Abstract
GTPases of the Rho family are transducers of extracellular signals and control cellular processes such as organization of the actin cytoskeleton, motility, adhesion and gene regulation. The Rho signalling pathway is activated, for example, by bioactive sphingolipids such as sphingosine-1-phosphate (SPP) or by overexpression of Rho family members in tumorigenesis and metastases. Here, we show that stimulation of the Rho signalling pathway induces translocation of the transcriptional LIM-only coactivator FHL2 to the nucleus and subsequent activation of FHL2- and androgen receptor-dependent genes. Interestingly, prostate tumours overexpress Rho GTPases and display altered cellular localization of FHL2 concomitant with tumour dedifferentiation. SPP-induced FHL2 activation is mediated by Rho GTPases, but not by the GTPases Cdc42, Rac1 or Ras, and depends on Rho-kinase. In addition, Rho signalling influences other transcriptional coactivators, thus pointing to a general regulatory role for Rho GTPases in cofactor function. In summary, our data propose a yet undescribed signalling pathway in which the coactivator FHL2 acts as a novel molecular transmitter of the Rho signalling pathway, thereby integrating extracellular cues into altered gene expression.
Keywords: androgen receptor/FHL2/nuclear translocation/small GTPases/transcriptional coactivator
Introduction
The transcriptional coactivator FHL2 is a LIM-only member of the LIM protein superfamily. LIM proteins are defined by the presence of one or multiple copies of the signature LIM domain. The LIM domain is a cysteine-rich motif with the consensus sequence CX2CX16–23HX2 CX2CX2CX16–21CX(C,H,D) that coordinately binds two zinc atoms and mediates protein–protein interactions (Bach, 2000). Based on the number of LIM domains and the presence or absence of additional motifs, LIM proteins are grouped into different classes (Bach, 2000). One class comprises LIM-only proteins that are composed solely of up to five LIM domains. A newly identified subclass of LIM-only proteins contains four and a half LIM domains and are designated as FHL proteins. The five members of the FHL subclass (FHL1–4 and ACT) display a restricted expression pattern in tissues such as striated muscle, heart, prostate, ovary and testis (Morgan and Madgwick, 1996, 1999a,b; Chan et al., 1998; Fimia et al., 1999; Müller et al., 2000). Very little is known about the biological function of FHL proteins but, recently, ACT, FHL3 and FHL2 were characterized as coactivators for the transcription factors CREM/CREB and androgen receptor (AR), respectively (Fimia et al., 1999, 2000; Müller et al., 2000). FHL2 contains a strong, autonomous transactivation function and binds specifically to the AR in vitro and in vivo (Müller et al., 2000). In an agonist- and activation function-2 (AF-2)-dependent manner, FHL2 selectively increases the transcriptional activity of the AR (Müller et al., 2000).
An emerging question in transcriptional regulation is whether extracellular signals might be transmitted into altered gene expression programmes by the modulation of cofactor activity. Indeed, recent data show that coactivators of the p160 family such as SRC-1 or AIB1 are direct targets of mitogen-activated protein (MAP) kinases which modulate the transcriptional activity of these coactivators (Font de Mora and Brown, 2000; Rowan et al., 2000). In addition, p38 MAP kinase signalling was shown to relieve negative constraints mediated by a putative repressor from PGC-1, thereby inducing coactivator function (Knutti et al., 2001). An alternative strategy to regulate cofactor function is employed by corepressors such as class II histone deacetylases (HDACs) during striated muscle cell differentiation. CaM kinase-dependent phosphorylation of nuclear class II HDACs allows binding to 14-3-3 proteins, dissociation from transcriptional complexes and sequestration of HDACs into the cytoplasm (McKinsey et al., 2000). Translocation into a different cellular compartment was also demonstrated to be the mechanism of action for the transcriptional regulator CASK/LIN-2, which can be translocated from the cell membrane to the nucleus (Hsueh et al., 2000).
Interestingly, FHL2 was shown to bind to integrins and is found in focal adhesions in cultured cells (Wixler et al., 2000; Li et al., 2001a). Since transcriptional coactivators function in the nucleus, whereas localization of FHL2 seems to be both nuclear and non-nuclear, we aimed to correlate FHL2 localization with transcriptional activity. The association of FHL2 with the cytoskeleton led us to explore a possible involvement of Rho family GTPases in the regulation of FHL2 function. The Rho family GTPases, which include RhoA, Rac1 and Cdc42, work as molecular switches, being inactive when bound to GDP and active when bound to GTP. The exchange of GTP for GDP induces a conformational change that allows the GTPases to bind effector molecules, such as protein kinases, and initiate downstream signalling events (Hall, 1998; Bishop and Hall, 2000). Rho family GTPases are well known for their ability to regulate actin cytoskeletal remodelling in response to extracellular signals, thereby promoting changes in cell morphology, adhesion and motility (Hall, 1998). In addition, by affecting multiple signalling pathways, Rho family members regulate cell cycle progression, cellular transformation and metastasis, and have been implicated in transcriptional regulation (Bar-Sagi and Hall, 2000). Recent data show that RhoA-, Rac1- and Cdc42-dependent activation of the transcription factor serum response factor (SRF) is mediated by actin treadmilling (Sotiropoulos et al., 1999). The same Rho family GTPases were also reported to down-regulate oestrogen receptor activity (Su et al., 2001). In contrast, RhoA-specific activation of the ERK6 (p38γ) MAP kinase pathway leads to the stimulation of the transcription factors ATF-2, MEF-2A and GATA-4 (Charron et al., 2001; Marinissen et al., 2001).
Here, we demonstrate that stimulation of the Rho signalling pathway by extracellular stimuli such as the bioactive lipids lysophosphatidic acid (LPA) or sphingosine-1-phosphate (SPP) induces gene expression dependent on the transcriptional coactivator FHL2. SPP-induced FHL2 activation is mediated exclusively by Rho GTPases, but not by the closely related small GTPases Cdc42, Rac1 or Ras, and depends on Rho-kinase. Interestingly, prostate tumours overexpress Rho GTPases and display altered cellular localization of FHL2 concomitant with tumour dedifferentiation. Consequently, we show that stimulation of the Rho signalling pathway induces translocation of the coactivator FHL2 to the nucleus and transcriptional activation of FHL2- and AR-dependent reporter genes. Rho signalling not only affects the activity of FHL2 but also influences other transcriptional cofactors such as FHL3 or p300, thus revealing a general signalling pathway linking Rho to transcriptional regulation.
Results
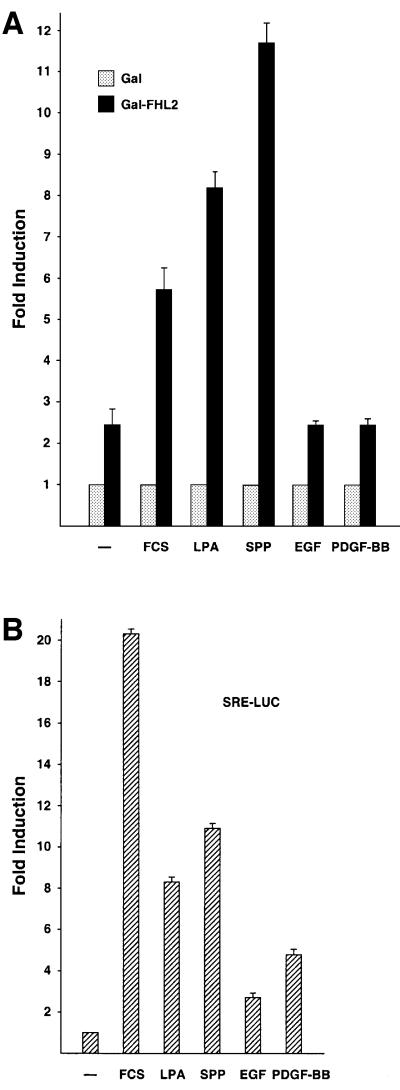
We were interested in analysing whether FHL2 might be involved in signal-regulated gene expression. To address this issue, we first performed transient transfection experiments with Gal-FHL2 expression constructs and asked whether the transcriptional activity of Gal-dependent reporter genes might be regulated by extracellular signals. Indeed, stimulation of serum-starved NIH 3T3 cells with fetal calf serum (FCS) for 3 h led to a significant increase in Gal-FHL2-dependent reporter activity (Figure 1A). Growth factors such as epidermal growth factor (EGF) or platelet-derived growth factor (PDGF)-BB have no effect on Gal-FHL2 activity although these growth factors were active and stimulated an SRF-dependent reporter control (Figure 1B). Gal-FHL2 activity is also not altered during the cell cycle, as tested either with synchronized cells after different time points of G1 release or by using mimosine and nocodazole which block at the G1/S and G2/M borders, respectively (data not shown). However, when we applied the bioactive lipids LPA or SPP, Gal-FHL2-dependent reporter activity is stimulated robustly (Figure 1A). Stimulation by either FCS or SPP follows a rapid and transient time course. Gal-FHL2-dependent transcriptional activity is already significantly increased after 2 h, peaks at ∼3–4 h and returns to unstimulated levels after 24 h (data not shown). Immunofluorescence analyses confirmed that the cellular localization of Gal-FHL2 corresponds to that of endogenous FHL2 (compare Figures 4A and 5B).
