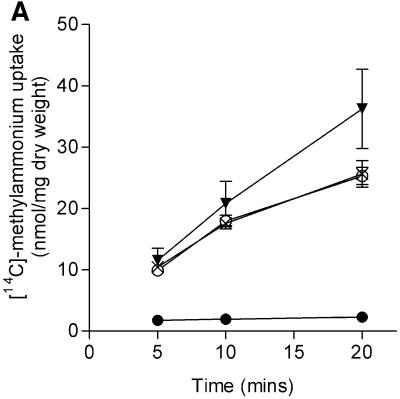
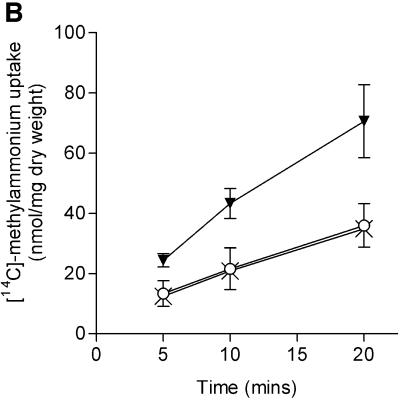
We examined two isogenic sets of strains, one derived from ET8000 and one from YMC10, for the effects of ΔglnK and ΔglnB mutations on uptake (Figure 6A and B). In each case, the ΔglnB strain was indistinguishable from the wild type, whereas the activity of the ΔglnK strain was considerably elevated (50% in the ET8000 background, Figure 6A; and 100% in the YMC10 background, Figure 6B). In principle, this effect of the ΔglnK mutation could arise in a number of ways. It could be due to a direct effect on the activity of AmtB or to an indirect effect (through NtrB and NtrC) leading to elevated glnK–amtB transcription. Alternatively, as methylammonium is metabolized by glutamine synthetase to methylglutamine and the transport assay effectively measures metabolically trapped methylammonium (Soupene et al., 1998), there is also the potential for effects on the activity of glutamine synthetase. The effects of glnK mutants of E.coli on both glnK–amtB transcription and glutamine synthetase activity have been assessed previously and, in each case, the mutant was shown to have little if any effect (Atkinson and Ninfa, 1998). Indeed, the most marked effect is on glnK–amtB transcription where the glnK mutant decreases, rather than increases, expression. To confirm independently that the levels of AmtB are unaffected by the glnK mutation, we used western blots to assess the levels of AmtB present in whole-cell extracts of wild type, and ΔglnK and ΔglnB mutants. We found no significant differences in the levels of AmtB in each of the strains (data not shown). We therefore conclude that the elevated transport activity of the glnK mutant is attributable to an increase in the activity of AmtB.
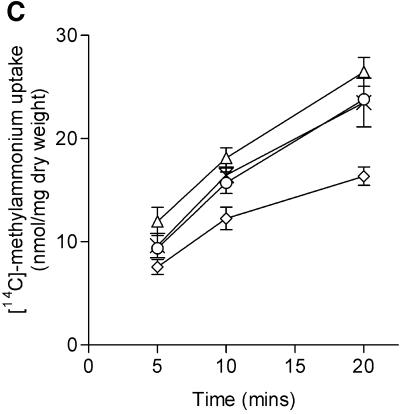
Fig. 6. The GlnK protein affects the methylammonium transport activity of AmtB. [14C]methylammonium transport activities were determined as described in Materials and methods. Strains used were as follows. (A) ET8000, wild type (open circles); PT8000, ΔglnB (crosses); GT1002, ΔglnK (inverted trangles); and GT1000, ΔglnK amtB (filled circles). (B) YMC10, wild type (open circles); RB9060, ΔglnB (crosses); and WCH30, ΔglnK (inverted triangles). (C) ET8000, wild type (open circles); ET8000 (pWSK29), wild type (upright triangles); ET8000 (pTA40), wild type (pglnK+) (crosses); and ET8000 (pTA43), wild type (pglnKY51F) (diamonds). (D) ET8000, wild type (open circles); GT1000, ΔglnK amtB (filled circles); GT1000 (pGT11), ΔglnK amtB (glnK+ amtB+) (upright triangles); and GT1000 (pGT14), ΔglnK amtB (amtB+) (crosses).
The deduced negative effect of GlnK on AmtB was confirmed by examining the activities of strains in which GlnK levels were elevated. We introduced into a wild-type strain low-copy-number plasmids on which either glnK (pTA40) or a mutant glnK allele (glnKY51F, carried on pTA43) is expressed from the lacZ promoter. AmtB activity was unchanged in the presence of wild-type GlnK, but a non-uridylylatable form of the protein, GlnKY51F, which is expected to associate strongly with AmtB, significantly depressed activity (Figure 6C).
