Abstract
Targeting of DNA molecules to specific subcellular positions is essential for efficient segregation, but the mechanisms underlying these processes are poorly understood. In Escherichia coli, several plasmids belonging to different incompatibility groups (F, P1 and RK2) localize preferentially near the midcell and quartercell positions. Here we compare the relative positions of these three plasmids using fluorescence in situ hybridization. When plasmids F and P1 were localized simultaneously using differentially labeled probes, the majority of foci (∼75%) were well separated from each other. Similar results were found when we compared the subcellular localization of F with RK2, and RK2 with P1: regardless of the number of foci per cell or growth conditions, most of the foci (70–80%) were not in close proximity to one another. We also localized RK2 in Pseudomonas aeruginosa and Vibrio cholerae, and found that plasmid RK2 localization is conserved across bacterial species. Our results suggest that each plasmid has its own unique subcellular address, implying a mechanism for the stable co-existence of plasmids in which subcelluar targeting plays a major role.
Keywords: chromosome segregation/DNA replication/plasmid localization/plasmid stability
Introduction
Segregation of DNA to daughter cells prior to cell division is important in all organisms, but the mechanisms underlying these processes in bacteria are poorly understood. Many DNA molecules once assumed to be randomly distributed in bacterial cells are now known to display a remarkable degree of previously unappreciated organizational complexity (Webb et al., 1997; Jensen and Shapiro, 1999b; Sharpe and Errington, 1999; Dasgupta et al., 2000; Gordon and Wright, 2000; Hiraga, 2000; Shapiro and Losick, 2000; Donachie, 2001). Individual regions of bacterial chromosomes are positioned specifically within the cell and can move rapidly from one location to another (Gordon et al., 1997; Lewis and Errington, 1997; Webb et al., 1997, 1998; Sharpe and Errington, 1998; Jensen and Shapiro, 1999a; Roos et al., 2001), despite the fact that bacteria lack obvious homologs of motor proteins or the proteins comprising the spindle apparatus. For example, in Bacillus subtilis and Escherichia coli, the origins of replication are anchored temporarily near the midcell and, after duplication, migrate rapidly to positions near the cell poles (Gordon et al., 1997; Webb et al., 1997, 1998; Niki and Hiraga, 1998).
In B.subtilis, DNA replication occurs in relatively stationary complexes located either near midcell or near the future midpoints of the daughter cells (one-quarter and three-quarters cell positions) (Lemon and Grossman, 1998, 2000). A stationary replisome situated near midcell might act as a biosynthetic motor that extrudes newly duplicated chromosomes in opposite directions (Dingman, 1974; Losick and Shapiro, 1998; Cook, 1999; Lemon and Grossman, 2001). The Bacillus origins duplicated near midcell are directed away from the centrally located replisome and are captured by unknown mechanisms near the one-quarter and three-quarters cell positions. The factory model of replication is likely to apply to many organisms, including Caulobacter crescentus and E.coli, where evidence suggests that organization and directionality of newly extruded DNA may be provided by proteins involved in nucleoid folding/condensation (Jensen and Shapiro, 1999a; Koppes et al., 1999; Onogi et al., 1999; Brendler et al., 2000; Dasgupta et al., 2000; Hiraga, 2000; Holmes and Cozzarelli, 2000; Sawitzke and Austin, 2000, 2001). The replication proteins of C.crescentus are also localized in replication factories; however, in contrast to the stationary factories of B.subtilis, the C.crescentus replisomes migrate during the cell cycle from the cell pole to midcell (Jensen et al., 2001).
Despite these advances in our understanding of the in vivo dynamics of chromosomal DNA, little is known about the mechanisms by which DNA molecules are targeted to specific positions within the cell or how they move from one location to another. Several plasmids have been localized in E.coli by either fluorescence in situ hybridization (FISH) or by tagging with green fluorescent protein (GFP)–LacI after insertion of a lacO array (Niki and Hiraga, 1997; Jensen and Gerdes, 1999; Jensen and Shapiro, 1999b; Gordon and Wright, 2000; Hiraga, 2000; Weitao et al., 2000; Pogliano et al., 2001). Plasmids F, P1 and RK2 localize near midcell and, after duplication, migrate with rapid kinetics to the one-quarter and three-quarters cell positions (Gordon et al., 1997; Niki and Hiraga, 1997; Pogliano et al., 2001). Localization of F and RK2 is coordinated with the host cell cycle such that the newborn cells contain a single midcell focus, whereas cells that have progressed further through the cell cycle contain two foci located near the one-quarter and three-quarters cell positions (Gordon et al., 1997; Niki and Hiraga, 1997; Pogliano et al., 2001). RK2 localization is also coordinated with the rate of growth, so that the number of foci per cell increases with increasing growth rate, and decreases when cells enter stationary phase (Pogliano et al., 2001). These highly organized localization patterns suggest that physiological cues about the host cell cycle feed into the regulatory networks controlling plasmid copy number and distribution (Bingle and Thomas, 2001). Partitioning systems encoded by these plasmids have been proposed to play a role in mediating localization to these positions (Niki and Hiraga, 1997; Bignell et al., 1999; Erdmann et al., 1999).