Fig. 1. Activation of FHL2 in response to extracellular stimuli in NIH 3T3 cells. (A) Cells were transfected with the Gal-dependent reporter G5E1b-LUC and expression plasmids for either Gal-FHL2 or the Gal4 DNA-binding domain (Gal) and stimulated with either 10% FCS, 10 µM LPA, 1.2 µM SPP, 50 ng/ml EGF or 50 ng/ml PDGF-BB for 3 h. Cells were serum-starved in 0.5% FCS for 25 h before stimulation. The relative luciferase activity of Gal was set to 1. (B) Stimuli (as in A) were controlled for activity on an SRE-LUC promoter.
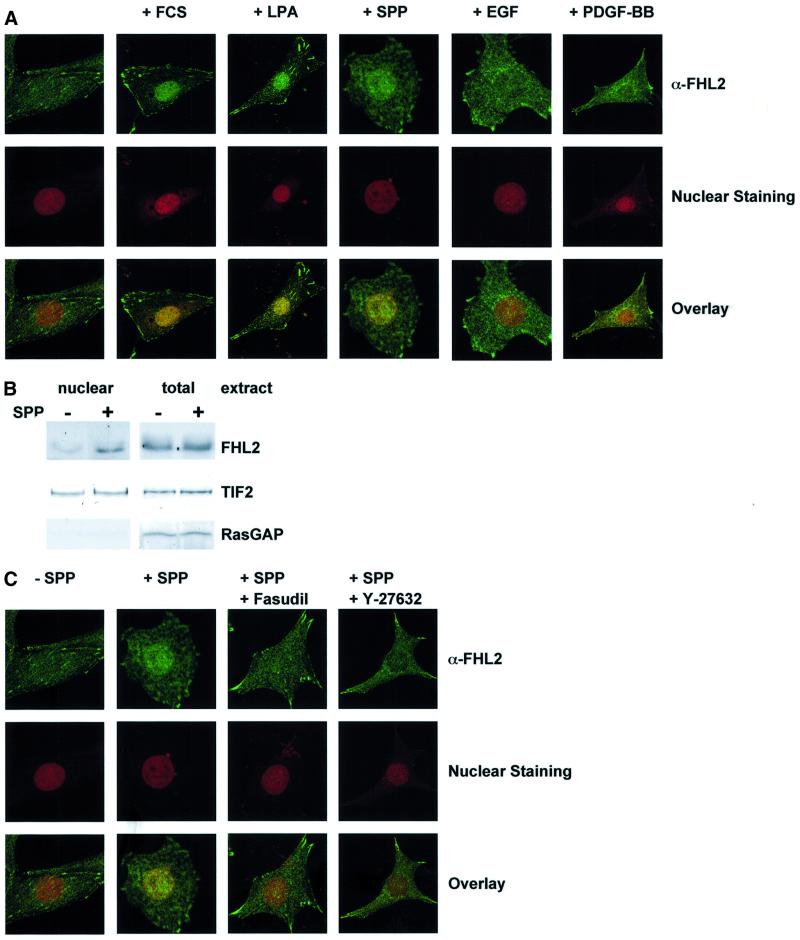
Fig. 4. Endogenous FHL2 becomes nuclear after stimulation of the Rho signalling pathway. (A) Confocal laser scanning images of FHL2 expression (green) in serum-starved NIH 3T3 cells. Cells were treated with either vehicle or 10% FCS, 10 µM LPA, 0.6 µM SPP, 50 ng/ml EGF or 50 ng/ml PDGF-BB for 2 h. Nuclei were stained with propidium iodide (red). (B) Western blot analysis of nuclear and total extracts of NIH 3T3 cells with or without 2 h of stimulation with 0.6 µM SPP. Fractionation was controlled by using nuclear (TIF2) and cytoplasmic (RasGAP) marker proteins. (C) Nuclear localization of FHL2 is inhibited by pre-incubation of NIH 3T3 cells with the Rock inhibitors Fasudil or Y-27632 (10 µM each). Confocal laser scanning images of FHL2 expression (green) in serum-starved NIH 3T3 cells treated for 2 h with either vehicle (–SPP) or 0.6 µM SPP (+SPP). Nuclei were stained with propidium iodide (red).
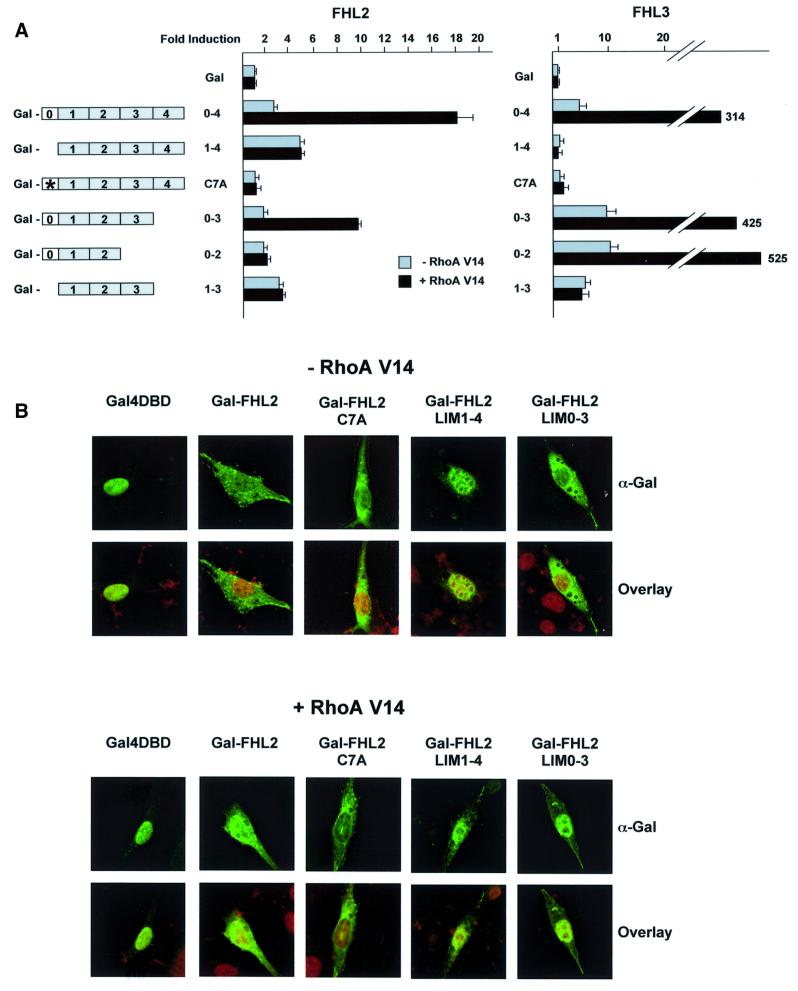
Fig. 5. Characterization of the FHL2 LIM domains involved in Rho signalling. (A) The N-terminal half LIM domain and LIM domain 3 are required for activation of FHL2 by Rho. The activity of the different Gal-FHL2 and Gal-FHL3 deletion and point mutants (schematic representation on the left) were analysed on the Gal-dependent reporter G5E1b-LUC in the absence (–) or presence (+) of constitutively active RhoA V14 in NIH 3T3 cells. The relative luciferase activity of Gal was set to 1. (B) Localization of Gal-FHL2 mutants corroborates that the N-terminal LIM domain 0 and LIM domain 3 are essential for Rho-induced nuclear localization. Confocal laser scanning microscopy of NIH 3T3 cells transfected with Gal-FHL2 mutants and constitutively active RhoA V14 as indicated. Cells were stained with α-Gal antibody (green). Nuclei were visualized with propidium iodide (red).