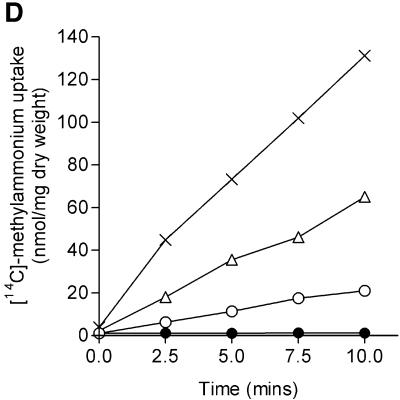
The negative effect of GlnK on AmtB activity was also apparent when we compared plasmids expressing either amtB alone (pGT14) or the complete glnK–amtB operon (pGT11) into strain GT1000, which carries a chromosomal glnK–amtB deletion (Figure 6D). The presence of multicopy glnK–amtB gave an activity 4-fold greater than wild type, but when glnK was deleted from the plasmid and amtB was expressed alone from the same native promoter, the activity was elevated 9-fold. Hence AmtB is significantly more active in the absence of GlnK.
Discussion
In almost all members of the bacteria and archaea, genes encoding the high-affinity ammonium transporter AmtB are linked to and potentially co-transcribed with glnK, which encodes a member of the PII signal transduction protein family (Thomas et al., 2000a; Arcondéguy et al., 2001). We have now shown that in E.coli and in A.vinelandii this genetic linkage almost certainly reflects a physical interaction between the two proteins such that GlnK can be sequestered specifically to the cell membrane by AmtB. The affinity of non-uridylylated GlnK for AmtB appears to be considerably greater than that of the uridylylated protein, such that the interaction of GlnK with AmtB is promoted by an increase in the intracellular nitrogen status. AmtB activity increases in the absence of GlnK, indicating that GlnK acts as a negative regulator of the transporter.
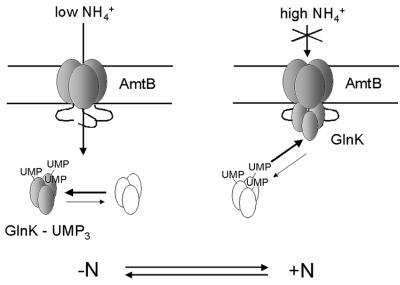
We propose that the physiological role of this interaction is to modulate the activity of the transporter so as to optimize ammonium uptake under all growth conditions, and a model for this regulation is outlined in Figure 7. The transcriptional control of amtB by the nitrogen regulation (NtrB/NtrC) system ensures that amtB is only expressed in nitrogen-limited growth conditions. However, during growth in nitrogen limitation, GlnK can fine-tune the activity of AmtB in response to minor or transient fluctuations in ammonium availability. In nitrogen-limited conditions, GlnK is predominantly in its fully uridylylated state and is not strongly membrane associated. AmtB is then active and will effectively scavenge ammonium from the surrounding medium. When the cellular nitrogen status changes, e.g. as a consequence of a sudden rise in the availability of extracellular ammonium, GlnK is deuridylylated rapidly and the unmodified form of the protein then associates tightly with AmtB in the inner membrane. This association reduces the activity of AmtB, and ammonium is no longer actively transported into the cell. This process is rapidly reversible, but in the event that the nitrogen status remains high, transcription of the glnK–amtB operon will cease due to dephosphorylation of the activator protein NtrC. GlnK previously has been proposed to act as a negative regulator of AmtB activity in the α-proteobacterium Azospirillum brasilense (de Zamaroczy, 1998). In this organism, a mutation in glnZ (the A.brasilense homologue of glnK) more than doubles the rate of [14C]methylammonium uptake and the presence of additional copies of glnZ in either wild-type or glnZ mutant strains reduces uptake. These data are entirely consistent with the data and the model proposed here for E.coli.
Fig. 7. Model for regulation of the interaction between GlnK and AmtB. In conditions of nitrogen limitation (–N), GlnK is predominantly in its fully uridylylated state and is not strongly membrane associated (non-shaded GlnK indicates the minority species in each situation). AmtB is active and will effectively scavenge ammonium from the surrounding medium. When the cellular nitrogen status changes, e.g. as a consequence of a sudden rise in the availability of extracellular ammonium, GlnK is deuridylylated rapidly and the unmodified form of the protein associates tightly with AmtB in the inner membrane. This association reduces the activity of AmtB and ammonium is no longer transported actively into the cell. This process is rapidly reversible, but in the event that the nitrogen status remains high, transcription of the glnK–amtB operon will cease due to dephosphorylation of the activator protein NtrC.