A single bacterial strain can stably maintain many different plasmids only if each belongs to a different incompatibility group, but the relationship between incompatibility groups and subcellular localization has never been fully understood (Novick, 1987; Nordstrom and Austin, 1989). One model proposed that each compatible plasmid is tethered to a different receptor encoded by the host cell, but an alternative model suggested that a single host protein or structure serves as a common receptor with which many plasmids interact (Novick, 1987; Nordstrom and Austin, 1989; Austin and Nordstrom, 1990). Therefore, the subcellular positioning of different plasmid molecules in the same cell could have profound implications for how plasmids interact with each other, including how they compete with one another for replication or how frequently they undergo recombination. Yet, such models of in vivo plasmid dynamics remain speculative, partly because of our limited understanding of the underlying basis of plasmid localization and movement in bacteria. Even less is known about plasmid localization in organisms besides E.coli.
Here we simultaneously localize pairs of plasmids (F, P1 and RK2) by FISH to address two questions. First, do different plasmids recognize the same, conserved structure at midcell (and therefore co-localize) or does each target to separate structures located in the vicinity of the cell midpoint? Secondly, do different plasmids move from midcell to quartercell simultaneously? We show that plasmids belonging to different incompatibility groups are not targeted to the same receptor inside the cell, and that they segregate at different times relative to each other. We also show that RK2 localization is conserved between the Gram-negative bacteria Pseudomonas aeruginosa, Vibrio cholerae and E.coli. Our results provide visual evidence for another mechanism of plasmid incompatibility in which compatible plasmids are tethered to unique receptors in the cell, that are separated both spatially and temporally from one another.
Results
Plasmids F and P1 are low-copy plasmids, present at one or two copies per chromosome equivalent, while RK2 is a multicopy plasmid present at five to eight copies per chromosome equivalent. When each plasmid is localized individually in cells grown in minimal media, most cells contain a single focus located near midcell or two foci located near the one-quarter and three-quarters cell positions (Gordon et al., 1997; Niki and Hiraga, 1997; Pogliano et al., 2001; Figure 1). The similarities in F, P1 and RK2 localization could indicate that each plasmid is tethered to a common structure or receptor within the cell. If this is the case, then signals from two different plasmids that are labeled with different fluorescent probes should co-localize. Alternatively, each plasmid may be targeted independently and separately from one another. In this case, signals from two differently labeled plasmids will not fully overlap, except by chance due to the small size of the cells and the limited resolution of light microscopy. To determine if plasmids F, P1 and RK2 are localized in close proximity to one another within the cell, we performed dual labeling FISH experiments. We compared the localization properties of a mini-P1 plasmid (λ-P1:5R), a mini-F plasmid (pOX38Kan) and the 60 kb RK2 plasmid or a 20 kb RK2 derivative (pRK290).
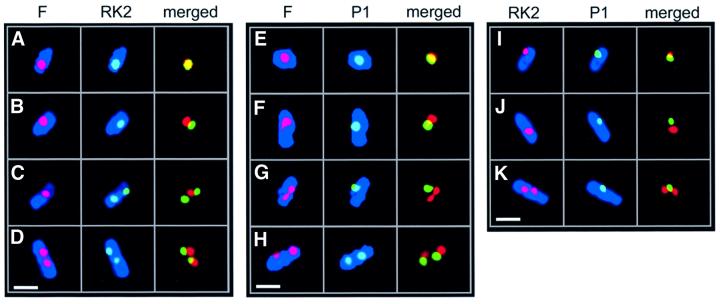
Fig. 1. Fluorescence micrographs showing dual labeling of plasmids F, P1 and RK2 in E.coli by FISH. Individual red (left columns) and green (middle columns) foci are shown with blue membranes, or merged together without membrane staining (right columns). Overlapping green and red foci appear yellow. Cell membranes were stained with MTG and are shown in blue false-color. White bars = 1 µm. (A–D) Plasmids F (red) and RK2 (green) showing cells (JP941) containing one fluorescent focus for each plasmid (A and B), a single F focus flanked by two RK2 foci (C) and two fluorescent foci for each plasmid (D). (E–H) Plasmids F (red) and P1 (green) showing cells (JP821) containing two overlapping fluorescent foci located near midcell (E), two non-overlapping foci (F), a single P1 focus and two F foci (G), and four non-overlapping foci (H). (I–K) Plasmids RK2 (red) and P1 (green) showing cells (JP869) containing two foci that overlap (I), two separated foci (J) and a single midcell P1 focus flanked by two RK2 foci (K).