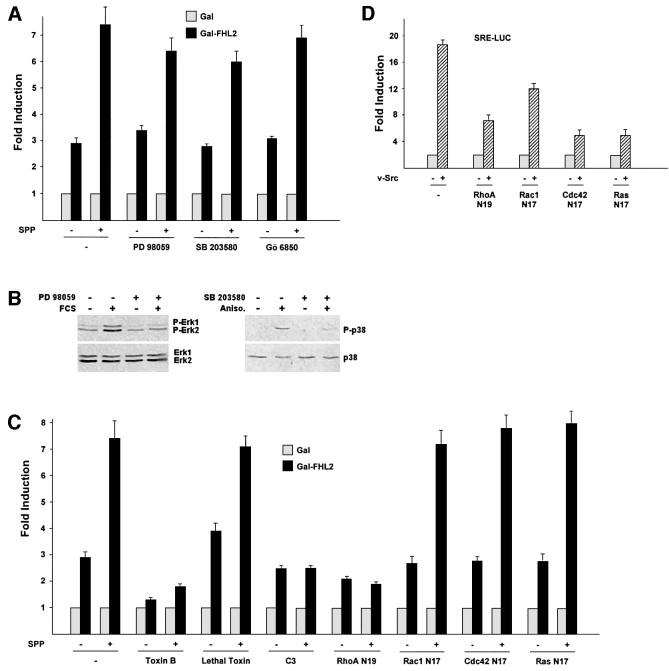
SPP and LPA are serum components that activate small GTPases of the Rho family via G-protein-coupled receptors (Pyne and Pyne, 2000). To elucidate the relevant signalling pathways that are involved in the activation of FHL2-dependent gene expression by SPP, we utilized specific inhibitors of major signalling pathways. Blocking the MAP kinase pathway by the MEK1 inhibitor PD 98059 or the use of the p38 MAP kinase inhibitor SB 203580 does not interfere with the transcriptional activation by SPP (Figure 2A). Stimulation of Gal-FHL2-dependent reporter activity is also not mediated by protein kinase C (PKC), since Gö 6850, an inhibitor of all PKCs (Martiny-Baron et al., 1993), shows no inhibitory effect (Figure 2A). Furthermore, co-transfection of the catalytical subunit of protein kinase A does not stimulate reporter activity, indicating that this pathway is also not involved (data not shown). In addition, constitutively active MKK3/6 with or without co-expression of p38γ does not influence Gal-FHL2 activity (Figure 3C and data not shown). The specificity of constitutively active MKK3/6 is controlled by the known ability of these proteins to activate a serum response element (SRE)-dependent reporter gene (data not shown). To control for the specificity of the MAP kinase inhibitors, we show that PD 98059 blocks FCS-induced phosphorylation of Erk1 and Erk2 and that SB 203580 blocks anisomycin-induced phosphorylation of p38 (Figure 2B).
Fig. 2. The Rho pathway in NIH 3T3 cells specifically mediates activation of FHL2. (A) Gal-FHL2-dependent reporter gene activation was analysed with or without 3 h of stimulation with 0.6 µM SPP. Activation was not significantly altered in the presence of either PD 98059 (10 µM), SB 203580 (10 µM) or Gö 6850 (1 µM). (B) Western blot controls show inhibition of FCS-mediated Erk and anisomycin-mediated p38 phosphorylation by PD 98059 or SB 203580 (10 µM each), respectively. The upper panel depicts the phospho forms of Erk1, -2 or p38, the lower panel total Erk1, -2 or p38. (C) Inhibitors of the Rho pathway block activation of the Gal-FHL2-dependent reporter gene. Activation was analysed with or without 3 h of stimulation with 0.6 µM SPP in the presence of either toxin B (10 ng/ml), lethal toxin (20 ng/ml) or C3 exoenzyme (0.5 µg/ml), or upon co-transfection with dominant-negative mutants of RhoA (RhoA N19), Rac1 (Rac1 N17), Cdc42 (Cdc42 N17) or Ras (Ras N17). (D) Specificity control for the dominant-negative mutants of RhoA (RhoA N19), Rac1 (Rac1 N17), Cdc42 (Cdc42 N17) or Ras (Ras N17). SRE-LUC is activated by co-transfection of v-Src.
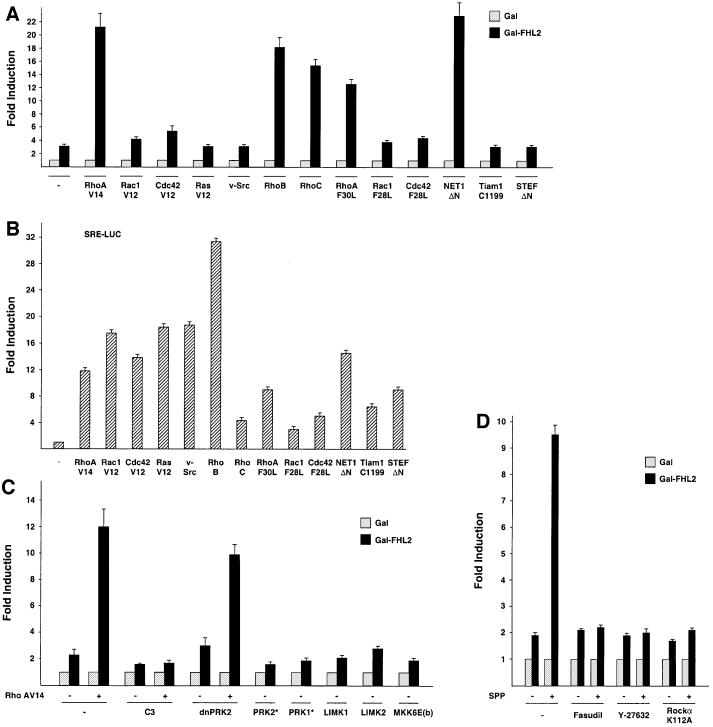
Fig. 3. Only Rho and Rho-specific GEFs activate FHL2 in NIH 3T3 cells. (A) Gal-FHL2 activity was analysed in the presence of expression plasmids coding for small GTPases or GEFs: constitutively active mutants of RhoA (RhoA V14), Rac1 (Rac1 V12), Cdc42 (Cdc42 V12), Ras (Ras V12), Src (v-Src), wild-type RhoB and RhoC, fast cycling mutants of RhoA (RhoA F30L), Rac1 (Rac1 F28L), Cdc42 (Cdc42 F28L) or the constitutively active GEFs NET1ΔN, Tiam1 C1199 or STEFΔN. (B) The activity of the expression plasmids (as in A) was controlled on a SRE-LUC promoter. (C) Influence of Rho signalling on Gal-FHL2 activity. Expression plasmids coding for C3 exoenzyme, dominant-negative PRK2 (dnPRK2), constitutively active PRK2 (PRK2*) and PRK1 (PRK1*), LIM kinase-1 (LIMK1), LIM kinase-2 (LIMK2) or constitutively active MKK6b [MKK6E(b)] were analysed for their ability to influence Gal-FHL2 activity. As indicated (–/+), constitutively active RhoA (RhoA V14) was co-transfected. (D) Involvement of Rock in Gal-FHL2 activation was analysed using either the Rock inhibitors Fasudil or Y-27632 (10 µM each) or upon co-transfection of dominant-negative Rockα (Rockα K112A). As indicated (–/+), cells were untreated or stimulated for 3 h with 0.6 µM SPP.
Next, we tested the signalling pathway of endogenous small GTPases. Toxin B from Clostridium difficile, a specific inhibitor of Rho, Rac1 and Cdc42 GTPases (Busch and Aktories, 2000), blocks both basal and SPP-mediated activation of Gal-FHL2-dependent reporter expression (Figure 2C). In contrast, lethal toxin from Clostridium sordelli, which inhibits Ras, Rac1 and Cdc42 but not Rho (Busch and Aktories, 2000), fails to do so. Clostridium botulinum C3 exoenzyme, which is a specific RhoA, -B and -C inhibitor (Busch and Aktories, 2000) completely blocks SPP-mediated activation of Gal-FHL2-dependent reporter expression. C3 exoenzyme also abrogates Gal-FHL2 activation by constitutively active RhoA (Figure 3C). Accordingly, co-transfection of RhoA N19, a dominant-negative mutant of RhoA, completely inhibits the transcriptional activation by SPP, whereas the dominant-negative mutants Rac1 N17, Cdc42 N17 or Ras N17 fail to show any inhibitory effect (Figure 2C). As demonstrated in Figure 2D, all dominant-negative mutants are functional in our assay system and down-regulate, as recently described, the v-Src-induced activity of an SRE-LUC reporter (Chiariello et al., 2001). Taken together, these data demonstrate that the bioactive lipid SPP stimulates signalling by Rho and that blocking of the endogenous Rho signalling pathway severely impairs FHL2 transactivation.