Whilst we consider that regulation of AmtB activity is likely to be the primary function of the GlnK–AmtB interaction, another significant consequence of GlnK sequestration will be to deplete the cytoplasmic GlnK pool rapidly in response to an increase in extracellular ammonium. This would have the effect of amplifying the regulatory properties of GlnK with respect to any cytoplasmic targets. To date, only one specific cytoplasmic target of GlnK is known, the nitrogen fixation regulatory protein complex NifLA (Jack et al., 1999). In Klebsiella pneumoniae, GlnK is required to relieve the inhibitory effects of NifL on the activator protein NifA and, somewhat surprisingly, uridylylation of GlnK is not required to regulate this process (He et al., 1998). This led to the question of how NifL-mediated inhibition could be restored when ammonium is added back to a nitrogen-limited medium and to the suggestion that GlnK might be proteolysed or covalently modified by a mechanism other than uridylylation upon replenishment of ammonium (He et al., 1998). Our present observations offer an alternative explanation in that the sequestration of deuridylylated GlnK by AmtB would rapidly lower the cytoplasmic GlnK pool and thereby release NifL to inhibit NifA activity. A further implication of this mechanism is that the pivotal role of AmtB in such a process would give the transporter the appearance of acting as a sensor of extracellular ammonium, a role that had been suggested previously for Amt proteins in R.capsulatus (Yakunin and Hallenbeck, 2000) and in S.cerevisiae (Lorenz and Heitman, 1998).
The C-terminal region of E.coli AmtB is required for the membrane sequestration of GlnK and potentially could constitute the specific site of interaction. In S.cerevisiae Mep proteins, a point mutation in this region can have a transdominant phenotype, suggesting that C-terminal residues are involved in interactions between subunits of the transporter (Marini et al., 2000b). In E.coli, deletion of this region does not abolish transport, though it does significantly reduce it. Hence, one function of this region of the transporter could be regulatory, and it is notable that whilst the C-terminal region in E.coli AmtB is conserved in Amt proteins, many members of the family have considerably extended C-terminal domains of up to 150 residues. These domains could potentially constitute interaction sites for regulatory proteins.
We suggest that the interaction of GlnK and Amt proteins is a general phenomenon that is fundamental to the biology of bacterial and archaeal ammonium transporters. In those organisms in which multiple copies of the amtB gene are present, e.g. Archaeoglobus fulgidus and Azoarcus, a linked glnK homologue is also duplicated, suggesting that the two proteins co-evolved. The major exception to this situation is in the cyanobacteria, which have a single PII gene (glnB) that is not amt linked and which can have multiple homologues of amtB (Montesinos et al., 1998). The conservation of the glnK–amtB operon leads us to suggest that PII proteins originally evolved together with the Amt proteins and that the primary role of the PII protein was to regulate the activity of the ammonium transporter. Based on this model, the PII paralogues that are now found in the bacteria and the archaea, namely the GlnB proteins of the Proteobacteria and the NifI proteins in the nitrogen fixation gene clusters of some archaea (Arcondéguy et al., 2001), are likely to be the result of subsequent gene duplication and specialization. Of particular interest in this context is the fact that the PII proteins are trimers and to date the reason for this quaternary structure has not been apparent, particularly as none of their known targets has trigonal symmetry. However, we have recently shown that the purified AmtB protein from E.coli is also trimeric (D.Blakey, A.Leach, G.H.Thomas, G.Coutts, K.Findlay and M.Merrick, in preparation) and hence, if GlnK did evolve in concert with AmtB, its trimeric structure may reflect a symmetry required for optimal interaction between the two proteins.
In E.coli, the interaction of GlnK with AmtB apparently is modulated by uridylylation, and a generalization of our model would imply that covalent modification of the PII protein is required to regulate interaction with the transporter. In the cyanobacteria, PII is modified by phosphorylation of Ser49 rather than uridylylation of Tyr51 (Forchhammer and Tandeau de Marsac, 1994) and this could serve the same purpose. However, it is not known whether the PII proteins of archaea are subject to covalent modification. A uridylyltransferase (GlnD homologue) is not present in the archaea and their PII proteins do not contain a potential phosphorylation site, i.e. a conserved serine residue, in the T-loop. It is also possible that the transporter plays an active role in the interaction with GlnK and that their association is also modulated by conformational changes in the transporter in response to increased levels of extracellular ammonium.
