Abstract
Previous studies have suggested that cell cycle-dependent changes in the affinity of the origin recognition complex (ORC) for chromatin are involved in regulating initiation of DNA replication. To test this hypothesis, chromatin lacking functional ORCs was isolated from metaphase hamster cells and incubated in Xenopus egg extracts to initiate DNA replication. Intriguingly, Xenopus ORC rapidly bound to hamster somatic chromatin in a Cdc6-dependent manner and was then released, concomitant with initiation of DNA replication. Once pre-replication complexes (pre-RCs) were assembled either in vitro or in vivo, further binding of XlORC was inhibited. Neither binding nor release of XlORC was affected by inhibitors of either cyclin-dependent protein kinase activity or DNA synthesis. In contrast, inhibition of pre-RC assembly, either by addition of Xenopus geminin or by depletion of XlMcm proteins, augmented ORC binding by inhibiting ORC release. These results demonstrate a programmed release of XlORC from somatic cell chromatin as it enters S phase, consistent with the proposed role for ORC in preventing re-initiation of DNA replication during S phase.
Keywords: cell cycle/DNA replication/initiation/origin recognition proteins/Xenopus
Introduction
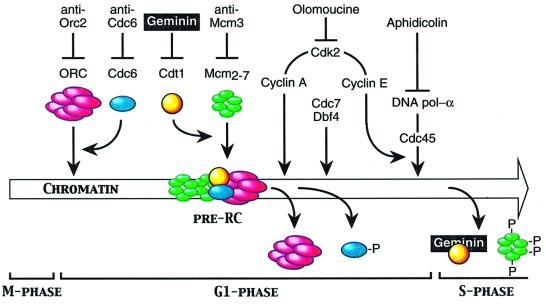
Initiation of DNA replication in eukaryotes is a highly conserved, multi-step process designed to restrict initiation events to once per replication origin per S phase. Homologs of the proteins involved in this process in budding yeast are found throughout the eukaryotic kingdom, and the sequence of events by which these proteins initiate DNA replication is remarkably similar (summarized in Figure 8; reviewed in Bogan et al., 2000; Kelly and Brown, 2000; Blow, 2001). First, the six proteins that comprise the origin recognition complex (ORC) bind to replication origins in the chromosomal DNA. Next, Cdc6 and Cdt1/RLF-B proteins load mini-chromosome maintenance (Mcm) proteins 2–7 onto the ORC/chromatin site to form a pre-replication complex (pre-RC). Activation of this complex begins when Cdc6 is released by the protein kinase, Cdk2/Cyclin A, and replaced by Cdc45 with the help of the protein kinases Cdc7/Dbf4 and Cdk2/cyclin E. Cdc45 allows DNA polymerase-α:DNA primase to bind to the complex and initiate DNA synthesis.
Fig. 8. ORC cycle. Cdc6 facilitates binding of Orc proteins to somatic cell chromatin. Mcm proteins trigger release of Orc proteins from somatic cell chromatin after assembly of pre-replication complexes, thus preventing re-initiation of DNA replication during S phase. Re-initiation is also prevented by loss of Cdc6 and inhibition of Cdt1 by geminin.
Multiple safeguards exist to prevent re-replication of the same DNA within a single cell cycle. In yeast, regulation of DNA replication begins by regulating the ability of Cdc6 and Cdt1/RLF-B to assemble Mcm2–7 at the ORC/chromatin site. Cdk1/cyclin B simultaneously promotes mitosis and inhibits binding of Cdc6 to ORC by phosphorylating Cdc6. When S phase begins, Cdc6 is again phosphorylated, released from pre-RCs, ubiquitylated and degraded (Drury et al., 2000 and references therein). A similar mechanism exists in frogs and mammals with the additional step that phosphorylated Cdc6 is transported out of the nucleus (Coverley et al., 2000; Mendez and Stillman, 2000; Petersen et al., 2000 and references therein). Nevertheless, under conditions where Cdc6 cannot be phosphorylated and remains in the nucleus during S phase, re-initiation of DNA replication still does not occur, revealing the existence of other regulatory mechanisms (Pelizon et al., 2000; Petersen et al., 2000). One of these mechanisms is inhibition of Cdt1/RLF-B activity by geminin, a protein produced during the S to M transition (Wohlschlegel et al., 2000; Tada et al., 2001). Another appears to be regulation of ORC activity itself.
In both budding and fission yeast, all of the ORC subunits remain tightly bound to chromatin throughout the cell cycle (Kong and DePamphilis, 2001 and references therein). Nevertheless, in Saccharomyces cerevisiae, re-initiation within a single S phase is prevented by a combination of ORC phosphorylation, downregulation of Cdc6 activity and nuclear exclusion of Mcm proteins (Nguyen et al., 2001). In metazoans, the affinity for chromatin of one or more ORC subunits appears to change during the cell cycle. In Xenopus, Orc proteins in activated eggs bind to sperm chromatin, but Orc proteins in meiotic eggs do not (Coleman et al., 1996; Hua and Newport, 1998; Findeisen et al., 1999; Rowles et al., 1999), and Orc proteins in cultured Xenopus cells are localized in the nucleus throughout interphase but move to the cytoplasm during mitosis (Romanowski et al., 1996). In Drosophila, Orc2 remains bound to chromosomes throughout the cell cycle (Pak et al., 1997), whereas the amount of nuclear-bound DmOrc1 is greatest during late G1 and S phases (Asano and Wharton, 1999). In mammals, the cellular concentrations of Orc1 and Orc2 remain constant throughout the cell cycle (Ritzi et al., 1998; Saha et al., 1998; Mendez and Stillman, 2000; Natale et al., 2000), but Orc1 is selectively released from chromatin during the S- to M-phase transition (Natale et al., 2000; Kreitz et al., 2001; Li and DePamphilis, 2002) and sequestered from reassociation by mono-ubiquitylation (Li and DePamphilis, 2002). This is consistent with the selective release of Orc1 from purified human ORC (Dhar et al., 2001a; Vashee et al., 2001) and would account for reports that M-phase chromatin does not contain functional ORCs (Yu et al., 1998; Li et al., 2000; Natale et al., 2000) and that an ORC-like footprint at the human lamin B2 origin disappears during mitosis (Abdurashidova et al., 1998). ORC activity is restored during the transition from M to G1 phase, concomitant with the reappearance of Orc1 tightly bound to chromatin, and pre-RCs at specific genomic sites (Li et al., 2000; Natale et al., 2000). Therefore, initiation of DNA replication appears to be regulated through cell cycle-dependent changes in ORC activity that delay reassembly of pre-RCs until DNA replication and mitosis have been completed and a nuclear membrane has formed.
Two critical questions arose from these observations. Are changes in the association of ORC with chromatin a natural occurrence in the sequence of events leading to DNA replication, and if so, what regulates association of ORC with chromatin? To address these questions, we took advantage of the facts that both Orc proteins and chromatin structure are highly conserved among vertebrates, and that both hamster metaphase chromatin (Yu et al., 1998; Natale et al., 2000; Li and DePamphilis, 2002) and Xenopus sperm chromatin (Blow, 2001) lack functional ORCs. This allowed us to monitor the affinity of XlOrc proteins for somatic cell chromatin as a function of specific cell cycle events by incubating hamster metaphase chromatin in Xenopus egg extract under conditions that initiate a single round of semi-conservative DNA replication (Gilbert et al., 1995a; Li et al., 2000). The results revealed changes in the affinity of the Xenopus ORC for somatic cell chromatin that paralleled the behavior of ORC subunits in vivo, and demonstrated that ORC can cycle on and off of somatic cell chromatin as cells transit from M to S phase.
Results
Binding and release of Orc proteins to somatic cell chromatin
To confirm that hamster metaphase chromatin lacks functional ORCs, Xenopus egg extract was depleted of Orc proteins by incubation with immobilized anti-XlOrc2 antibodies. As reported previously (Romanowski et al., 1996; Rowles et al., 1996; Tugal et al., 1998), both Orc1 and Orc2 were removed from the extract (Figure 1A). However, ∼50% of the Orc4 remained, revealing that not all of the XlOrc proteins exist in a single stable complex. Nevertheless, replication of either sperm chromatin or hamster metaphase chromatin was completely inhibited in the depleted extract, and this inhibition did not result from failure to assemble chromatin into a nuclear structure (Figure 1B).