To evaluate further the specificity of Rho, we tested constitutively active mutants of Rho, Rac1, Cdc42, Ras and Src. Only the constitutively active RhoA mutant (RhoA V14) but not the respective Rac1, Cdc42, Ras or Src mutants, induces Gal-FHL2-dependent gene expression (Figure 3A). We also tested the potential of wild-type RhoB and RhoC to activate Gal-FHL2-regulated reporters. As shown in Figure 3A, both wild-type RhoB and RhoC activate reporter expression to an extent similar to constitutively active RhoA. In addition, we also used the fast cycling mutants of RhoA (RhoA F30L), Rac1 (Rac1 F28L) and Cdc42 (Cdc42 F28L) which maintain normal GTP hydrolysis, but are independent of GDP–GTP exchange factors (GEFs) (Lin et al., 1999). Again, only RhoA F30L induces transcriptional activity by Gal-FHL2 (Figure 3A). GEFs bind small GTPases of the Rho family and are involved in promoting nucleotide exchange (Cerione and Zheng, 1996). Thus, we tested whether expression of GEFs is able to promote Gal-FHL2-dependent gene expression by acting on endogenous Rho GTPases. The activated form of the Rho-specific GEF NET1 (NET1ΔN) potently stimulated the Gal-FHL2-dependent reporter, whereas the activated forms of the Rac1- or Rac1- and Cdc42-specific GEFs Tiam1 (Tiam C1199) and STEF (STEFΔN), respectively, failed (Figure 3A). As expected (Sotiropoulos et al., 1999; Chiariello et al., 2001), v-Src, all GTPases or mutants thereof, or the GEFs activate the expression of an SRE-LUC reporter (Figure 3B). Although there might be extensive cross-talk between Cdc42, Rac1 and Rho in certain cell types (Bishop and Hall, 2000), our data show that FHL2 activation in NIH 3T3 cells is mediated exclusively by members of the Rho family, such as RhoA, RhoB or RhoC.
To delineate further the pathway downstream of Rho, we assayed several signalling molecules (Bishop and Hall, 2000). Neither LIM kinase-1, LIM kinase-2, PKC-related kinase 1 (PRK1), and -2 (PRK2), MKK6b (Figure 3C), DMPK, PAK, CRIK, MRCKα nor MKK3b (data not shown) are able to induce Gal-FHL2-regulated expression. In contrast, Fasudil, an inhibitor of Rho-kinase (Rock), completely blocks the transcriptional activation by SPP (Figure 3D) as does the Rock inhibitor Y-27632 (Ishizaki et al., 2000) (Figure 3D). Furthermore, the kinase-dead Rockα mutant K112A inhibits SPP-mediated reporter activation (Figure 3D). This finding indicates a clear difference from the activation of the transcription factor SRF by Rho that is not blocked by the Rock inhibitor Y-27632 (Sahai et al., 1999). SRF furthermore is activated by LIM-kinases 1/2, G-actin and actin treadmilling (Sotiropoulos et al., 1999), which is not the case for FHL2 (Figure 3C and data not shown). The specificity of PRK1/2 and LIM kinases-1/2 in our assay system is controlled by the known ability of these proteins to activate either the c-jun promoter or an SRE-dependent reporter gene (data not shown).
FHL2 recently was reported to bind to integrins and is found in focal adhesions in cultured cells (Wixler et al., 2000; Li et al., 2001a). Since transcriptional coactivators function in the nucleus whereas localization of FHL2 seems to be both nuclear and non-nuclear, we hypothesized that the coactivator FHL2 might be involved in transducing extracellular signals to the nucleus. We therefore tested whether SPP induces nuclear translocation of FHL2. Using two different approaches, immunofluorescence analyses and cell fractionation followed by western blotting, we analysed the subcellular distribution of FHL2. In serum-starved NIH 3T3 cells, endogenous FHL2 is localized in focal adhesions and diffusely in the cytoplasm (Figure 4A). Confocal laser scanning microscopy reveals little FHL2 immunoreactivity in the nucleus. Within 2 h of SPP stimulation, significant immunoreactivity is detected in the nucleus (Figure 4A). Also, FCS and LPA, but neither EGF nor PDGF-BB, stimulation leads to nuclear translocation of FHL2 (Figure 4A) consistent with the pattern of transcriptional activation of Gal-FHL2 (Figure 1A). To demonstrate nuclear translocation of FHL2 alternatively, we performed cellular fractionation of SPP-treated NIH 3T3 cells (Figure 4B). As shown above, SPP stimulation induces nuclear translocation of FHL2 within 2 h. Cellular fractionation was controlled by analysis of nuclear (TIF2) and cytoplasmic (RasGAP) marker proteins. We next tested whether Rho-kinase inhibitors can influence the nuclear translocation of FHL2. Nuclear immunoreactivity of FHL2 after SPP stimulation is not detected when the cells were pre-treated with the Rock inhibitors Fasudil or Y-27632 (Figure 4C). Since transcriptional activity and nuclear translocation of FHL2 are regulated by the same stimuli, our results therefore suggest that at least one mechanism that accounts for the transcriptional activation of FHL2-dependent genes is nuclear translocation.
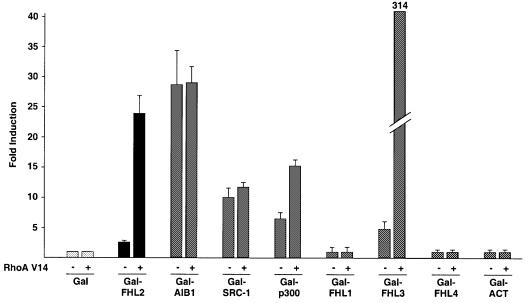
To address the question which LIM domains of FHL2 are required for the activation by Rho, we generated several N- and C-terminal deletion mutants as well as point mutants in the individual LIM domains. All Gal-FHL2 fusion proteins were expressed at similar levels (data not shown). Deletion of the N-terminal half LIM domain results in a mutant (Gal-FHL2LIM1–4) that is more active than wild-type FHL2 but not stimulated further by RhoA V14 (Figure 5A). Removal of LIM domain 4 (Gal-FHL2LIM0–3), however, generates a mutant that is still inducible by RhoA V14, indicating that LIM domain 4 is not required for this activation (Figure 5A). Further deletions of either LIM domains 0 and 4 (Gal-FHL2LIM1–3) or LIM domains 3 and 4 (Gal-FHL2LIM0–2) abolishes inducibility by RhoA V14 although these mutants exhibit considerable basal level activity. To analyse the function of the N-terminal half LIM domain (LIM0) in the context of the full-length protein, we generated the point mutant Gal-FHL2 C7A that disrupts the integrity of the first zinc finger. This mutant is no longer inducible by RhoA V14 and is transcriptionally inactive (Figure 5A). In addition, we tested the corresponding mutants of FHL3, the only other FHL family member that can be induced by Rho (see Figure 8). Again, the N-terminal half LIM domain is required for activation by Rho (Figure 5A).
Fig. 8. Cofactor activation by Rho signalling. Gal fusion proteins of the coactivators AIB1, SRC-1 and p300 or the FHL family members FHL1–4 and ACT were tested on the Gal-dependent reporter G5E1b in NIH 3T3 cells with constitutively active RhoA V14 as indicated (–/+).
To correlate the results of the deletion analysis with the cellular localization of the various Gal-FHL2 proteins, we performed confocal laser scanning microscopy and immunofluorescence analysis with Gal-DNA-binding domain (DBD), full-length Gal-FHL2 and the various mutants thereof (Figure 5B). As expected, Gal-DBD is localized exclusively in the nucleus. In clear contrast, Gal-FHL2 becomes localized prominently in the nucleus only when the cells were stimulated with RhoA V14 (Figure 5B). The transcriptionally hyperactive mutant Gal-FHL2LIM1–4 is already nuclear in the absence of Rho stimulation and Rho signalling does not change its subcellular distribution (Figure 5B). In addition, the mutant protein Gal-FHL2 C7A, which is transcriptionally inactive, is present in the cytoplasma and does not translocate to the nucleus even when stimulated with RhoA V14 (Figure 5A). Accordingly, all other Gal-FHL2 mutants, which do not respond to Rho, also fail to translocate to the nucleus (data not shown). However, the Rho-inducible mutant Gal-FHL2LIM0–3 translocates into the nucleus when stimulated with RhoA V14 (Figure 5B). Taken together, our data indicate that the intact N-terminal half LIM domain together with LIM domain 3 is necessary to respond to the Rho signalling pathway. Consequently, mutants that fail to respond to Rho do not translocate to the nucleus.