Dynamic changes in the subcellular localization of proteins are emerging as significant features in bacteria with respect to both cellular physiology and cell differentiation (Shapiro and Losick, 2000). A number of examples of membrane sequestration of regulatory proteins have been described. The transcriptional repressor of the Salmonella typhimurium proline utilization (put) operon, PutA, shuttles between the membrane and the cytoplasm depending on the intracellular concentration of proline (Muro-Pastor et al., 1997). Similarly, in the presence of glucose, the E.coli repressor protein Mlc binds to the activated glucose transporter PtsG, thereby becoming sequestered to the membrane and allowing derepression of Mlc-regulated promoters (Lee et al., 2000; Tanaka et al., 2000). In the case of GlnK and AmtB, the cytoplasmic component is a signal transduction protein rather than a transcriptional regulator but, as with Mlc, the system potentially provides a means not only of responding to the presence of an extracellular substrate but also of sensing how much is entering the cell. One can imagine that such mechanisms may be much more widespread and indeed the potential role of transport proteins as part of signal transduction pathways in bacteria and archaea is perhaps considerably greater than has been recognized so far. One possible example, which also involves a PII protein, is in the cyanobacterium Synechococcus, where GlnB has been shown to regulate nitrate uptake and might interact with the C-terminus of one of the ATP-binding subunits of the nitrate transporter NrtC (Kobayashi et al., 1997; Hisbergues et al., 1999).
Materials and methods
Strains and plasmids
The bacterial strains and plasmids used in this study are listed in Table I. The E.coli strains were grown routinely in Luria medium (Luria broth or Luria agar LA). For growth under nitrogen limitation, an altered M9 medium was used in which the ammonia was replaced by 200 µg/ml glutamine (M9Gln medium). Ammonia shock was achieved by addition of NH4Cl to a final concentration of 30 mM. Azotobacter vinelandii was grown in Burk’s sucrose medium supplemented with 25 mM ammonium acetate for +N conditions and 200 µg/ml glutamine for –N conditions. Antibiotics were used at the following concentrations: chloramphenicol 30 µg/ml and kanamycin 1 µg/ml.
Table I. Bacterial strains and plasmids used in this study.
| Strain/plasmid | Genotype or phenotype | Reference |
|---|---|---|
| E.coli | ||
| ET8000 | rbs lacZ::IS gyrA hutCK | Jayakumar et al. (1986) |
| AT8000 | rbs lacZ::IS gyrA hutCK ΔglnB1, ΔamtB | Reyes-Ramirez et al. (2001) |
| FT8000 | rbs lacZ::IS gyrA hutCK ΔglnB1, ΔglnK1 | Reyes-Ramirez et al. (2001) |
| GT1000 | rbs lacZ::IS gyrA hutCK ΔglnKamtB | this work |
| GT1001 | rbs lacZ::IS gyrA hutCK ΔamtB | Thomas et al. (2000b) |
| GT1002 | rbs lacZ::IS gyrA hutCK ΔglnK1 | this work |
| PT8000 | rbs lacZ::IS gyrA hutCK ΔglnB1 | Reyes-Ramirez et al. (2001) |
| RB9060 | ΔlacU169 endA1 thi-1 hsdR17 supE44 hutCK ΔglnB2306 | Bueno et al. (1985) |
| WCH30 | ΔlacU169 endA1 thi-1 hsdR17 supE44 hutCK ΩGmr ΔglnK1 | Arcondéguy et al. (1999) |
| YMC10 | ΔlacU169 endA1 thi-1 hsdR17 supE44 hutCK | Chen et al. (1982) |
| A.vinelandii | ||
| UW136 | Rifr | Bishop and Brill (1977) |
| MV560 | Rifr ΔamtB1::KIXX | Meletzus et al. (1998) |
| Plasmids | ||
| pGC2 | glnK amtB1 in pACYC184 | this work |
| pGC4 | glnK amtB4 in pACYC184 | this work |
| pGT11 | glnK amtB in pWSK29 | this work |
| pGT14 | amtB in pWSK29 | Thomas et al. (2000b) |
| pMM280 | glnK amtB3 in pACYC184 | this work |
| pTA40 | glnK in pWSK30 | this work |
| pTA43 | glnKY51F in pWSK30 | this work |
Plasmid pGT11, which contains the complete glnK–amtB operon, was created by subcloning an SspI–BamHI fragment from pWVH141 (van Heeswijk et al., 1996) into SspI–BamHI-cut pWSK29 (Wang and Kushner, 1991). pGC2 was derived from pGT11 in two steps: first, the HindIII–BamHI 1.8 kb fragment of pGT11 was cloned into pACYC184 (Chang and Cohen, 1978) to give pGC1. Secondly, using the primers AMT1 (GGGCCATCAGTTGCTGG) and AMT2 (CCGGATCCTTACAGATCTGCGTTATAGGCATTCTCGCC; BamHI and BglII sites underlined and termination codon italicized), the 3′ end of amtB was amplified on a PvuI–BamHI fragment. This fragment then replaced the wild-type PvuI–BamHI fragment, giving pGC2 in which the BamHI site was moved closer to the termination codon of amtB and a unique BglII site encoding two additional residues (aspartate and leucine) was introduced 5′ to the stop codon. The modified amtB gene in pGC2 was designated amtB1.