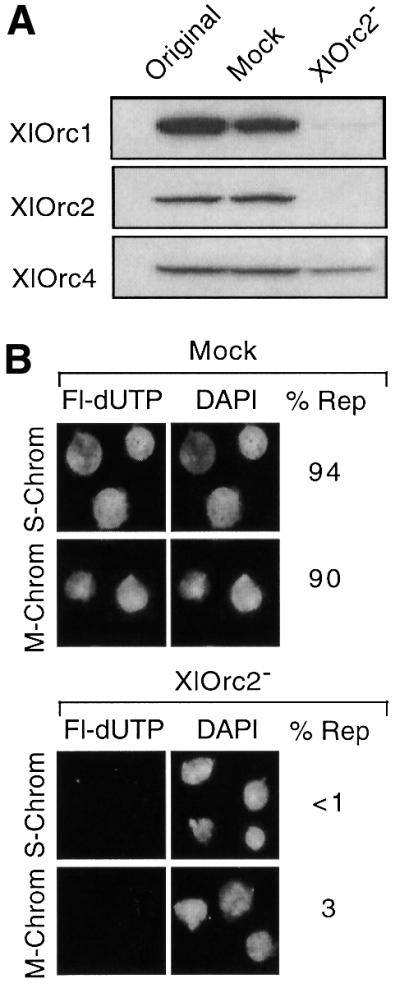
Fig. 1. Hamster metaphase chromatin and Xenopus sperm chromatin lacked functional ORCs. (A) Xenopus egg extract, mock-depleted extract and XlOrc2-depleted extract were fractionated by SDS–PAGE, and the relative amounts of XlOrc1, XlOrc2 and XlOrc4 were determined by immunoblotting. (B) Either hamster metaphase (M-Chrom) or Xenopus sperm (S-Chrom) chromatin was incubated for 1.5 h in either mock-depleted or XlOrc2-depleted extracts containing Fl-dUTP. Nuclei were stained with DAPI to visualize DNA. The percentage of fluorescent-labeled pseudonuclei (% Rep) is given for each sample (>100 nuclei were scored in each case).
These results confirmed that hamster metaphase chromatin lacks functional ORCs, and implied that XlOrc proteins can bind to hamster metaphase chromatin and initiate DNA replication. To test this hypothesis, hamster metaphase chromatin was incubated in a Xenopus egg extract under low salt conditions that allowed initiation of DNA replication. At the times indicated, nuclei were isolated under the same low salt conditions. Nuclear proteins not bound to chromatin were released by addition of Triton X-100 to permeabilize the nuclei, and the nuclei were recovered by sedimentation. Chromatin-bound proteins in the pellet were subjected to SDS–PAGE followed by immunoblotting with antisera specific for the indicated protein.
Hamster metaphase chromatin did not contain proteins that cross-reacted with XlOrc1, XlOrc2 or XlOrc4 (Figure 2A). However, within the first 10–20 min of incubation, all three proteins bound to the chromatin rapidly, and they were greatly enriched in the chromatin fraction. All three proteins then began to dissociate from the chromatin, concomitant with the onset of DNA replication (Figure 2B). Since all three proteins behaved similarly, they appeared to bind to and then dissociate from hamster metaphase chromatin as an intact complex.
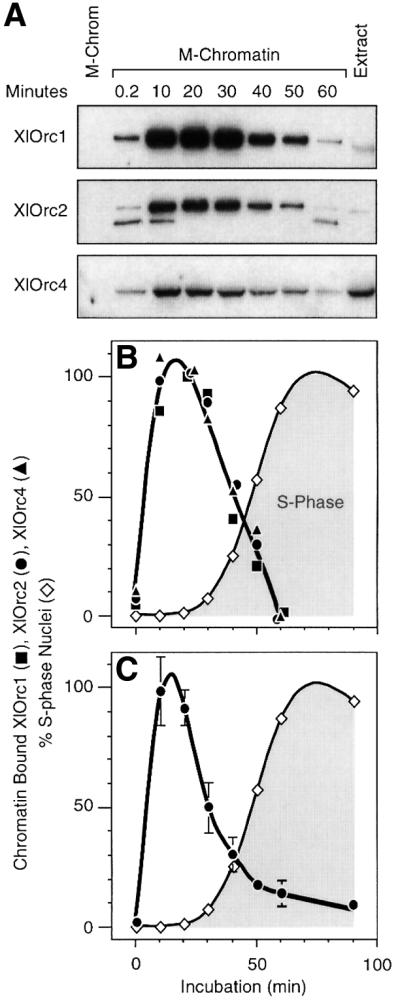
Fig. 2. XlOrc proteins were rapidly bound to, and then released from, somatic cell chromatin. (A) Hamster metaphase chromatin (M-Chromatin) was incubated in Xenopus egg extract (Extract) for the times indicated, and the relative amount of chromatin-bound proteins in each sample was determined. (B) Immunoblotting data for each protein were quantified by densitometry, normalized to the amount of histone H3 in the sample (data not shown) and then expressed relative to the maximum value. The peak level for each protein was defined as 100%. The fraction of nuclei incorporating Fl-dUTP was determined for each sample (% S-phase Nuclei). The mean value for the three XlOrc proteins in each sample was plotted. (C) XlOrc2 results from seven independent experiments were normalized to their 20 min time points and then averaged together (± SEM).
Some variation was observed among different preparations of Xenopus egg extract and of hamster metaphase chromatin. Nevertheless, the same basic cycle of XlOrc binding and release was routinely observed (Figure 2C). Analysis of seven independent experiments revealed that the time required for maximum XlOrc binding was essentially the same (10–20 min), although the time required for complete XlOrc release varied from 1 to 2 h.
The loss of chromatin-bound ORC did not result from Orc degradation, because egg extracts contained several protease inhibitors, and the total concentration of Orc proteins remained constant during these incubations. Furthermore, in experiments described below, the amount of Orc bound to hamster metaphase chromatin increased 4-fold in the presence of geminin or in the absence of Mcm proteins, and in the absence of Mcm proteins, remained constant for the next hour. Finally, the same experiments were also carried out with Xenopus sperm chromatin. As reported previously (Romanowski et al., 1996; Rowles et al., 1996; Jares and Blow, 2000), XlOrc1 and XlOrc2 rapidly bound to sperm chromatin during the first 30 min in Xenopus egg extract, and remained bound (data not shown). Therefore, association of XlORC with sperm chromatin differed from its association with somatic cell chromatin (see Discussion).
Anti-XlOrc2 antibodies recognized two proteins bound to either hamster metaphase chromatin or sperm chromatin (Figure 2A). The slower migrating band, which was the predominant form of XlOrc2 in egg extracts, corresponded to XlOrc2 on the basis of its mobility relative to XlOrc2 synthesized in vitro. The faster migrating form of XlOrc2 only appeared at the beginning of the incubation and then again after DNA replication was well under way, suggesting that it was a cell cycle-dependent modification of Orc2.
Release of ORC from chromatin was independent of DNA synthesis, Cdk activity and de novo protein synthesis
To determine whether or not release of XlORC from chromatin required DNA replication, hamster metaphase chromatin was incubated in Xenopus egg extract containing either 50 µg/ml aphidicolin (a specific inhibitor of replicative DNA polymerases such as α, δ and ε) or 1 mM olomoucine (a specific inhibitor of cyclin-dependent protein kinases). In both cases, DNA synthesis was reduced to <1% of the control. Neither inhibitor affected the time course shown in Figure 2 for the binding of XlOrc proteins to and their release from hamster metaphase chromatin (data not shown). Thus, release of XlOrc proteins from chromatin did not require either DNA synthesis or activation of pre-RCs by Cdk2. Similar results were observed when 250 µg/ml cycloheximide, a specific inhibitor of ribosomal translocation, was included in the assay, revealing that neither binding nor release of ORC required de novo protein synthesis (data not shown).