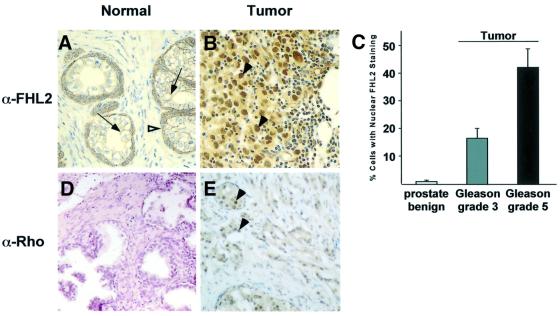
Next, we were interested in knowing whether nuclear translocation of FHL2 not only reflects the in vitro situation but also occurs in vivo. Since FHL2 is expressed in prostate (Müller et al., 2000), we analysed the localization of endogenous FHL2 in human prostate in normal and pathological situations. We performed immunohistochemical analyses of the FHL2 expression pattern in tissue sections of normal human prostate and prostate cancer obtained from radical prostatectomy specimens (n = 11). In the secretory epithelium of normal human prostate tissue, practically the entire FHL2 immunoreactivity is confined to the cell membrane (Figure 6A, arrows). Intense cytoplasmatic FHL2 expression is detected in cells of the normal basal compartment (Figure 6A, open arrowhead). The cancer specimens contained areas of well-differentiated tubular carcinoma (Gleason grade 3) in all 11 radical prostatectomy cases, and of low-differentiated carcinoma (Gleason grade 5) in seven cases. A significant increase in nuclear FHL2 staining is observed in these areas (Figure 6B, arrowheads) and correlates with the loss of tubular and glandular structures. Statistical analysis of the number of cells with nuclear FHL2 shows an increase from 1.5 ± 0.5% of epithelial cell nuclei in benign prostate to 16.7 ± 3.6% in nuclei of Gleason grade 3 carcinoma and further to 42.3 ± 7.4% in nuclei of the more malignant Gleason grade 5 carcinoma (Figure 6C).
Fig. 6. Cellular localization of FHL2 changes during prostate cancer progression (A–C) and correlates with increased Rho expression (D and E). (A) In the secretory epithelium of normal prostate, FHL2 immunoreactivity is confined almost entirely to the cell membrane (arrows). Intense cytoplasmatic staining is detected in the basal cell compartment (open arrowhead). (B) Strong nuclear (arrowheads) and cytoplasmatic staining in undifferentiated prostate carcinoma (Gleason grade 5). (C) Statistical analysis of cells with nuclear FHL2 staining in benign prostate, prostate carcinoma Gleason grade 3 and Gleason grade 5. (D) Immunohistochemistry with an α-Rho(A, -B, -C) antibody shows very weak staining in normal prostate. (E) In undifferentiated prostate carcinoma (Gleason grade 5), pan-Rho staining increases robustly and is strongly nuclear (arrowheads).
Members of the Rho family are important for migration and in vivo dissemination of cancer cells (Clark et al., 2000) and frequently are overexpressed in many human tumours (Fritz et al., 1999). We therefore compared Rho expression in normal prostate versus prostate tumours with a pan-Rho-specific antibody in the same cancer specimens used above. In tissue sections of normal human prostate, very little Rho immunoreactivity is detected (Figure 6D). However, in low-differentiated carcinomas (Gleason grade 5), a dramatic increase in Rho immunoreactivity is observed in those areas (Figure 6E, arrowheads) which correlate with the loss of tubular and glandular structures. Taken together, our data reveal both plasmalemmal and nuclear localization of FHL2 in vivo and indicate that increasing nuclear localization correlates with progression to a highly malignant phenotype of prostate carcinoma cells, which is paralleled by increased expression of Rho GTPase proteins.
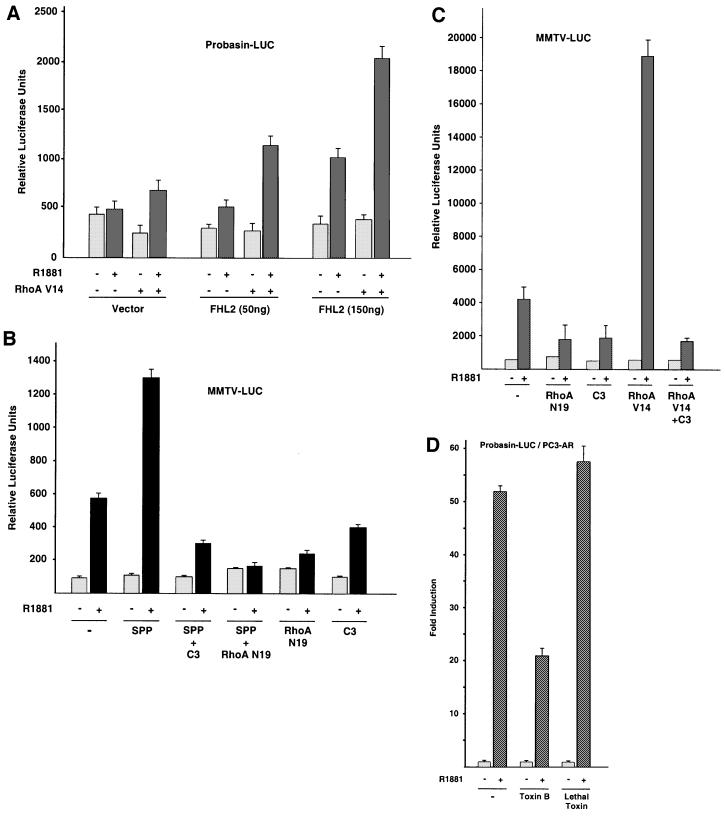
The results shown in Figure 6 suggest that stimulation of the Rho signalling pathway should be paralleled by altered activity of FHL2-regulated prostate-specific target genes. To examine the effect of Rho signalling on a naturally occurring gene, the probasin promoter was chosen. Since the prostate-specific probasin gene is regulated selectively by the combined action of AR and FHL2 (Müller et al., 2000), we tested constitutively active RhoA on a probasin reporter. In the presence of suboptimal concentrations of the synthetic agonist R1881, the AR is only marginally active (Figure 7A). Co-expression of the AR and FHL2 results in an agonist-dependent coactivation, which is up-regulated further in the presence of constitutively active RhoA V14 (Figure 7A). The fact that Rho signalling not only activates Gal-FHL2-dependent reporters but also induces expression of the known AR target gene probasin in an agonist- and FHL2-dependent manner supports a role for Rho in FHL2-mediated gene regulation.
Fig. 7. Rho signalling activates FHL2- and AR-dependent reporter genes. (A) Specific activation of the AR target gene probasin-LUC by co-expression of AR, FHL2 and constitutively active RhoA V14. 293 cells were harvested 20 h after addition of 10–10 M of the AR-agonist R1881. (B) The endogenous Rho signalling pathway suffices to activate the AR. 293 cells co-transfected with the MMTV-LUC reporter and AR were stimulated for 20 h with (+) or without (–) 10–9 M R1881. As indicated, cells were either stimulated for 20 h with 1.2 µM SPP or co-transfected with expression plasmids coding for C3 exoenzyme or dominant-negative RhoA (RhoA N19). (C) C3 exoenzyme or dominant-negative RhoA N19 blocks activation of AR by constitutively active RhoA V14. 293 cells were co-transfected with the MMTV-LUC reporter and AR, and 10–9 M R1881 was added for 20 h as indicated (–/+). (D) Blocking of the endogenous Rho signalling by toxin B but not by lethal toxin inhibits activation of the endogenous AR. PC3-AR cells were harvested 20 h after addition of 10–9 M of the AR-agonist R1881.
To test whether signal transduction by the endogenous Rho signalling pathway is sufficient to activate AR-dependent gene regulation, we stimulated 293 cells, which express FHL2 endogenously, with physiological concentrations of SPP. As shown in Figure 7B, SPP stimulation further increases the agonist-dependent activation of an MMTV reporter. Importantly, both agonist- and SPP-induced reporter activity is blocked by the Rho-specific inhibitor C3 exoenzyme (Figure 7B). In addition, the dominant-negative RhoA mutant RhoA N19 completely blocks basal and SPP-stimulated MMTV-LUC activity (Figure 7B). C3 exoenzyme also blocks the agonist-dependent coactivation in the presence of constitutively active RhoA V14 on the MMTV reporter (Figure 7C). The Rock inhibitor Fasudil also blocks SPP-induced reporter activity (data not shown). Endogenous AR activity in the prostate cell line PC3-AR is also inhibited specifically by toxin B but not by the Ras, Rac1 and Cdc42 inhibitor lethal toxin (Figure 7D). Taken together, these results show that the endogenous Rho signalling pathway suffices to regulate AR activity.