A short linking amino acid-coding sequence was added to the C-terminus of AmtB by the ligation of an oligonucleotide cassette into pGC2 cut with BglII and BamHI. The cassette was produced by annealing AMT3 (GATCAAGCGCACCAGCCCGCTCAGGCAGATCTCTCGAGCTAAG) and AMT4 (GATCCTTAGCTCGAGAGATCTGCCTGAGCGGGCTGGTGCGCTT) to produce pGC5. A His6 tag [made by annealing oligos AMT5 (GATCATCACCACCATCATCATCAC TAAG) and AMT6 (GATCCTTAGTGATGATGGTGGTGAT)] was added to the C-terminus of the protein by the ligation of the oligonucleotide cassette between the BglII and BamHI sites in pGC5 to give a further allele of amtB,amtB3, encoded on pMM280.
The plasmid pGC4 was created by using two primers Amt1 (GGGCCATCAGTTGCTGG) and Amt5 (CCGGATCCTTAGCTCGAGGTCAAGATCCGCCAATTTGTAGCC; BamHI and XhoI sites underlined and termination codon italicized) to amplify part of the end of amtB. This fragment was then ligated into pGC2 (glnK amtB1) on a PvuI–BamHI fragment to give pGC4 (glnK amtB4), which encodes GlnK and a truncated form of AmtB comprising the wild-type sequence to residue Thr402 with a newly introduced XhoI site resulting in one extra codon (Ser403) just prior to the stop codon.
Plasmid pTA40 was constructed by cloning an EcoRI–SacI fragment carrying glnK from pWVH149 (van Heeswijk et al., 1996) into pWSK30 (Wang and Kushner, 1991) so that glnK is expressed from placZ. The derivative plasmid pTA43 is identical to pTA40 but contains a site-directed mutation that converts the TAC codon for Y51 to a TTC codon, giving F51.
Strain GT1000 containing an unmarked deletion that removes the majority of the glnK–amtB operon was constructed in a manner comparable with that used previously to construct the unmarked amtB deletion strain GT1001 (Thomas et al., 2000b). PCR primers GlnKNo (5′-CGCGGATCCGCTGGTCGAGACGCCGCAAA-3′) and GlnKNi (5′-CCCATCCTCTAGACTTAAACACAGGGA-3′) were used, with plasmid pWVH141 as template DNA, to amplify a 500 bp region upstream of glnK, which ends with the first six codons of glnK. A similar pair of primers, AmtBCi (5′-TGTTTAAGTCTAGAGGATGGGGATGTCAACAGCCACGGCGAG-3′) and AmtBCo (5′-CGCGGATCCGCGCAAATGGTAGC-3′), was used to amplify a 500 bp region extending from the last seven codons of amtB into the downstream tesB gene. The two PCR products were purified, mixed and used as templates for a second PCR using primers GlnKNo and AmtBCo. The resulting PCR product was digested with BamHI and cloned into BamHI-cut pKO3 (Link et al., 1997). This unmarked deletion of glnK–amtB was then crossed onto the chromosome of strain ET8000. Resolution of the recombination was checked extensively by PCR. The unmarked deletion of glnK (ΔglnK1) that is not polar on amtB was transduced from strain WCH30 into ET8000 selecting for gentamicin-resistant mutants. Candidate transductants were checked using PCR and the resultant strain was designated GT1002.
In vivo [14C]methylamine transport assays
These assays were performed as described previously using cells grown in M9Gln medium (Jack et al., 1999; Thomas et al., 2000b). [14C]methylamine hydrochloride (2.15 Gbq/mmol) was obtained from Amersham Pharmacia, UK.
Western blotting
Protein concentrations of various cell fractions were determined using the Sigma Bradford reagent/Bio-Rad protein assay system using bovine serum albumin as a standard. In all cases, 5 µg of total protein was separated by either SDS–PAGE (15% polyacrylamide) or native PAGE (7.5% polyacrylamide). After transfer to a nitrocellulose membrane (Hybond ECL nitrocellulose membrane; Amersham), the proteins were reacted with either anti-PII antibody (initially raised against purified E.coli GlnB protein, but also cross-reactive against E.coli and A.vinelandii GlnK), anti-AmtB antibody or anti-(tetra-)His antibody (Qiagen). Antibody against E.coli AmtB was a polyclonal antibody raised in rats against purified protein prepared in this laboratory (D.Blakey, A.Leach, G.H.Thomas, G.Coutts, K.Findlay and M.Merrick, in preparation). A 100 µl aliquot of whole-cell extract from strain FT8000 (ΔglnB,ΔglnK) was added routinely to the anti-PII primary incubations to reduce non-specific interactions. Signals were then detected using the ECL system (Amersham).