Binding of ORC to chromatin was facilitated by Cdc6 protein
To determine whether or not XlCdc6 affected either the binding of XlORC to chromatin or its subsequent release, hamster metaphase chromatin was incubated in Xenopus egg extract that had been depleted of XlCdc6 protein. These XlCdc6-depleted extracts contained <1% of the XlCdc6, but >90% of the XlOrc2 and XlMcm3, when compared with mock-depleted extracts (Figure 3A). As expected, stable binding of Mcm proteins onto sperm chromatin was not detected in XlCdc6-depleted extracts, and the fraction of sperm chromatin nuclei that underwent DNA replication in XlCdc6-depleted extracts was <1% of mock extracts (data not shown).
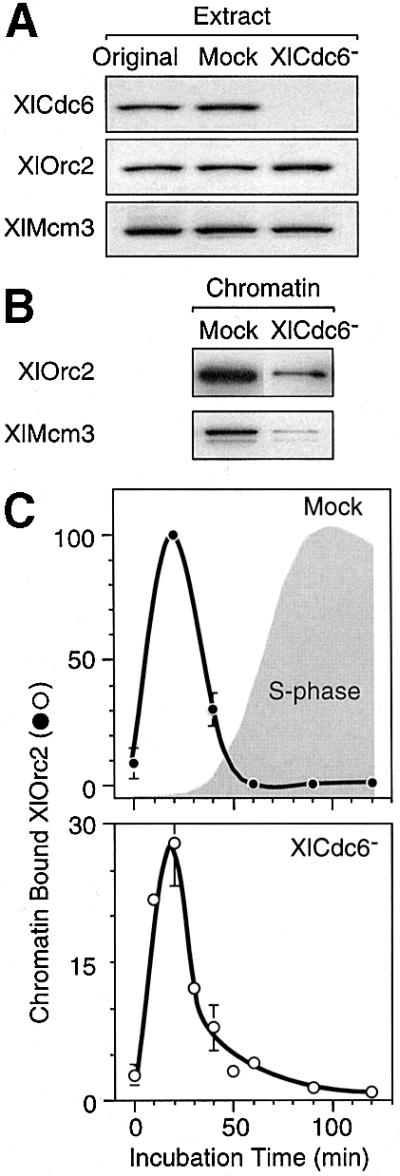
Fig. 3. Cdc6 facilitated binding of ORC to chromatin, but not its release. (A) Samples of the original Xenopus egg extract, mock-depleted extract or XlCdc6-depleted extract were fractionated by gel electrophoresis, and Xenopus Cdc6, Orc2 and Mcm3 were detected by immunoblotting. (B) Hamster chromatin-bound XlOrc2 and XlMcm3 was assayed after a 1 h incubation. (C) Relative amounts of hamster chromatin-bound XlOrc2 in mock-depleted and XlCdc6-depleted extracts were determined, as in Figure 2. Immunoblotting data for each protein were quantified by densitometry, normalized to the amount of histone H3 present in the sample, and then expressed relative to the maximum value observed in mock-depleted extracts.
When hamster metaphase chromatin was incubated in XlCdc6-depleted extracts, the amount of chromatin-bound XlOrc2 was reduced, on average, to 30% of the mock-depleted control, but the time course for release of chromatin-bound XlOrc proteins remained unchanged (Figure 3B and C). Thus, XlCdc6 facilitated binding of XlORC to somatic cell chromatin, but XlCdc6 was not required for its subsequent release. This observation initially suggested that assembly of pre-RCs was not required for release of chromatin-bound ORC, because only limited binding of Mcm proteins onto hamster chromatin was detected in XlCdc6-depleted extracts (Figure 3B). However, ∼60% of these nuclei initiated DNA replication (compared with 85% in the mock extract), although they were not as intensely stained with fluorescein-12-dUTP (Fl-dUTP) as were nuclei assembled in mock extracts. This surprising result was explained by the presence of high concentrations of Cdc6 protein in hamster (data not shown) and human (Saha et al., 1998; Jiang et al., 1999; Mendez and Stillman, 2000; Petersen et al., 2000) metaphase cells, relative to interphase cells.
Release of ORC from chromatin was dependent on Mcm proteins
As expected, binding of ORC to hamster metaphase chromatin incubated in Xenopus egg extract preceded binding of Mcm proteins, and binding of Mcm proteins preceded binding of proliferating cell nuclear antigen (PCNA), which was accompanied by DNA replication (Figure 4A and B). Interestingly, ORC was released from chromatin concomitant with Mcm3 binding, suggesting that completion of pre-RC assembly triggered release of ORCs.
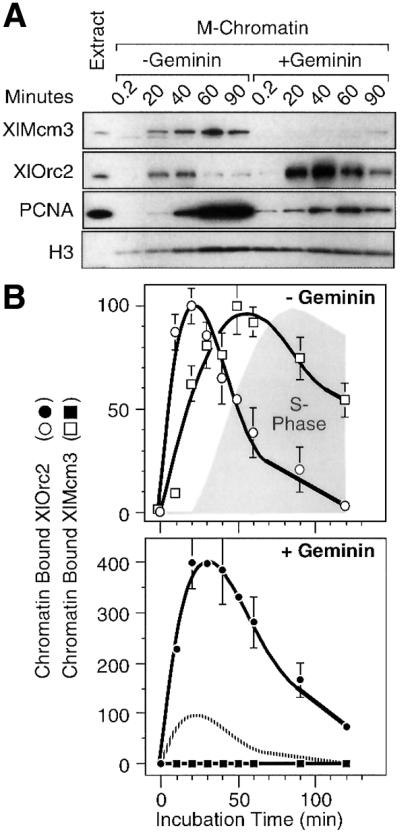
Fig. 4. Release of XlORC from somatic cell chromatin was inhibited by geminin. Hamster metaphase chromatin was incubated in Xenopus egg extract either in the presence (filled symbols) or absence (open symbols) of 40 nM Xenopus geminin. (A) Chromatin-bound XlMcm3, XlOrc2, PCNA and histone H3 were determined by immunoblotting. (B) Immunoblotting data were quantified by densitometry, normalized to the amount of histone H3 present, and then expressed relative to the maximum values in the ‘– Geminin’ control. Mean values (± SEM) from three independent experiments were plotted. The shaded area (S phase) indicates the percentage of nuclei that incorporated Fl-dUTP. The broken line in the ‘+ Geminin’ panel is the chromatin-bound XlOrc2 result in the ‘– Geminin’ panel.
To determine whether or not this hypothesis was correct, Xenopus geminin was added to the extract. Previous studies with Xenopus sperm chromatin have shown that geminin is a cellular protein that specifically binds Cdt1(RLF-B) (Wohlschlegel et al., 2000; Tada et al., 2001). Since Cdt1(RLF-B), like Cdc6, is required for stable binding of Mcm proteins to ORC/chromatin sites, geminin inhibits binding of Mcm proteins to chromatin and prevents initiation of DNA replication. Consistent with these results, addition of geminin inhibited binding of XlMcm3 to hamster chromatin (Figures 4A, B and 5A), and this inhibition could be reversed by addition of Cdt1/RLF-B (Figure 5A). Consequently, addition of geminin inhibited DNA replication (Figure 5B), resulting in reduced binding of PCNA to chromatin (Figures 4A and 5A). The inhibitory effects of geminin on DNA replication were also eliminated by addition of Cdt1 (Figure 5B).
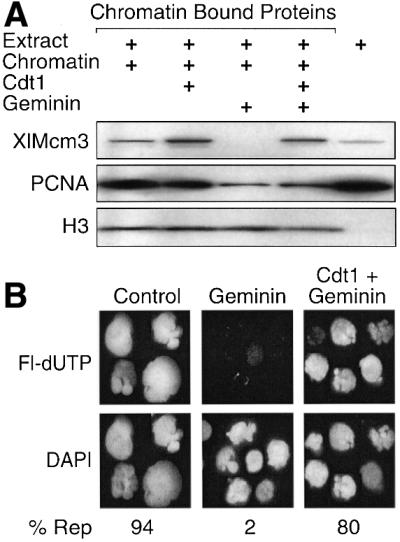
Fig. 5. Geminin inhibited Cdt1/RLF-B mediated loading of Mcm proteins onto somatic cell chromatin. (A) Hamster metaphase chromatin was incubated for 1 h in Xenopus egg extract. Aliquots were supplemented with 25 nM human Cdt1 or Xenopus geminin, or 25 nM each geminin and Cdt1. Chromatin-bound Mcm3, PCNA and histone H3 in each sample were determined. (B) Fraction of DAPI-stained nuclei that incorporated Fl-dUTP in 1.5 h (% Rep).