We finally asked whether Rho signalling influences the transcriptional activity of other coactivators. Figure 8 shows that the coactivators AIB1 or SRC-1 do not respond to constitutively active RhoA V14. In contrast, p300 is moderately and FHL3 is potently activated. Interestingly, the other members of the FHL family such as FHL1, FHL4 or ACT do not respond to RhoA V14 (Figure 8). While the mechanism of Rho signalling to p300 and FHL3 currently is not known in detail, activation of FHL2 by Rho signalling describes a novel pathway which may also regulate other coactivators.
Discussion
Our data show that extracellular signals present in FCS or bioactive sphingolipids such as LPA or SPP activate FHL2-dependent transcription, at least in part, by promoting nuclear translocation of FHL2. Detailed analyses of the intracellular signalling pathway reveals that Gal-FHL2 stimulation by SPP is mediated via the endogenous Rho signalling pathway. Toxins such as toxin B that abrogate Rho GTPase signalling, or C3 exoenzyme, the most specific inhibitor of Rho function, potently inhibit SPP-mediated activation of Gal-FHL2-dependent reporter expression. As a control, lethal toxin, which inhibits Ras, Rac1 and Cdc42, but not Rho, fails to do so. We then used an extensive series of dominant-negative, constitutively active and fast cycling mutants of Ras, RhoA, Rac1 or Cdc42 to show that Gal-FHL2 stimulation by SPP indeed is mediated by Rho only. This conclusion is supported further by the inability of PDGF-BB and constitutively active Src to stimulate FHL2. Both were reported recently to signal through the GTPase Rac1 (Chiariello et al., 2001). The activity of small GTPases is regulated by GEFs, which are specific for individual GTPase family members (Cerione and Zheng, 1996). When analysing the potential of GEFs to activate the endogenous signalling factors necessary for Gal-FHL2-dependent activation, only the Rho-specific GEFs such as mNET1 were active in our assays (see Figure 3A). This not only indicates the specificity of FHL2-mediated transcriptional regulation by Rho but again demonstrates that stimulation of the endogenous Rho signalling pathway suffices for activation.
In sharp contrast, serum growth factors such as EGF or PDGF-BB neither activate FHL2-dependent reporter genes nor induce nuclear translocation of FHL2. Accordingly, constitutively active forms of Ras fail to activate FHL2-dependent transcription. Also, neither dominant-negative Ras nor the specific MEK inhibitor PD 98059 influences stimulation of Gal-FHL2 activity by SPP. In addition, both PDGF-BB and constitutively active Src fall short in activation. Taken together, these results demonstrate that FHL2-induced transcriptional activation is independent of the Ras–Raf–MEK–ERK signalling pathway. Therefore, signalling to FHL2 is clearly different from the MAP kinase-dependent regulation of the p160 cofactors SRC-1 or AIB1 (Font de Mora and Brown, 2000; Rowan et al., 2000). The p38α/β (SAPK2a/b) inhibitor SB 203580 does not block stimulation of Gal-FHL2 activity by SPP. Since SB 203580 does not inhibit p38γ/δ (SAPK3/4) (Davies et al., 2000), we excluded the upstream activators MKK3/6 of these kinases as regulators of Gal-FHL2-dependent transcription. Our data demonstrate that the mechanism of FHL2-dependent activation is clearly distinct from the p38 SAPK-regulated activation of the coactivator PGC-1 (Knutti et al., 2001) or the p38γ-dependent Rho signalling leading to the stimulation of the transcription factors ATF-2, MEF-2A and GATA-4 (Charron et al., 2001; Marinissen et al., 2001). Since the specific Rock-I and Rock-II inhibitor Y-26732 does not inhibit JNK phosphorylation (Sahai et al., 1999), but blocks FHL2-dependent reporter gene activation, JNK also seems not to be involved. This is supported further by the fact that Rac1, which is an upstream activator of JNK (Minden et al., 1995), also does not stimulate Gal-FHL2.
We next looked at signalling molecules involved in the Rho pathway. The combined use of either specific Rock inhibitors, such as Fasudil and Y-27632, or dominant-negative and constitutively active mutants show that Rock is a Rho effector leading to FHL2-dependent activation and nuclear translocation of FHL2. Since the Rock inhibitors Fasudil and Y-27632 also block PRK1/PKN and PRK2 kinase activity (Davies et al., 2000), it was important to show that constitutively active forms of both kinases do not alter FHL2, thus corroborating specificity. Furthermore, Gal-FHL2 stimulation was not affected in cells blocked at the G1/S and G2/M borders. In addition, the Rock inhibitorY-27632, which does not block cell cycle re-entry of NIH 3T3 cells (Sahai et al., 1999), efficiently blocks Gal-FHL2-dependent reporter gene activation. These results indicate that no special stage of the cell cycle or the microtubular cytoskeleton is required for FHL2 activation.
Until now, only a restricted number of transcription factors but no coactivators have been shown to be regulated by Rho family GTPases (Bar-Sagi and Hall, 2000; Charron et al., 2001). RhoA activation of the transcription factor SRF was shown to be dependent on changes in actin dynamics. Actin treadmilling and changes in the G-actin pool were characterized as important mediators (Sotiropoulos et al., 1999). Since one function of Rho is the reorganization of the actin cytoskeleton, we tested whether actin or actin treadmilling is required for the FHL2-dependent activation. In clear contrast to SRF, neither jasplakinolide, which binds to and stabilizes F-actin, nor swinholide, which sequesters G-actin as dimers (Sotiropoulos et al., 1999), influenced FHL2-dependent reporter genes significantly (data not shown). Moreover, the SRF activation pattern is clearly different from that of FHL2. SRF-dependent reporters that are activated by Rho are also activated by LIM kinases-1/2, Rac1, Cdc42 or Src and their respective GEFs (Sotiropoulos et al., 1999). Taken together, these results demonstrate that the regulation of SRF and FHL2 activity by Rho is distinct and that the regulation of actin dynamics is not sufficient to control FHL2 function.
Stimulation of the Rho signalling pathway by various treatments results in nuclear translocation of FHL2 (Figure 4 and data not shown). Our results strongly suggest that at least one mechanism which accounts for the transcriptional activation of FHL2 target genes is nuclear translocation. Interestingly, a growing number of LIM proteins including the transcriptional coactivators ARA55 (Fujimoto et al., 1999) or FHL3 (Li et al., 2001b) are localized in both the cytoplasma and the nucleus. So far, no mechanistic explanation has been provided for the control of the cellular localization of these LIM proteins, but it is tempting to speculate that Rho signalling might be involved.
Deletion analyses of FHL2 demonstrate that the N-terminal half LIM domain is required for the Rho-regulated localization of FHL2, since its deletion results in a mutant that is localized in the nucleus, transcriptionally active but no longer inducible by Rho. In contrast, the point mutant FHL2 C7A is locked in the cytoplasm, does not respond to Rho signalling and is transcriptionally inactive. In addition, LIM domain 3 is essential for full activation by Rho signalling. So far, we have not detected any modifications such as phosphorylation that might account for Rho-mediated nuclear translocation of FHL2. A possible scenario might be that FHL2 is retained in the cytoplasm by Rho-controlled protein–protein interactions.
Since FHL2 is expressed in prostate (Müller et al., 2000), we analysed expression of FHL2 and Rho in normal tissue and different stages of malignant carcinoma. Nuclear localization of FHL2 correlates with progression to a more malignant phenotype. In addition, we also observe a dramatic increase in Rho expression in the same tumour samples, and Rho overexpression correlates with the progression to a more malignant carcinoma phenotype. Interestingly, overexpression of RhoC was shown recently to be a prerequisite for metastatic growth (Clark et al., 2000), whereas the inhibitor of Rho-kinase Y-27632 retards migration and in vivo dissemination of human prostate cancer cells (Somlyo et al., 2000). SPP or LPA are components of the serum, but are also liberated in significant amounts in tumours (Pyne and Pyne, 2000), in which we observe strong nuclear FHL2 immunoreactivity (Figure 6 and data not shown). Stimulation of the Rho signalling pathway is paralleled by a transcriptional activation of the AR- and FHL2-dependent prostate-specific probasin promoter. AR is one of the key regulatory molecules important for the development and progression of prostate tumours (Montgomery et al., 2001). Since prostate tumours not only overexpress Rho GTPases, but also show a dramatic increase in nuclear FHL2, our results suggest a Rho-controlled mechanism that regulates FHL2- and AR-dependent gene activation and may add to tumour progression.