Fractionation
A pre-culture grown in Luria broth was used to inoculate 500 ml of modified M9 medium, M9Gln (or Burk’s sucrose medium –N for Azotobacter) using a 1:50 dilution. The cells were grown to an OD650 of 1.3–1.4 when they were harvested by centrifugation at 5000 r.p.m. at 4°C for 10 min in an SLA-1500 rotor and resuspended in 0.75 ml of 50 mM sodium phosphate buffer pH 7.0. For ammonia shock experiments, an initial 1 l culture was spilt into two cultures of 500 ml just prior to shock and one was treated by addition of NH4Cl to a final concentration of 30 mM and harvested 15 min later. Breakage was achieved by five 15 s sonications on ice in an MSE Soniprep 150 sonicator at an amplitude of 10 µm with 15 s intervals between bursts. The extract was clarified by centrifugation in a microfuge at 16 000 g for 4 min. A 1 ml aliquot of the supernatant was then transferred to an 11 × 34 mm polycarbonate ultracentrifuge tube (Beckman) and centrifuged at 250 000 g at 4°C for 30 min using a Beckman TLA-120.2 rotor in a Beckman TL-100 ultracentrifuge; the remaining low-speed supernatant was used as the whole-cell fraction. After ultracentrifugation, the top 0.1 ml was taken from the tube and used as the cytoplasmic fraction; the rest of the supernatant was disposed of. The membrane pellet was resuspended in 1 ml of 50 mM sodium phosphate buffer and centrifuged again at 250 000 g. The supernatant was discarded, and the membrane pellet was resuspended in 1 ml of 50 mM sodium phosphate buffer and centrifuged again at 250 000 g. The pellet from this third centrifugation was resuspended in 50 µl of 50 mM sodium phosphate buffer and used as the membrane fraction. The purity of the membrane fractions was confirmed by western blotting with an antibody against a known cytoplasmic protein, namely pyruvate formate lyase.
To wash the membranes stringently, the cells were treated as above but after the third centrifugation the membrane pellet was resuspended in 50 mM sodium phosphate, 600 mM NaCl buffer pH 7.0. This was then centrifuged twice at 250 000 g using the same high-salt buffer, after which the pellet was resuspended in 16 µl (or other suitable volume) of 50 mM sodium phosphate buffer.
Acknowledgments
Acknowledgements
The authors thank Christina Kennedy and Paul Rudnick for the gift of strain MV560, Tania Arcondéguy for plasmids pTA40 and pTA43, Gary Sawers for the gift of antibody against pyruvate formate lyase, Wally van Heewijk and Daniel Kahn for purified GlnK and antibody against E.coli GlnB, and Richard Little for advice on native gel electrophoresis. Barbara Reinhold-Hurek and Dietmar Martin are thanked for initially drawing our attention to the likely stability of the Glnk–AmtB complex. Sara Austin, Ray Dixon, Tracy Palmer, Richard Little and Gary Sawers are all thanked for helpful comments on the manuscript. G.C. acknowledges a BBSRC studentship. D.B. and M.M. were supported by a grant-in-aid from the BBSRC to the John Innes Centre. G.T. was supported by a grant from CEE (EURATINE:BIOTECH94-2310).