Inhibition of Cdt1 activity by geminin increased the amount of ORC bound to somatic cell chromatin ∼4-fold (Figure 4A), although the time course for ORC binding and release mirrored that observed in the absence of geminin (Figure 4B). Since Cdt1 activity was not required for binding Orc proteins to chromatin, the increased accumulation of Orc proteins on chromatin would only occur if inhibition of Cdt1 reduced the rate at which Orc proteins were released from chromatin. Thus, geminin augmented ORC binding by delaying ORC release. This conclusion suggested that release of Orc proteins was coupled to the binding of Mcm proteins to chromatin.
To test this hypothesis, the association of XlOrc proteins with hamster metaphase chromatin was examined in an Mcm3-depleted Xenopus egg extract. At least 95% of the XlMcm3 was removed from these extracts with no reduction in XlOrc2 (Figure 6A). Previous studies have shown that depletion of XlMcm3 protein from Xenopus egg extract also depletes the other five XlMcm proteins (Thommes et al., 1997). As expected, very little XlMcm3 was bound to hamster chromatin in XlMcm3-depleted extracts, and therefore DNA replication and PCNA binding to hamster chromatin was inhibited (Figure 6B). However, depletion of XlMcm proteins increased binding of XlOrc1 and XlOrc2 to hamster chromatin 4- to 5-fold (Figure 6B and C), consistent with the effects of geminin (Figure 4B), but prevented their release. Thus, the programmed release of XlORC from hamster chromatin was dependent on Cdt1-mediated interaction of Mcm proteins with ORC/chromatin sites.
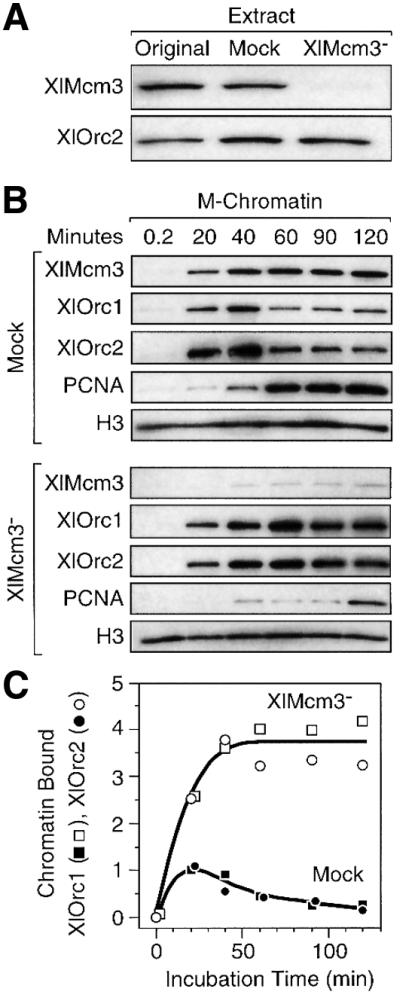
Fig. 6. Release of XlORC from hamster chromatin required XlMcm proteins. (A) Samples of the original Xenopus egg extract, mock-depleted extract and XlMcm3-depleted extract were fractionated by gel electrophoresis, and the relative amounts of XlMcm3 and XlOrc2 proteins present were determined by SDS–PAGE and immunoblotting. (B) Hamster metaphase chromatin was incubated in either mock-depleted or Mcm3-depleted Xenopus egg extract for the times indicated, and the amounts of chromatin-bound XlMcm3, XlOrc1, XlOrc2, PCNA and histone H3 proteins were determined. (C) Immunoblotting data were quantified by densitometry, normalized to the amount of histone H3 present, and then expressed relative to the amount of XlOrc2 protein detected after 20 min in the ‘Mock’-depleted extract. The mean from three independent experiments was plotted.
Assembly of pre-replication complexes inhibited further binding of Orc proteins
Completion of pre-RC assembly is marked by stable binding of Mcm and PCNA proteins to chromatin and the initiation of DNA replication. This occurred with hamster chromatin after 30 min of incubation in Xenopus egg extract (Figure 4) and was accompanied by release of XlORC. No additional XlORC bound to hamster chromatin despite the fact that the total concentration of XlORC in these extracts remained essentially constant during the incubation, suggesting that once pre-RCs were assembled, further ORC binding was inhibited until the next cell cycle. To test this hypothesis, binding of XlOrc2 to hamster chromatin was measured in nuclei isolated from late G1- and S-phase hamster cells, because previous studies have shown that functional pre-RCs appear at specific chromosomal sites in late G1 phase [3–4 h after cells are released from metaphase (Wu and Gilbert, 1996; Wu et al., 1998; Li et al., 2000; Natale et al., 2000; Okuno et al., 2001)].
Hamster metaphase chromatin, hamster late G1-phase nuclei, and hamster G1- to S-phase nuclei, prepared by permeabilizing cells with digitonin, were incubated in a Xenopus egg extract to initiate DNA replication. XlOrc2 entered the nuclei and bound to the chromatin, but the maximum levels of binding in late G1- to S-phase nuclei were 10- to 20-fold less than when metaphase chromatin was the substrate (Figure 7A). In fact, the greater the proportion of S-phase nuclei (Figure 7B), the less XlOrc2 bound to chromatin (Figure 7A). These results were confirmed by confocal microscopy of immunostained samples and quantification of the intensity of fluorescence from individual nuclei (data not shown). No differences were detected in the nuclear distribution of the fluorescence in each substrate. Therefore, once pre-RCs had been assembled in hamster nuclei, binding of XlORC was inhibited throughout the hamster genome.
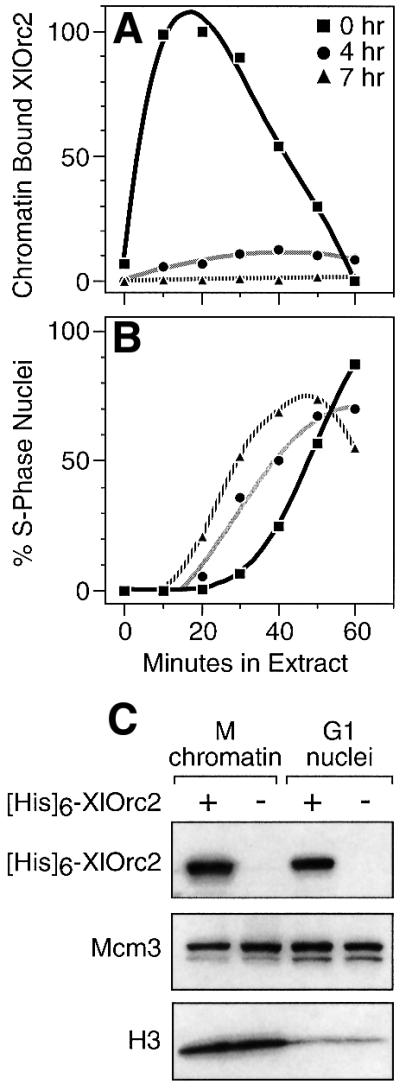
Fig. 7. Binding of XlOrc proteins to chromatin was inhibited in late G1- and S-phase hamster nuclei. Hamster cells arrested in metaphase (0 h; squares) were washed free of nocodazole and cultured in fresh medium for either 4 or 7 h (circles and triangles, respectively). Cells were permeabilized with digitonin and then incubated in Xenopus egg extract supplemented with Fl-dUTP for the times indicated. (A) Relative amounts of XlOrc2 bound to chromatin. (B) Fraction of nuclei undergoing DNA synthesis. (C) Relative amounts of Orc2 that accumulated in nuclei assembled around metaphase chromatin or in G1 nuclei after a 1 h incubation either in extract supplemented with 1 µg of His6-XlOrc2 (+) or in normal extract (–). Nuclei were isolated by sedimentation through a sucrose cushion and then subjected to SDS–PAGE. Anti-His6 or anti-Mcm3 antibodies were used to detect His6-XlOrc2 and Mcm3, respectively, by immunoblotting.