Simultaneous localization of F and RK2
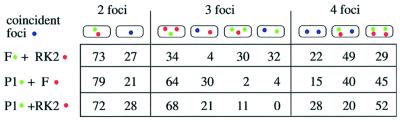
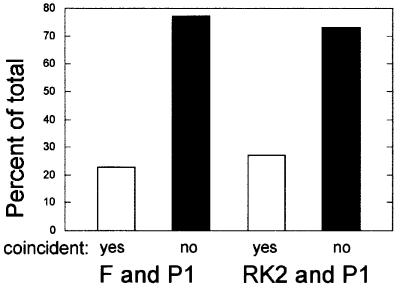
We localized F and RK2 replicons simultaneously in fixed cells of strain JP941 (containing pOX38Kan and pRK290), grown in minimal glucose media at 30°C, using FISH. The RK2 probe was labeled with Cy5 (green), the F probe was labeled with Cy3 (red) and the cell membranes were stained with Mitotracker Green FM (MTG) (blue; Figure 1A–D). As expected under these growth conditions, most of the cells contain one or two foci for each plasmid (Gordon et al., 1997; Niki and Hiraga, 1997; Pogliano et al., 2001). Even though RK2 is a multicopy plasmid, many copies of the plasmid cluster together at a few positions (midcell and quartercell) inside the cell (Pogliano et al., 2001). In cells containing one fluorescent signal for each plasmid, we could readily detect two foci that were overlapping (Figure 1A; yellow), as well as two foci that were not overlapping (Figure 1B). We quantitated the percentage of overlapping foci for cells containing a total of two, three or four foci in the same cell (Figure 2). In the majority (73%) of cells containing two foci (one focus for each plasmid), the signals from the two probes did not overlap (Figures 1B and 2), even though each focus was positioned near midcell. When four foci per cell were present (two for each plasmid), they often occurred near the quartercell positions as expected but, in most cases, either one (49%) or both (29%) pairs were well separated from each other (Figures 1D and 2). Identical results were obtained if the two fluorophores of the probes were reversed (not shown). These results demonstrate that F and RK2 replicons generally are not co-localized within the cell.
Fig. 2. Percentage of coincident plasmid foci from dual-labeling FISH experiments. Cells containing either one or two signals for each plasmid were categorized into groups containing a total of two, three or four foci, and the percentage of cells containing coincident (purple dot) or non-overlapping foci (red and green dots) was determined. A total of 578 cells were scored with n = 249 for P1 and RK2, n = 174 for P1 and F, and n = 155 for F and RK2.
Two fluorescent signals were overlapping ∼20–30% of the time (Figure 2). This degree of overlap could be due to the small diameter of E.coli (∼0.6 µm) and the limited resolution of light microscopy (0.2 µm). We estimate that two separate signals randomly positioned near midcell would appear to overlap by chance approximately one-third of the time, which may account for the fraction of overlapping foci observed here. Alternatively, plasmids may be targeted to the same region of the cell during a portion of their replication/segregation cycles.
Simultaneous localization of F and P1
We next determined if F and P1 were in close proximity or well separated from one another using a Cy3-labeled probe for F and a Cy5-labeled probe for P1. In cells of JP821 (containing pOX38Kan and λ-P1:5R) grown in minimal glucose media at 30°C, most contained one or two foci for each plasmid, with a single focus of P1 (green) or F (red) preferentially near midcell (Figure 1E and F). Sometimes (21%), these foci at midcell were overlapping (Figures 1E and 2), suggesting that they were too close together to be resolved by light microscopy. However, in the majority of cells (79%), the single P1 and single F foci did not overlap (Figures 1F and 2). When two foci per plasmid were present, the percentage of cells in which neither, one or both pairs of foci overlapped was 45, 40 and 15%, respectively (Figure 2). Therefore, F appears to be spatially separated from both P1 and RK2 in the majority of cells.