References
- Arcondéguy T., van Heeswijk,W.C. and Merrick,M. (1999) Studies on the roles of GlnK and GlnB in regulating Klebsiella pneumoniae NifL-dependent nitrogen control. FEMS Microbiol. Lett., 180, 263–270. [DOI] [PubMed] [Google Scholar]
- Arcondéguy T., Jack,R. and Merrick,M. (2001) PII signal transduction proteins: pivotal players in microbial nitrogen control. Microbiol. Mol. Biol. Rev., 65, 80–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. and Ninfa,A.J. (1998) Role of the GlnK signal transduction protein in the regulation of nitrogen assimilation in Escherichia coli. Mol. Microbiol., 29, 431–447. [DOI] [PubMed] [Google Scholar]
- Atkinson M. and Ninfa,A.J. (1999) Characterization of the GlnK protein of Escherichia coli. Mol. Microbiol., 32, 301–313. [DOI] [PubMed] [Google Scholar]
- Bender R.A., Janssen,K.A., Resnick,A.D., Blumenberg,M., Foor,F. and Magasanik,B. (1977) Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J. Bacteriol., 129, 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P.E. and Brill,W.J. (1977) Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J. Bacteriol., 130, 954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno R., Pahel,G. and Magasanik,B. (1985) Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J. Bacteriol., 164, 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr P.D., Cheah,E., Suffolk,P.M., Vasudevan,S.G., Dixon,N.E. and Ollis,D.L. (1996) X-ray structure of the signal transduction protein PII from Escherichia coli at 1.9 Å. Acta Crystallogr. D, 52, 93–104. [DOI] [PubMed] [Google Scholar]
- Chang A.C. and Cohen,S.N. (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol., 134, 1141–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.M., Backman,K. and Magasanik,B. (1982) Characterisation of a gene, glnL, the product of which is involved in the regulation of nitrogen utilisation in Escherichia coli. J. Bacteriol., 150, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar T., Snel,B., Huynen,M. and Bork,P. (1998) Conservation of gene order: a fingerprint of proteins that physically interact. Trends Biochem. Sci., 23, 324–328. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M. (1998) Structural homologues PII and PZ of Azospirillum brasilense provide intracellular signalling for selective regulation of various nitrogen-dependent functions. Mol. Microbiol., 29, 449–463. [DOI] [PubMed] [Google Scholar]
- Forchhammer K. and Tandeau de Marsac,N. (1994) The PII protein in the cyanobacterium Synechococcus sp. strain PCC 7942 is modified by serine phosphorylation and signals the cellular N-status. J. Bacteriol., 176, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer K., Hedler,A., Strobel,H. and Weiss,V. (1999) Heterotrimerization of PII-like signalling proteins: implications for PII-mediated signal transduction systems. Mol. Microbiol., 33, 338–349. [DOI] [PubMed] [Google Scholar]
- He L., Soupene,E., Ninfa,A.J. and Kustu,S. (1998) Physiological role for the GlnK protein of enteric bacteria: relief of NifL inhibition under nitrogen-limiting conditions. J. Bacteriol., 180, 6661–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisbergues M., Jeanjean,R., Joset,F., Tandeau de Marsac,N. and Bedu,S. (1999) Protein PII regulates both inorganic carbon and nitrate uptake and is modified by a redox signal in Synechocystis PCC 6803. FEBS Lett., 463, 216–220. [DOI] [PubMed] [Google Scholar]
- Howitt S. and Udvardi,M. (2000) Structure, function and regulation of ammonium transporters in plants. Biochim. Biophys. Acta, 1465, 152–170. [DOI] [PubMed] [Google Scholar]
- Hsieh M.H., Lam,H.M., van de Loo,F.J. and Coruzzi,G. (1998) A PII-like protein in Arabidopsis: putative role in nitrogen sensing. Proc. Natl Acad. Sci. USA, 95, 13965–13970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack R., de Zamaroczy,M. and Merrick,M. (1999) The signal transduction protein GlnK is required for NifL-dependent nitrogen control of nif expression in Klebsiella pneumoniae. J. Bacteriol., 181, 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggi R., Ybarlucea,W., Cheah,E., Carr,P.D., Edwards,K.J., Ollis,D. and Vasudevan,S.G. (1996) The role of the T-loop of the signal transducing protein PII from Escherichia coli. FEBS Lett., 391, 223–228. [DOI] [PubMed] [Google Scholar]