The extent of chromatin binding by XlOrc2 was not limited by the nuclear membrane, because XlOrc binding to each substrate had reached saturation (Figure 7A). Moreover, transport of Orc and other proteins into hamster nuclei did not vary significantly between metaphase chromatin that was assembled in vitro into nuclei and late G1-phase nuclei isolated from hamster cells. When extracts were supplemented with His6-XlOrc2, the tagged Orc2 protein accumulated in both types of nuclei, and the ratio of His6-XlOrc2 to histone H3 (i.e. the concentration of XlOrc2) was greater in the G1 nuclei (Figure 7C). A similar result was observed for Mcm3. Therefore, the reduced binding of XlOrc proteins to chromatin in hamster G1 nuclei did not result from reduced access of Orc proteins to chromatin.
Finally, although olomoucine did inhibit initiation of DNA replication in these nuclei (consistent with previous studies using the general protein kinase inhibitor 6-dimethylaminopurine; Gilbert et al., 1995a), it did not affect the levels of XlOrc2 binding to G1-phase chromatin (data not shown). Therefore, the mechanism that inhibited XlORC binding after pre-RCs had been assembled in vivo did not involve Cdk activity.
Discussion
The results presented here demonstrate a programmed release of XlORC from somatic cell chromatin as it enters S phase that is consistent with the role proposed for ORC in preventing re-initiation of DNA replication during S phase (Figure 8). Replication of metaphase chromatin from hamster cells in Xenopus egg extract required the presence of XlOrc proteins (Figure 1). XlORC subunits 1, 2 and 4 (and presumably 3, 5 and 6 as well) rapidly bound to the chromatin, initiated DNA replication, and were then released (Figure 2). Once pre-RCs were assembled either in Xenopus egg extracts or hamster cells, then binding of XlOrc proteins was inhibited (Figure 7). The release of one or more ORC subunits from somatic cell chromatin should not interfere with the ability of Cdc7/Dbf4 to load Cdc45 protein (Figure 8), because this step still occurs even when ORC is removed by salt elution (Jares and Blow, 2000).
These changes in the affinity of Orc proteins for somatic cell chromatin are consistent with the behavior of Orc proteins in vitro and in vivo. Xenopus Orc proteins exist as a stable complex that can be purified from Xenopus egg extract (Figure 1; Rowles et al., 1996; Tugal et al., 1998), and therefore it is not surprising that both XlOrc1 and XlOrc2 are localized in the nuclei of cultured Xenopus cells throughout interphase, but that both move to the cytoplasm during mitosis (Romanowski et al., 1996). In contrast, human Orc proteins exist as a stable complex of Orc2–5 to which human Orc1 binds only weakly (Dhar et al., 2001a; Vashee et al., 2001). Therefore, it is not surprising that hamster and human Orc1 is selectively released from chromatin when cells enter S phase, and then rebinds at the beginning of G1 phase (Natale et al., 2000; Kreitz et al., 2001; Li and DePamphilis, 2002). Furthermore, these differences between Xenopus and mammalian ORCs may account for reports that Xenopus ORC is weakly bound to randomly selected sites on chromatin in Xenopus eggs (Blow, 2001), whereas mammalian ORC is tightly bound to specific chromosomal loci in mammalian cells (DePamphilis, 1999; Li et al., 2000; Altman and Fanning, 2001). Thus, in contrast to yeast where all six ORC subunits are stably bound to chromatin throughout the cell cycle, ORC activity in some, or perhaps all, of the metazoa can be regulated during each cell cycle through selective dissociation and reassociation of one or more ORC subunits with chromatin.
Cell cycle-dependent binding of ORCs with somatic cell chromatin
The ability of XlORC to bind to somatic cell chromatin was facilitated by XlCdc6 (Figure 3). The requirement for XlCdc6 may well have been greater than observed here (∼3-fold) except for the fact that mammalian metaphase cells contained Cdc6 (Saha et al., 1998; Jiang et al., 1999; Mendez and Stillman, 2000; Petersen et al., 2000 and data not shown). Those ORCs that did bind were able to initiate replication, and then release from, chromatin. Both of these events involved Mcm proteins, even though the amount of bound Mcm3 was greatly reduced (Figure 3B), because depletion of XlMcm proteins inhibited both DNA replication and ORC release (Figure 6 and data not shown). This is consistent with reports that a 90% reduction in the amount of human Orc2 resulted in only a 50% reduction in the rate of human cell proliferation (Dhar et al., 2001b) and that 40% of the nuclei from G0 cells could still initiate DNA replication in an Mcm-depleted Xenopus egg extract, despite a 98% reduction in the amount of chromatin-bound Mcm proteins (Sun et al., 2000).
The ability of Cdc6 to facilitate ORC binding to chromatin is consistent with its ability to bind Orc1 (Saha et al., 1998; Wang et al., 1999) and with its presence in mitotic cells, where Cdc6 may facilitate reassociation of Orc1 with those ORC subunits that remain bound to chromatin throughout the cell cycle (Natale et al., 2000; Li and DePamphilis, 2002). Therefore, Cdc6 may play a role in determining where ORC is assembled on somatic cell chromatin in the metazoa, as it does in yeast (Mizushima et al., 2000). Moreover, the involvement of Cdc6 in ORC loading distinguishes its action from that of Cdt1.
Cell cycle-dependent release of ORCs from somatic cell chromatin
The stimulus for release of ORC from somatic cell chromatin was not initiation of DNA synthesis itself, because ORC release was not prevented by addition of either olomoucine or aphidicolin. Olomoucine inhibits Cdk2, which is required for release of Cdc6 protein and for Cdc45-mediated loading of DNA polymerase-α (Figure 8). Aphidicolin inhibits polymerase-α, which is required to initiate DNA synthesis at replication origins, as well as polymerase-δ, which is required for elongating nascent DNA strands, and polymerase-ε, which is required at some less well-defined step (Kelly and Brown, 2000). Therefore, neither extension of the short RNA-primed DNA chains that are synthesized in the presence of aphidicolin (Decker et al., 1987; Nethanel and Kaufmann, 1990; Gilbert et al., 1995b) nor Cdk2 activation of pre-RCs assembled in Xenopus egg extract (Jares and Blow, 2000; Walter, 2000) is required for release of Orc proteins (Figure 8).
Release of chromatin-bound ORC depended on interaction of Mcm proteins with ORC/chromatin sites, but not on stable binding of Mcm proteins to chromatin. ORC proteins were released concomitant with binding of Mcm proteins, and prior to initiation of DNA replication (Figure 4). Although inhibition of Cdt1 activity by geminin prevented stable binding of Mcm proteins to chromatin, it delayed, but did not prevent, release of ORC (Figure 4). The fact that geminin did not prevent ORC release, even when higher concentrations were used or when additional geminin was added at later times, suggested that only a brief interaction, rather than stable binding, of Mcm proteins with ORC/chromatin sites is required to release ORC. This hypothesis was confirmed by depleting the extract of Mcm3 (and presumably most, if not all, of Mcm2–7; Thommes et al., 1997). Under these conditions, ORC binding to chromatin was augmented to the same extent as with addition of geminin (4-fold), but ORC was not released (Figure 6). Furthermore, this conclusion is consistent with the fact that release of chromatin-bound ORC was not affected by depletion of XlCdc6, even though little XlMcm3 was stably bound to chromatin (Figure 3). Hamster metaphase chromatin preparations contained a high concentration of hamster Cdc6 and initiated DNA synthesis in Cdc6-depleted extracts at 70% of the level in a mock-depleted extract. Therefore, Mcm proteins in egg extracts are in great excess over what is required to initiate DNA synthesis, and hamster Cdc6 was able to use Xenopus Orc and Mcm proteins to assemble a limited number of pre-RCs.