Interestingly, the members of the FHL family differ in their response to Rho. FHL1, FHL4 and ACT are not activated, whereas FHL3 is strongly activated by Rho signalling. At the moment, it remains an open question whether structural differences are responsible for the marked difference. Since stimulation of Rho signalling not only influences FHL2 but also regulates the activity of the cofactors p300 and FHL3, our data imply that Rho GTPase signalling might regulate various other cofactors. In addition, a particular transcription factor such as the AR might be targeted through multiple Rho-stimulated cofactors, resulting in specific transcriptional responses. Although the Rho signalling pathway activates transcription factors such as SRF, MEF-2, ATF-2 or GATA-4, the role of coactivators in Rho signalling has not been described so far. Therefore, our results not only define FHL2 as the first transcriptional coactivator involved in the Rho signalling but also integrate cofactors in a novel pathway linking Rho signalling to the activation of transcription factors.
Materials and methods
Plasmids
The following plasmids were described previously: pCMXGal-FHL2, pCMXFHL2, pCMXGal4DBD, pSG5hAR, Gal-SRC-1, MMTV-LUC and G5E1b-LUC (Müller et al., 2000); RhoA V14, Rac V12, Cdc42 V12, Rac N17 and C3 (Hill et al., 1995); pSRE-LUC (Stratagene); RhoA F30L, Rac1 F28L and Cdc42 F28L (Lin et al., 1999); v-Src, Ras V12 obtained from T.Benzing, Freiburg; LMK1 and LMK2 (Sotiropoulos et al., 1999); Cdc42 N17 (Minden et al., 1995); mNET1ΔN (Alberts and Treisman, 1998); Tiam1 C1199 (Michiels et al., 1997); RhoB obtained from G.Fritz, Mainz; STEFΔN (Hoshino et al., 1999); PRK1 (Flynn et al., 1998); PRK2 (Gross et al., 2001); RhoAN19 and Rockα K112A (Leung et al., 1998); Gal-p300 obtained from R.Eckner, Zürich; and Gal-AIB1 (Font de Mora et al., 2000). To construct the Gal-FHL2 and Gal-FHL3 deletion and point mutants, the corresponding fragments (LIM0, amino acids 1–36; LIM1, amino acids 37–97; LIM2, amino acids 98–158; LIM3, amino acids 159–217; and LIM4, amino acids 218 to the end) were amplified by PCR and inserted at the BamHI–EcoRI sites of pCMXGalDBD. For Gal fusions of mFHL1, hFHL3, mFHL4 and mACT, the coding sequences from the respective pGBT9 plasmid (Fimia et al., 2000) were inserted in pCMXGal4DBD. For human RhoC, the coding sequence of pCB6-vsv-RhoC (obtained from J.H.Camonis, Paris) was inserted in pCMXPL2 (EcoRI–BamHI). All plasmids were verified by sequencing.
Cell lines and transfections
NIH 3T3 and 293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), and PC3-AR in Ham F-12 supplemented with 10% FCS (NIH 3T3) or 10% double-stripped FCS. Transient transfection assays were carried out in 12-well plates (5 × 104, 8 × 104 or 1 × 105 cells per well for NIH 3T3, 293 or PC3-AR, respectively). 293 and PC3-AR cells were transfected using the calcium phosphate precipitation method as described (Müller et al., 2000). NIH 3T3 cells were transfected using DOTAP (Roche) after changing the medium to 0.5% FCS/DMEM. The total amount of transfected DNA was kept constant (1 µg for NIH 3T3, 4 µg for 293) by adding the corresponding amounts of empty expression plasmids and pUC18. A total of 500 ng of reporter plasmids, 25 ng of AR expression plasmid, 50 ng of Gal fusion expression plasmids or 100 ng of each expression plasmid were transfected per well. Luciferase activity was assayed 24–30 h after transfection as described (Müller et al., 2000). All experiments were repeated at least five times. Chemicals were obtained as indicated: PDGF-BB (Life Technologies); PD 98059, SB 203580, anisomycin (Calbiochem); Fasudil [1-(5-iso quinolinesulfonyl)-1H-hexahydro-1,4-diazepine, dihydrochloride] (Toronto Research Chemicals); EGF and LPA (l-α-lysophosphatic acid, oleolyl sodium) (Sigma); sphingosine-1-phosphate and Y-27632 (BioMol). Toxin B, lethal toxin and C3 exoenyme were a kind gift from K.Aktories, Freiburg. Inhibitors or C3 were applied 30 or 120 min, respectively, before stimulation.
Generation of polyclonal FHL2-specific antibodies
LIM domain 4 of FHL2 was cloned in pRSET (Invitrogen) and expressed in Escherichia coli. Affinity-purified polyclonal antibodies from rabbit specifically recognize LIM domain 4 of human and mouse FHL2 but not any other FHL family member (data not shown).
Immunofluorescence and western blot analyses
For endogenous FHL2 staining in NIH 3T3 cells, the medium was changed to 0.5% FCS/DMEM 26 h before stimulation. Transfections were carried out on coverslips as described above. Cells were washed twice with phosphate-buffered saline (PBS), fixed with 5% parafomaldehyde/PBS for 10 min, permeabilized with 0.2% Triton X-100/PBS and blocked in 0.2% gelatine/PBS overnight. Staining with the polyclonal α-FHL2 antibody (diluted 1:300 in 0.2% gelatine/PBS) or α-Gal4 (sc-510; Santa Cruz; diluted 1:2000 in 0.2% gelatine/PBS) was followed by washing five times with PBS. The secondary Alexa 488-labelled antibody (Molecular Probes) was used at a dilution of 1:2000 in 0.2% gelatine/PBS followed by washing five times with PBS. Nuclei were stained with 1 µg/ml propidium iodide (Sigma) after 50 µg/ml RNase A digestion. Western blots with 10 µg of nuclear or 20 µg of total extract were performed as described (Müller et al., 2000). Antibodies were utilized as recommended: α-FHL2 monoclonal (Müller et al., 2000), TIF2 (BD Biosciences), RasGAP (Alexis Biochemicals), p38, phospho-p38, Erk1/2 and phospho-Erk1/2 (Cell Signalling Technology).
Immunohistochemistry
Stainings were performed using indirect immunoperoxidase and a protocol for antigen retrieval as described previously (Müller et al., 2000). Paraffin-embedded 4 µm tissue sections were pre-treated in a microwave oven twice for 5 min. α-FHL2 polyclonal antibody was used at a dilution of 1:600 and the α-pan-Rho(A, -B, -C) rabbit polyclonal antibody (Upstate Biotechnology) at a dilution of 1:200. Biotin-linked anti-rabbit IgG (1:500; Dako) was used as the secondary antibody and immunoreactions were visualized with the ABC complex diluted 1:50 in PBS (Vectastain, Vector). Negative controls were performed using equivalent amounts of non-specific rabbit immunoglobulins instead of primary antibodies. Immunohistochemical stainings were counterstained with haematoxilin or haematoxilin–eosin.
Acknowledgments
Acknowledgements
We thank K.Aktories, M.Brown, J.H.Camonis, P.Coffer, J.G.Collard, K.S.Erdmann, M.Follo, G.Fritz, G.Hama, L.Lim, D.Manor, M.Moser, E.Paggen, B.L.Quilliam, P.Sassone-Corsi, B.Schaefer, A.P.Soler and R.Treisman for generously providing reagents and support, the members of the Schüle lab for discussion, and C.Schüle for the art work. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB388) and the Schering AG, Berlin to R.S.