Simultaneous localization of RK2 and P1
From comparing cells containing the same number of F and RK2 or F and P1 foci, the above results suggested that these plasmids are targeted independently of one another. We next simultaneously localized P1 and RK2 plasmids in strain JP869 (containing λ-P1:5R and RK2) grown in minimal glucose media at 30°C. Using a Cy3-labeled probe for RK2 and a Cy5-labeled probe for P1, most of the cells contained one or two foci corresponding to each plasmid. When cells contained a single focus for P1 (green) and RK2 (red), some of the foci appeared to overlap (Figure 1I), but the majority (72%) did not (Figures 1J and 2). When four foci were present (two for each plasmid), the largest class of cells (52%) contained four foci that did not overlap (Figure 2), while one pair of foci did not overlap in 20% of the cells (Figure 2). Taken together, our results suggest that plasmids F, P1 and RK2 are generally not co-localized inside the cell, despite their generally similar localization patterns.
Relative timing of plasmid segregation
A substantial number of cells (between 40 and 60%) contained three foci, suggesting that segregation of the different plasmids is asynchronous. For F and P1, most of these cells (94%) contained two F foci flanking a single P1 focus (Figures 1G and 2), suggesting that the F plasmids segregated before P1. For RK2 and P1, 89% of the cells with three foci contained two RK2 foci flanking a single P1 focus (Figures 1K and 2). P1 segregation thus appeared to lag behind RK2 in most of the cells. In comparison, for F and RK2, 62% of the cells with three foci contained two F foci flanking a single RK2 focus. Therefore, individual plasmids may be separated temporally as well as spatially.
Cell division is not required for plasmid segregation
Given the location of F, P1 and RK2, a central question is whether cell division is required for plasmid localization or segregation. Previous studies found that segregation of a mini-P1 plasmid tagged with GFP–LacI is inhibited when cell division is blocked by β-lactams specific for FtsI (Gordon et al., 1997). In contrast, segregation of a mini-F plasmid tagged with GFP–LacI was unaffected, suggesting that each plasmid is targeted in the cell by a different mechanism, only one of which is dependent upon cell division. Since our co-localization results support the proposal that different plasmids are targeted to different structures, we sought to determine if plasmid RK2 is affec ted by inhibitors of cell division. We also re-examined the effect of cell division inhibition on F and P1, since a more recent study suggested that some P1 plasmids may not require cell division for segregation (Erdmann et al., 1999).
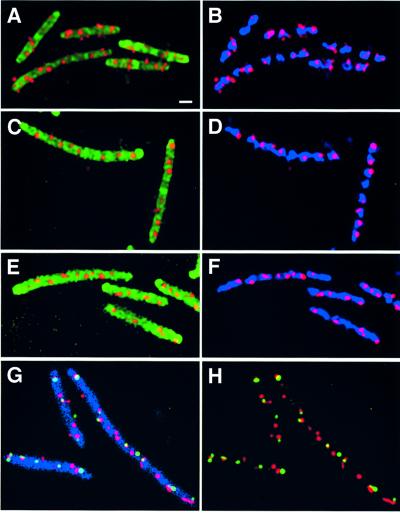
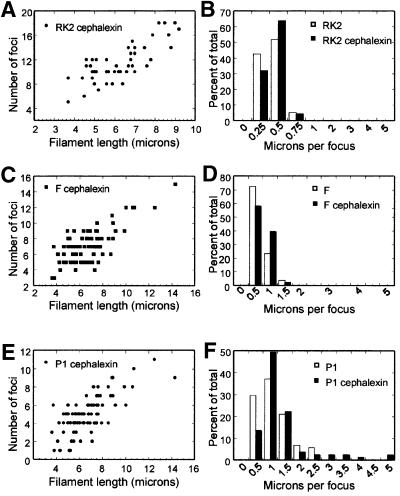
We localized RK2 in E.coli cell filaments formed by inactivating cell division with the antibiotic cephalexin. Cell growth and chromosome segregation continue in the absence of cell division, and long, multinucleate filaments are produced. Localization of RK2 in LB at 30°C in the presence of cephalexin is shown in Figure 3A and B. The cell membranes are shown in green, the nucleoids are shown in blue and the foci are shown in red. RK2 foci were distributed regularly along the entire length of the filament, indicating that targeting of each cluster of RK2 plasmids was unaffected by the inactivation of cell division. Foci always occurred in association with the bacterial nucleoid and were rarely observed in the spaces between the nucleoids (Figure 3). Although cells grown in LB contained many RK2 foci as expected (Pogliano et al., 2001), the number of foci per cell increased with filament length (Figure 4A). The number of foci per micron of cell length remained unchanged after cephalexin treatment (Figure 4B), suggesting that RK2 does not require cell division for segregation.