The dependency of ORC release on the interaction of Mcm proteins with ORC/chromatin sites would account for the observed variation in the rate of ORC release among different preparations of egg extract. Geminin accumulates during S, G2 and M phases, and is degraded during the M- to G1-phase transition (McGarry and Kirschner, 1998; Tada et al., 2001). Metaphase-arrested eggs activated in vitro by addition of Ca2+ often gave the slowest ORC release, while extracts stored at low temperature for several weeks to several months often gave the fastest release, consistent with differences in the levels of geminin present.
The conclusion that ORC is released upon loading of Mcm proteins at ORC/chromatin sites was further supported by the observation that ORC binding to somatic cell chromatin was inhibited once pre-RCs were established either in vitro or in vivo. In egg extract, chromatin-bound ORC decreased after 20–30 min of incubation (Figure 2), despite the fact that these extracts contained a vast excess of ORC whose level remained unchanged during the course of the incubation. Therefore, as soon as pre-RCs were assembled and DNA replication had begun (Figures 2 and 4), ORC stopped binding to somatic cell chromatin in vitro. Similarly, binding of XlORC to chromatin in nuclei isolated from late G1-phase hamster cells, which already contained pre-RCs that had been assembled in vivo, was severely inhibited relative to chromatin in metaphase cells (Figure 7). The mechanism that prevents ORC from binding to chromatin following pre-RC assembly is not known. However, it does not require Cdk activity, because levels of olomoucine that completely blocked DNA replication did not affect either XlORC binding to, or XlORC release from, M-, G1- or S-phase substrates. In mammalian cells, Orc1 protein is released and mono-ubiquitylated during S phase, which may serve to prevent reassociation of Orc1 with those ORC subunits that remain bound to chromatin (Li and DePamphilis, 2002).
Comparison of somatic cell chromatin with sperm and yeast chromatin
All previous studies on the binding of Orc proteins to chromatin in Xenopus egg extract were carried out using lysolecithin-demembranated sperm as the substrate. In contrast to the results reported here using digitonin-permeabilized metaphase hamster cells as the substrate, both XlOrc1 and XlOrc2 were reported to bind rapidly to sperm chromatin and remain bound there for at least 2 h (Romanowski et al., 1996; Rowles et al., 1996; Jares and Blow, 2000). We confirmed these results using sperm chromatin (data not shown). Furthermore, in contrast to the Cdc6-facilitated binding of XlORC to somatic cell chromatin reported here, depletion of XlCdc6 did not affect XlOrc binding to sperm chromatin (Coleman et al., 1996 and data not shown). Since both sperm chromatin and hamster metaphase chromatin are rapidly assembled into nuclei by Xenopus egg extract (Figure 1), any differences in the binding of XlORC to sperm chromatin compared with somatic cell chromatin most likely reflect differences in chromatin composition.
Sperm chromatin undergoes extensive remodeling during its first 30 min in a Xenopus egg extract (Philpott and Leno, 1992). Nucleoplasmin-dependent decondensation of sperm chromatin is a prerequisite for XlORC binding and subsequent assembly of pre-RCs (Gillespie and Blow, 2000). Remodeling involves displacement of sperm- specific protamines, uptake of core histones H2A and H2B (histones H3 and H4 are already present) and linker histone B4 (histone H1 is absent from both Xenopus sperm and Xenopus eggs), as well as phosphorylation of core histones and uptake of HMG-2 protein (Dimitrov et al., 1994). Sperm chromatin is not converted into somatic cell chromatin until after the midblastula transition, when histone H1 is expressed, the rate of cell division slows down and a G1 phase first appears in the cell division cycle.
Previous studies have shown that after the stable loading of Mcm proteins at ORC/chromatin sites (i.e. licensing) has occurred in either Xenopus egg extracts (Hua and Newport, 1998; Rowles et al., 1999) or yeast (Donovan et al., 1997), ORC can be eluted from the chromatin by exposure either to high levels of Cdk2 or to ∼0.3 M NaCl, leaving chromatin-bound XlMcm proteins capable of initiating DNA replication. This weakened association of ORC with sperm chromatin was independent of DNA replication, protein synthesis, cyclin-dependent kinases and Cdc7, but required Cdc6, Mcm2–7 and Cdt1 activity, suggesting that Mcm2–7 binding to chromatin weakens the association of ORC with sperm chromatin and thus made it more sensitive to elution with salt. These observations are similar to those reported here for the programmed release of Xenopus ORC from hamster somatic cell chromatin. Similarly, regulation of DNA replication in yeast through ORC phosphorylation (Nguyen et al., 2001) may affect the association of ORC with chromatin and thus account for its change in salt sensitivity. Interestingly, yeast chromatin, like Xenopus sperm chromatin, lacks a classical histone H1 linker (Landsman, 1996). We propose that changes in the association of ORC with chromatin are a universal mechanism for preventing re-initiation of DNA replication during S phase that occur upon completion of pre-RC assembly. ORC stability determines whether or not one or more Orc proteins are released, while chromatin composition and structure determine the affinity of binding. The transitions from a salt-stable to a salt-labile ORC/chromatin site observed with both Xenopus sperm and yeast chromatin are simply different manifestations of the programmed release of one or more ORC subunits from somatic cell chromatin in mammalian cells (Natale et al., 2000; Kreitz et al., 2001; Li and DePamphilis, 2002) and in Xenopus egg extracts (reported here).
Materials and methods
Chromatin and nuclei
Sperm chromatin consisted of lysolecithin-demembranated Xenopus sperm (Smythe and Newport, 1991). Hamster metaphase chromatin consisted of metaphase-arrested CHO C400 cells permeabilized with 40 µg/ml digitonin (Wu et al., 1997; Natale et al., 2000). G1- and G1/S-phase CHO C400 cells were obtained by replating metaphase-arrested cells in fresh medium, collecting them either 4 or 7.5 h later, and permeabilizing them with digitonin. Permeabilized cells were washed three times in transport buffer, stored at –80°C in transport buffer containing 10% glycerol, and then thawed on ice when needed (usually within 3 days).
Xenopus egg extracts
Metaphase extracts were prepared from unactivated Xenopus eggs, and interphase extracts from activated eggs (Li et al., 2000; Sun et al., 2000). After thawing, metaphase extracts were activated with 0.3 mM CaCl2 for 10 min at room temperature, and supplemented with 250 µg/ml cycloheximide, 24 mM phosphocreatine and 30 µg/ml creatine phosphokinase (Sigma). Calcium activation was omitted with interphase extracts. Metaphase extracts gave optimum synchronization, but may have contained high levels of geminin (McGarry and Kirschner, 1998; Tada et al., 2001). About 5000 metaphase chromatin bodies or G1-phase nuclei were routinely used per microliter of extract, although results did not vary significantly using 1000–10 000 nuclei/µl of extract.
Immunodepletion of Xenopus egg extracts was carried out as described (Coleman et al., 1996). An anti-XlOrc2 antiserum (Natale et al., 2000) was conjugated to protein A–Sepharose beads (Amersham Pharmacia Biotech; 2 vols of antisera/1 vol. of beads for 30 min at room temperature). Interphase extracts supplemented with 250 µg/ml cycloheximide, 24 mM phosphocreatine and 30 µg/ml creatine phosphokinase were added to XlOrc2–beads (60% beads/40% extract) and the mixture rotated for 45 min at 4°C. Each extract was depleted twice. Mock-depleted extracts were depleted in parallel using protein A–Sepharose beads alone or coupled with anti-mouse IgG antibody (Sigma), with similar results. For two depletions of 130 µl extracts, 20 µg of affinity-purified anti-XCdc6 antibody were conjugated with 26 µl of beads. For two depletions of 125 µl extracts, 200 µl of anti-XMcm3 antiserum were conjugated with 50 µl of beads.
DNA replication
DNA replication was measured by supplementing Xenopus egg extract with 10 µM Fl-dUTP (Roche), incubating the extract with chromatin at 22°C for the times indicated, and then determining the fraction of labeled nuclei by fluorescence microscopy (Sun et al., 2000).