References
- Alberts A.S. and Treisman,R. (1998) Activation of RhoA and SAPK/JNK signalling pathways by the RhoA-specific exchange factor mNET1. EMBO J., 17, 4075–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach I. (2000) The LIM domain: regulation by association. Mech. Dev., 91, 5–17. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D. and Hall,A. (2000) Ras and Rho GTPases: a family reunion. Cell, 103, 227–238. [DOI] [PubMed] [Google Scholar]
- Bishop A.L. and Hall,A. (2000) Rho GTPases and their effector proteins. Biochem. J., 348, 241–255. [PMC free article] [PubMed] [Google Scholar]
- Busch C. and Aktories,K. (2000) Microbial toxins and the glycosylation of rho family GTPases. Curr. Opin. Struct. Biol., 10, 528–535. [DOI] [PubMed] [Google Scholar]
- Cerione R.A. and Zheng,Y. (1996) The Dbl family of oncogenes. Curr. Opin. Cell Biol., 8, 216–222. [DOI] [PubMed] [Google Scholar]
- Chan K.K., Tsui,S.K., Lee,S.M., Luk,S.C., Liew,C.C., Fung,K.P., Waye,M.M. and Lee,C.Y. (1998) Molecular cloning and characterization of FHL2, a novel LIM domain protein preferentially expressed in human heart. Gene, 210, 345–350. [DOI] [PubMed] [Google Scholar]
- Charron F., Tsimiklis,G., Arcand,M., Robitaille,L., Liang,Q., Molkentin,J.D., Meloche,S. and Nemer,M. (2001) Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev., 15, 2702–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiariello M., Marinissen,M.J. and Gutkind,J.S. (2001) Regulation of c-myc expression by PDGF through Rho GTPases. Nature Cell Biol., 3, 580–586. [DOI] [PubMed] [Google Scholar]
- Clark E.A., Golub,T.R., Lander,E.S. and Hynes,R.O. (2000) Genomic analysis of metastasis reveals an essential role for RhoC. Nature, 406, 532–535. [DOI] [PubMed] [Google Scholar]
- Davies S.P., Reddy,H., Caivano,M. and Cohen,P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J., 351, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimia G.M., De Cesare,D. and Sassone-Corsi,P. (1999) CBP-independent activation of CREM and CREB by the LIM-only protein ACT. Nature, 398, 165–169. [DOI] [PubMed] [Google Scholar]
- Fimia G.M., De Cesare,D. and Sassone-Corsi,P. (2000) A family of LIM-only transcriptional coactivators: tissue-specific expression and selective activation of CREB and CREM. Mol. Cell. Biol., 20, 8613–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn P., Mellor,H., Palmer,R., Panayotou,G. and Parker,P.J. (1998) Multiple interactions of PRK1 with RhoA. Functional assignment of the Hr1 repeat motif. J. Biol. Chem., 273, 2698–2705. [DOI] [PubMed] [Google Scholar]
- Font de Mora J. and Brown,M. (2000) AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol., 20, 5041–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G., Just,I. and Kaina,B. (1999) Rho GTPases are over-expressed in human tumors. Int. J. Cancer, 81, 682–687. [DOI] [PubMed] [Google Scholar]
- Fujimoto N., Yeh,S., Kang,H.Y., Inui,S., Chang,H.C., Mizokami,A. and Chang,C. (1999) Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J. Biol. Chem., 274, 8316–8321. [DOI] [PubMed] [Google Scholar]
- Gross C., Heumann,R. and Erdmann,K.S. (2001) The protein kinase C-related kinase PRK2 interacts with the protein tyrosine phosphatase PTP-BL via a novel PDZ domain binding motif. FEBS Lett., 496, 101–104. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hill C.S., Wynne,J. and Treisman,R. (1995) The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell, 81, 1159–1170. [DOI] [PubMed] [Google Scholar]
- Hoshino M., Sone,M., Fukata,M., Kuroda,S., Kaibuchi,K., Nabeshima,Y. and Hama,C. (1999) Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J. Biol. Chem., 274, 17837–17844. [DOI] [PubMed] [Google Scholar]
- Hsueh Y.P., Wang,T.F., Yang,F.C. and Sheng,M. (2000) Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature, 404, 298–302. [DOI] [PubMed] [Google Scholar]
- Ishizaki T., Uehata,M., Tamechika,I., Keel,J., Nonomura,K., Maekawa,M. and Narumiya,S. (2000) Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol., 57, 976–983. [PubMed] [Google Scholar]
- Knutti D., Kressler,D. and Kralli,A. (2001) Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc. Natl Acad. Sci. USA, 98, 9713–9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T., Chen,X.Q., Tan,I., Manser,E. and Lim,L. (1998) Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol. Cell. Biol., 18, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.Y. et al. (2001a) Translocation of a human focal adhesion LIM-only protein, FHL2, during myofibrillogenesis and identification of LIM2 as the principal determinants of FHL2 focal adhesion localization. Cell Motil. Cytoskel., 48, 11–23. [DOI] [PubMed] [Google Scholar]
- Li H.Y., Ng,E.K., Lee,S.M., Kotaka,M., Tsui,S.K., Lee,C.Y., Fung,K.P. and Waye,M.M. (2001b) Protein–protein interaction of FHL3 with FHL2 and visualization of their interaction by green fluorescent proteins (GFP) two-fusion fluorescence resonance energy transfer (FRET). J. Cell. Biochem., 80, 293–303. [PubMed] [Google Scholar]
- Lin R. et al. (1999) Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J. Biol. Chem., 274, 23633–23641. [DOI] [PubMed] [Google Scholar]
- Marinissen M.J., Chiariello,M. and Gutkind,J.S. (2001) Regulation of gene expression by the small GTPase Rho through the ERK6 (p38γ) MAP kinase pathway. Genes Dev., 15, 535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz,M.G., Mischak,H., Blumberg,P.M., Kochs,G., Hug,H., Marme,D. and Schächtele,C. (1993) Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J. Biol. Chem., 268, 9194–9197. [PubMed] [Google Scholar]
- McKinsey T.A., Zhang,C.L., Lu,J. and Olson,E.N. (2000) Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature, 408, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F., Stam,J.C., Hordijk,P.L., van der Kammen,R.A., Ruuls-Van Stalle,L., Feltkamp,C.A. and Collard,J.G. (1997) Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and C-Jun NH2-terminal kinase activation. J. Cell Biol., 137, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A., Lin,A., Claret,F.X., Abo,A. and Karin,M. (1995) Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell, 81, 1147–1157. [DOI] [PubMed] [Google Scholar]
- Montgomery J.S., Price,D.K. and Figg,W.D. (2001) The androgen receptor gene and its influence on the development and progression of prostate cancer. J. Pathol., 195, 138–146. [DOI] [PubMed] [Google Scholar]
- Morgan M.J. and Madgwick,A.J. (1996) Slim defines a novel family of LIM-proteins expressed in skeletal muscle. Biochem. Biophys. Res. Commun., 225, 632–638. [DOI] [PubMed] [Google Scholar]
- Morgan M.J. and Madgwick,A.J. (1999a) The fourth member of the FHL family of LIM proteins is expressed exclusively in the testis. Biochem. Biophys. Res. Commun., 255, 251–255. [DOI] [PubMed] [Google Scholar]
- Morgan M.J. and Madgwick,A.J. (1999b) The LIM proteins FHL1 and FHL3 are expressed differently in skeletal muscle. Biochem. Biophys. Res. Commun., 255, 245–250. [DOI] [PubMed] [Google Scholar]
- Müller J.M. et al. (2000) FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J., 19, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne S. and Pyne,N. (2000) Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol. Ther., 88, 115–131. [DOI] [PubMed] [Google Scholar]
- Rowan B.G., Garrison,N., Weigel,N.L. and O’Malley,B.W. (2000) 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol. Cell. Biol., 20, 8720–8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E., Ishizaki,T., Narumiya,S. and Treisman,R. (1999) Transformation mediated by RhoA requires activity of ROCK kinases. Curr. Biol., 9, 136–145. [DOI] [PubMed] [Google Scholar]
- Somlyo A.V., Bradshaw,D., Ramos,S., Murphy,C., Myers,C.E. and Somlyo,A.P. (2000) Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem. Biophys. Res. Commun., 269, 652–659. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A., Gineitis,D., Copeland,J. and Treisman,R. (1999) Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell, 98, 159–169. [DOI] [PubMed] [Google Scholar]
- Su L.F., Knoblauch,R. and Garabedian,M.J. (2001) Rho GTPases as modulators of the estrogen receptor transcriptional response. J. Biol. Chem., 276, 3231–3237. [DOI] [PubMed] [Google Scholar]
- Wixler V., Geerts,D., Laplantine,E., Westhoff,D. Smyth,N., Aumailley,M., Sonnenberg,A. and Paulsson,M. (2000) The LIM-only protein DRAL/FHL2 binds to the cytoplasmic domain of several α and β integrin chains and is recruited to adhesion complexes. J. Biol. Chem., 275, 33669–33678. [DOI] [PubMed] [Google Scholar]