- Jayakumar A., Schulman,I., Macneil,D. and Barnes,E.M. (1986) Role of the Escherichia coli glnALG operon in regulation of ammonium transport. J. Bacteriol., 166, 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Peliska,J.A. and Ninfa,A.J. (1998) Enzymological characterization of the signal-transducing uridylyltransferase/uridylyl-removing enzyme (EC 2.7.7.59) of Escherichia coli and its interaction with the PII protein. Biochemistry, 37, 12782–12794. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Rodriguez,R., Lara,C. and Omata,T. (1997) Involvement of the C-terminal domain of an ATP-binding subunit in the regulation of the ABC-type nitrate/nitrite transporter of the cyanobacterium Synechococcus sp. strain PCC 7942. J. Biol. Chem., 272, 27197–27201. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Boos,W., Bouche,J.P. and Plumbridge,J. (2000) Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J., 19, 5353–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A.J., Phillips,D. and Church,G.M. (1997) Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol., 179, 6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M.C. and Heitman,J. (1998) The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J., 17, 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini A.-M. and André,B. (2000) In vivo N-glycosylation of the Mep2 high-affinity ammonium transporter of Saccharomyces cerevisiae reveals an extracytosolic N-terminus. Mol. Microbiol., 38, 552–564. [DOI] [PubMed] [Google Scholar]
- Marini A.-M., Matassi,G., Raynal,V., André,B., Cartron,J.-P. and Chérif-Zahar,B. (2000a) The human Rhesus-blood-group associated RhAG protein and a novel kidney homologue promote ammonium transport in yeast. Nature Genet., 26, 341–344. [DOI] [PubMed] [Google Scholar]
- Marini A.-M., Springael,J.Y., Frommer,W.B. and André,B. (2000b) Cross-talk between ammonium transporters in yeast and interference by the soybean SAT1 protein. Mol. Microbiol., 35, 378–385. [DOI] [PubMed] [Google Scholar]
- Meletzus D., Rudnick,P., Doetsch,N., Green,A. and Kennedy,C. (1998) Characterization of the glnK–amtB operon of Azotobacter vinelandii. J. Bacteriol., 180, 3260–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos M.L., Muro-Pastor,A.M., Herrero,A. and Flores,E. (1998) Ammonium/methylammonium permeases of a cyanobacterium. Identification and analysis of three nitrogen-regulated amt genes in Synechocystis sp. PCC 6803. J. Biol. Chem., 273, 31463–31470. [DOI] [PubMed] [Google Scholar]
- Muro-Pastor A.M., Ostrovsky,P. and Maloy,S. (1997) Regulation of gene expression by repressor localization: biochemical evidence that membrane and DNA binding by the PutA protein are mutually exclusive. J. Bacteriol., 179, 2788–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak A.A., Jiang,P. and Ninfa,A.J. (2000) The Escherichia coli PII signal transduction protein regulates the activities of the two-component system transmitter protein NRII by direct interaction with the kinase domain of the transmitter module. Biochemistry, 39, 13450–13461. [DOI] [PubMed] [Google Scholar]
- Reyes-Ramirez F., Little,R. and Dixon,R. (2001) Role of Escherichia coli nitrogen regulatory genes in the nitrogen response of the Azotobacter vinelandii NifL–NifA complex. J. Bacteriol., 183, 3076–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M.H. Jr et al. (1999) Phylogenetic characterisation of novel transport protein families revealed by genome analysis. Biochim. Biophys. Acta, 1422, 1–56. [DOI] [PubMed] [Google Scholar]
- Shapiro L. and Losick,R. (2000) Dynamic spatial regulation in the bacterial cell. Cell, 100, 89–98. [DOI] [PubMed] [Google Scholar]
- Soupene E., He,L., Yan,D. and Kustu,S. (1998) Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc. Natl Acad. Sci. USA, 95, 7030–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Kimata,K. and Aiba,H. (2000) A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J., 19, 5344–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Coutts,G. and Merrick,M. (2000a) The glnKamtB operon: a conserved gene pair in prokaryotes. Trends Genet., 16, 11–14. [DOI] [PubMed] [Google Scholar]
- Thomas G., Mullins,J.G.L. and Merrick,M. (2000b) Membrane topology of the Mep/Amt family of ammonium transporters. Mol. Microbiol., 37, 331–344. [DOI] [PubMed] [Google Scholar]
- van Heeswijk W.C., Hoving,S., Molenaar,D., Stegeman,B., Kahn,D. and Westerhoff,H.V. (1996) An alternative PII protein in the regulation of glutamine synthetase in Escherichia coli. Mol. Microbiol., 21, 133–146. [DOI] [PubMed] [Google Scholar]
- van Heeswijk W.C., Wen,D., Clancy,P., Jaggi,R., Ollis,D.L., Westerhoff,H.V. and Vasudevan,S.G. (2000) The Escherichia coli signal transducers PII (GlnB) and GlnK form heterotrimers in vivo: fine tuning the nitrogen signal cascade. Proc. Natl Acad. Sci. USA, 97, 3942–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.F. and Kushner,S.R. (1991) Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene, 100, 195–199. [PubMed] [Google Scholar]
- Xu Y., Cheah,E., Carr,P.D., van Heeswijk,W.C., Westerhoff,H.V., Vasudevan,S.G. and Ollis,D. (1998) GlnK, a PII-homologue: structure reveals ATP binding site and indicates how the T-loops may be involved in molecular recognition. J. Mol. Biol., 282, 149–165. [DOI] [PubMed] [Google Scholar]
- Yakunin A.F. and Hallenbeck,P.C. (2000) AmtB is necessary for NH4+ induced nitrogenase switch-off and ADP-ribosylation in Rhodobacter capsulatus. In Pedrosa,F.O., Hungria,M., Yates,M.G. and Newton,W.E. (eds), Nitrogen Fixation: From Molecules to Crop Productivity. Kluwer Academic, Dordrecht, The Netherlands, pp. 95–96.