Chromatin-bound proteins
Chromatin or nuclei was incubated in Xenopus egg extract at 22°C for the times indicated. Nuclei were isolated by diluting the reaction in 10 vols of modified nuclear isolation buffer (NIB-A) (50 mM HEPES–KOH pH 7.6, 50 mM KCl, 5 mM MgCl2, 0.5 mM spermidine, 0.15 mM spermine, 1 mM ATP, 2 mM dithiothreitol, and 1 µg/ml each aprotinin, leupeptin and pepstatin) followed by centrifugation (6000 g at 4°C) through 15% sucrose in NIB-A (NIB-A-Su). Chromatin-bound fractions were obtained by extracting the nuclei with 0.5% Triton X-100 in NIB-A for 5 min at 4°C, followed by centrifugation through NIB-A-Su. Samples were subjected to electrophoresis in 4–20% pre-cast gradient polyacrylamide gels (Invitrogen). Specific proteins were detected by immunoblotting with the antiserum indicated followed by reaction with the appropriate secondary antibody conjugated with horseradish peroxidase, and detected with SuperSignal WestPico chemiluminescent substrate (Pierce) (Natale et al., 2000; Sun et al., 2000). Signals were quantified by densitometry using NIH-IMAGE software. Anti-XlOrc1 serum was provided by J.J.Blow (Rowles et al., 1996), anti-XlOrc4 serum by T.Tugal (Tugal et al., 1998), and anti-XlCdc6 and anti-XlMcm3 by T.Coleman. Anti-MmMcm3, anti-HsCdc6, anti-MmPCNA and anti-MmH3 were purchased from Santa Cruz Biotechnology, and anti-polyhistidine monoclonal antibody from Roche.
Recombinant proteins
His6-XlOrc2 was expressed in Sf9 cells using a baculovirus expression vector (Carpenter et al., 1996) extracted with B100 buffer, bound to Ni-NTA–agarose (Qiagen) and then eluted with B100 containing 200 mM imidazole. His6-XlGeminin-DEL was expressed using a bacterial expression vector (McGarry and Kirschner, 1998) and purified on Ni-NTA–agarose. GST–HsCdt1 was expressed using a bacterial expression vector (Wohlschlegel et al., 2000) and purified on glutathione–Sepharose 4B resin (Amersham Pharmacia Biotech). All steps were carried out at 4°C.
Acknowledgments
Acknowledgements
We thank D.Natale and W.Stünkle for help in preparation of Xenopus egg extracts, J.J.Blow for the XlOrc1 expression vector and anti-XlOrc1 antiserum, T.Tugal for the XlOrc4 expression vector and anti-XlOrc4 antiserum, T.J.McGarry for the Xlgeminin expression vector, A.Dutta for the HsCdt1 expression vector, M.Dasso for Xenopus sperm chromatin, and A.Vassilev and X.-H.Zhang for assistance in preparing recombinant proteins.
References
- Abdurashidova G., Riva,S., Biamonti,G., Giacca,M. and Falaschi,A. (1998) Cell cycle modulation of protein–DNA interactions at a human replication origin. EMBO J., 17, 2961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A.L. and Fanning,E. (2001) The Chinese hamster dihydrofolate reductase replication origin β is active at multiple ectopic chromosomal locations and requires specific DNA sequence elements for activity. Mol. Cell. Biol., 21, 1098–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M. and Wharton,R.P. (1999) E2F mediates developmental and cell cycle regulation of ORC1 in Drosophila. EMBO J., 18, 2435–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow J.J. (2001) Control of chromosomal DNA replication in the early Xenopus embryo. EMBO J., 20, 3293–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogan J.A., Natale,D.A. and Depamphilis,M.L. (2000) Initiation of eukaryotic DNA replication: conservative or liberal? J. Cell Physiol., 184, 139–150. [DOI] [PubMed] [Google Scholar]
- Carpenter P.B., Mueller,P.R. and Dunphy,W.G. (1996) Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature, 379, 357–360. [DOI] [PubMed] [Google Scholar]
- Chong J.P., Thommes,P., Rowles,A., Mahbubani,H.M. and Blow,J.J. (1997) Characterization of the Xenopus replication licensing system. Methods Enzymol., 283, 549–564. [DOI] [PubMed] [Google Scholar]
- Coleman T.R., Carpenter,P.B. and Dunphy,W.G. (1996) The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell, 87, 53–63. [DOI] [PubMed] [Google Scholar]
- Coverley D., Pelizon,C., Trewick,S. and Laskey,R.A. (2000) Chromatin-bound Cdc6 persists in S and G2 phases in human cells, while soluble Cdc6 is destroyed in a cyclin A–cdk2 dependent process. J. Cell Sci., 113, 1929–1938. [DOI] [PubMed] [Google Scholar]
- Decker R.S., Yamaguchi,M., Possenti,R., Bradley,M.K. and DePamphilis, M.L. (1987) In vitro initiation of DNA replication in simian virus 40 chromosomes. J. Biol. Chem., 262, 10863–10872. [PubMed] [Google Scholar]
- DePamphilis M.L. (1999) Replication origins in metazoan chromosomes: fact or fiction? BioEssays, 21, 5–16. [DOI] [PubMed] [Google Scholar]
- Dhar S.K., Delmolino,L. and Dutta,A. (2001a) Architecture of the human origin recognition complex. J. Biol. Chem., 276, 29067–29071. [DOI] [PubMed] [Google Scholar]
- Dhar S.K., Yoshida,K., Machida,Y., Khaira,P., Chaudhuri,B., Wohlschlegel,J.A., Leffak,M., Yates,J. and Dutta,A. (2001b) Replication from oriP of Epstein–Barr virus requires human ORC and is inhibited by geminin. Cell, 106, 287–296. [DOI] [PubMed] [Google Scholar]
- Dimitrov S., Dasso,M.C. and Wolffe,A.P. (1994) Remodeling sperm chromatin in Xenopus laevis egg extracts: the role of core histone phosphorylation and linker histone B4 in chromatin assembly. J. Cell Biol., 126, 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan S., Harwood,J., Drury,L.S. and Diffley,J.F. (1997) Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc. Natl Acad. Sci. USA, 94, 5611–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury L.S., Perkins,G. and Diffley,J.F. (2000) The cyclin-dependent kinase Cdc28p regulates distinct modes of Cdc6p proteolysis during the budding yeast cell cycle. Curr. Biol., 10, 231–240. [DOI] [PubMed] [Google Scholar]
- Findeisen M., El-Denary,M., Kapitza,T., Graf,R. and Strausfeld,U. (1999) Cyclin A-dependent kinase activity affects chromatin binding of ORC, Cdc6 and MCM in egg extracts of Xenopus laevis. Eur. J. Biochem., 264, 415–426. [DOI] [PubMed] [Google Scholar]
- Gilbert D.M., Miyazawa,H. and DePamphilis,M.L. (1995a) Site-specific initiation of DNA replication in Xenopus egg extract requires nuclear structure. Mol. Cell. Biol., 15, 2942–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D.M., Neilson,A., Miyazawa,H., DePamphilis,M.L. and Burhans, W.C. (1995b) Mimosine arrests DNA synthesis at replication forks by inhibiting deoxyribonucleotide metabolism. J. Biol. Chem., 270, 9597–9606. [DOI] [PubMed] [Google Scholar]
- Gillespie P.J. and Blow,J.J. (2000) Nucleoplasmin-mediated chromatin remodelling is required for Xenopus sperm nuclei to become licensed for DNA replication. Nucleic Acids Res., 28, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X.H. and Newport,J. (1998) Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J. Cell Biol., 140, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jares P. and Blow,J.J. (2000) Xenopus cdc7 function is dependent on licensing but not on XORC, XCdc6, or CDK activity and is required for XCdc45 loading. Genes Dev., 14, 1528–1540. [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Wells,N.J. and Hunter,T. (1999) Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc. Natl Acad. Sci. USA, 96, 6193–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T.J. and Brown,G.W. (2000) Regulation of chromosome replication. Annu. Rev. Biochem., 69, 829–880. [DOI] [PubMed] [Google Scholar]
- Kong D. and DePamphilis,M.L. (2001) Site-specific DNA binding of the Schizosaccharomyces pombe origin recognition complex is determined by the Orc4 subunit. Mol. Cell. Biol., 21, 8095–8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitz S., Ritzi,M., Baack,M. and Knippers,R. (2001) The human origin recognition complex protein 1 dissociates from chromatin during S phase in HeLa cells. J. Biol. Chem., 276, 6337–6342. [DOI] [PubMed] [Google Scholar]
- Landsman D. (1996) Histone H1 in Saccharomyces cerevisiae: a double mystery solved? Trends Biochem. Sci., 21, 287–288. [PubMed] [Google Scholar]
- Li C.-J. and DePamphilis,M.L. (2002) Mammalian Orc1 protein is selectively released from chromatin and ubiquitinated during the S to M transition in the cell division cycle. Mol. Cell. Biol., 22, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.J., Bogan,J.A., Natale,D.A. and DePamphilis,M.L. (2000) Selective activation of pre-replication complexes in vitro at specific sites in mammalian nuclei. J. Cell Sci., 113, 887–898. [DOI] [PubMed] [Google Scholar]
- Lygerou Z. and Nurse,P. (1999) The fission yeast origin recognition complex is constitutively associated with chromatin and is differentially modified through the cell cycle. J. Cell Sci., 112, 3703–3712. [DOI] [PubMed] [Google Scholar]
- McGarry T.J. and Kirschner,M.W. (1998) Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell, 93, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Mendez J. and Stillman,B. (2000) Chromatin association of human origin recognition complex, cdc6 and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol., 20, 8602–8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima T., Takahashi,N. and Stillman,B. (2000) Cdc6p modulates the structure and DNA binding activity of the origin recognition complex in vitro. Genes Dev., 14, 1631–1641. [PMC free article] [PubMed] [Google Scholar]
- Natale D.A., Li,C.J., Sun,W.H. and DePamphilis,M.L. (2000) Selective instability of Orc1 protein accounts for the absence of functional origin recognition complexes during the M-G1 transition in mammals. EMBO J., 19, 2728–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethanel T. and Kaufmann,G. (1990) Two DNA polymerases may be required for synthesis of the lagging DNA strand of simian virus 40. J. Virol., 64, 5912–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.Q., Co,C. and Li,J.J. (2001) Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature, 411, 1068–1073. [DOI] [PubMed] [Google Scholar]
- Okuno Y., McNairn,A.J., den Elzen,N., Pines,J. and Gilbert,D.M. (2001) Stability, chromatin association and functional activity of mammalian pre-replication complex proteins during the cell cycle. EMBO J., 20, 4263–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak D.T., Pflumm,M., Chesnokov,I., Huang,D.W., Kellum,R., Marr,J., Romanowski,P. and Botchan,M.R. (1997) Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell, 91, 311–323. [DOI] [PubMed] [Google Scholar]
- Pelizon C., Madine,M.A., Romanowski,P. and Laskey,R.A. (2000) Unphosphorylatable mutants of Cdc6 disrupt its nuclear export but still support DNA replication once per cell cycle. Genes Dev., 14, 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B.O., Lukas,J., Sorensen,C.S., Bartek,J. and Helin,K. (1999) Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J., 18, 396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B.O. et al. (2000) Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev., 14, 2330–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott A. and Leno,G.H. (1992) Nucleoplasmin remodels sperm chromatin in Xenopus egg extracts. Cell, 69, 759–767. [DOI] [PubMed] [Google Scholar]
- Ritzi M., Baack,M., Musahl,C., Romanowski,P., Laskey,R.A. and Knippers,R. (1998) Human minichromosome maintenance proteins and human origin recognition complex 2 protein on chromatin. J. Biol. Chem., 273, 24543–24549. [DOI] [PubMed] [Google Scholar]
- Romanowski P., Madine,M.A., Rowles,A., Blow,J.J. and Laskey,R.A. (1996) The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin. Curr. Biol., 6, 1416–1425. [DOI] [PubMed] [Google Scholar]
- Rowles A., Chong,J.P., Brown,L., Howell,M., Evan,G.I. and Blow,J.J. (1996) Interaction between the origin recognition complex and the replication licensing system in Xenopus. Cell, 87, 287–296. [DOI] [PubMed] [Google Scholar]
- Rowles A., Tada,S. and Blow,J.J. (1999) Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J. Cell Sci., 112, 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P., Chen,J., Thome,K.C., Lawlis,S.J., Hou,Z.H., Hendricks,M., Parvin,J.D. and Dutta,A. (1998) Human CDC6/Cdc18 associates with Orc1 and cyclin–cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol. Cell. Biol., 18, 2758–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe C. and Newport,J.W. (1991) Systems for the study of nuclear assembly, DNA replication and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol., 35, 449–468. [DOI] [PubMed] [Google Scholar]
- Sun W., Hola,M., Pedley,K., Tada,S., Blow,J.J., Todorov,I.T., Kearsey,S.E. and Brooks,R.F. (2000) The replication capacity of intact mammalian nuclei in Xenopus egg extracts declines with quiescence, but the residual DNA synthesis is independent of Xenopus MCM proteins. J. Cell Sci., 113, 683–695. [DOI] [PubMed] [Google Scholar]
- Tada S., Li,A., Maiorano,D., Mechali,M. and Blow,J.J. (2001) Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nature Cell Biol., 3, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommes P., Kubota,Y., Takisawa,H. and Blow,J.J. (1997) The RLF-M component of the replication licensing system forms complexes containing all six MCM/P1 polypeptides. EMBO J., 16, 3312–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugal T., Zou-Yang,X.H., Gavin,K., Pappin,D., Canas,B., Kobayashi,R., Hunt,T. and Stillman,B. (1998) The Orc4p and Orc5p subunits of the Xenopus and human origin recognition complex are related to Orc1p and Cdc6p. J. Biol. Chem., 273, 32421–32429. [DOI] [PubMed] [Google Scholar]
- Vashee S., Simancek,P., Challberg,M.D. and Kelly,T.J. (2001) Assembly of the human origin recognition complex. J. Biol. Chem., 276, 26666–26673. [DOI] [PubMed] [Google Scholar]
- Walter J.C. (2000) Evidence for sequential action of cdc7 and cdk2 protein kinases during initiation of DNA replication in Xenopus egg extracts. J. Biol. Chem., 275, 39773–39778. [DOI] [PubMed] [Google Scholar]
- Wang B., Feng,L., Hu,Y., Huang,S.H., Reynolds,C.P., Wu,L. and Jong,A.Y. (1999) The essential role of Saccharomyces cerevisiae CDC6 nucleotide-binding site in cell growth, DNA synthesis and Orc1 association. J. Biol. Chem., 274, 8291–8298. [DOI] [PubMed] [Google Scholar]
- Wohlschlegel J.A., Dwyer,B.T., Dhar,S.K., Cvetic,C., Walter,J.C. and Dutta,A. (2000) Inhibition of eukaryotic DNA replication by geminin binding to cdt1. Science, 290, 2309–2312. [DOI] [PubMed] [Google Scholar]
- Wu J.R. and Gilbert,D.M. (1996) A distinct G1 step required to specify the Chinese hamster DHFR replication origin. Science, 271, 1270–1272. [DOI] [PubMed] [Google Scholar]
- Wu J.R., Yu,G. and Gilbert,D.M. (1997) Origin-specific initiation of mammalian nuclear DNA replication in a Xenopus cell-free system. Methods, 13, 313–324. [DOI] [PubMed] [Google Scholar]
- Wu J.R., Keezer,S.M. and Gilbert,D.M. (1998) Transformation abrogates an early G1-phase arrest point required for specification of the Chinese hamster DHFR replication origin. EMBO J., 17, 1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Wu,J.R. and Gilbert,D.M. (1998) Analysis of mammalian origin specification in ORC-depleted Xenopus egg extracts. Genes Cells, 3, 709–720. [DOI] [PubMed] [Google Scholar]