Fig. 3. Fluorescence micrographs of FISH experiments showing plasmid localization in cells grown in LB at 30°C and treated with cephalexin two generations prior to collecting samples for microscopy. The outlines of the filaments were visualized by staining the cell membranes with MTG and appear green in (A), (C) and (E) and blue in (G). The nucleoids were stained with DAPI and appear blue in (B), (D) and (F). (A and B) RK2 plasmids form regularly spaced foci (red) along the length of the filaments of strain JP704. (C and D) P1 plasmids form foci (red) regularly distributed along the entire length of the filaments of strain JP857. (E and F) F plasmids form foci (red) along the entire length of the filaments of strain JP911. (G and H) Dual labeling of F (red) and P1 (green) in cephalexin-induced filaments of JP821. (G) F plasmids (red) and P1 plasmids (green) form foci that are regularly spaced along the length of the filaments (blue). (H) F and P1 alone (no membranes) showing that most of the foci do not overlap. Bar in (A) = 1 µm. All panels are at the same scale.
Fig. 4. Segregation of F, P1 and RK2 in the presence of the cell division inhibitor cephalexin. (A) The number of RK2 foci per cell increases with cell length in filaments of strain JP704 formed by treatment with cephalexin in LB at 30°C. (B) The number of RK2 foci per cell was normalized to cell length (microns per focus) and plotted versus the percentage of total cells. Strain JP704 was grown in LB at 30°C in the absence (white bars) or presence (black bars) of cephalexin. For (A) and (B), a total of 59 wild-type cells containing 148 foci and 47 filaments containing 540 foci were measured. (C–F) The effect of cephalexin on F and P1 distribution in filaments of strain JP821 grown in LB at 30°C. A total of 108 wild-type (untreated) cells containing 191 P1 and 287 F foci were measured. A total of 81 cephalexin-treated cells (filaments) containing 408 P1 and 583 F foci were measured. (C) The number of F foci per cell increases with cell length in cephalexin-induced filaments of JP821. The number of foci per filament is plotted versus filament length. (D) The number of F foci per cell was normalized to cell length (microns per focus) and plotted versus the percentage of total cells. Untreated cells (white bars) have approximately the same number of microns per focus as cephalexin-treated cells (black bars). (E) The number of P1 foci per cell increases with cell length in cephalexin-induced filaments of JP821. The number of foci per filament is plotted versus filament length. (F) The number of P1 foci per cell was normalized to cell length (microns per focus) and plotted versus the percentage of total cells. Untreated cells (white bars) range from 0.5 to 2.5 µm per focus, whereas a small percentage of cephalexin-treated cells (black bars) range up to 5 µm per focus.
We compared F, P1 and RK2 localization in the presence of cephalexin. In our experiments, F (Figure 3E and F) formed foci that were regularly spaced along the length of the filament and were associated with the nucleoid region, in agreement with previous results (Gordon et al., 1997). However, P1 localization was more complex. Some filaments had very few foci, as seen previously (Gordon et al., 1997), but the majority contained many regularly spaced P1 foci (Figure 3C and D) similar to F. To understand better the effect of cephalexin on P1 segregation, we quantitated F and P1 co-localization after cephalexin treatment.
The numbers of F and P1 foci per filament are plotted versus filament length in Figure 4C and E. For P1, the number of foci per filament ranged from one to 11, with only a small percentage of filaments having only one (5%) or two foci (7%), while 12% contained ≥8 foci (Figure 4E). These results appear similar to F (Figure 4C); however, differences between the two plasmids become more apparent when foci number is normalized to cell length (Figure 4D and F). For P1, most of the filaments (90%) had a micron per focus ratio of ≤2, with the remainder containing up to 5 µm per focus (Figure 4F, black bars). In comparison, when F was localized in these same filaments, 100% of the filaments had a micron per focus ratio of <2 (Figure 4D). Taken together, our results suggest that, like the E.coli nucleoid, plasmids F and RK2 do not require cell division for segregation, whereas segregation of P1 is inhibited in a small (10%) percentage of the population. This percentage might vary substantially between different mini-P1 plasmids, with growth conditions or with the methods used for plasmid visualization (Gordon et al., 1997; Erdmann et al., 1999).
When F and P1 were localized simultaneously in cephalexin-treated cells (Figure 3G and H), the majority (77%) of F and P1 foci did not overlap (Figures 3G and H, and 5). Similarly, 73% of RK2 and P1 foci were non-overlapping in cephalexin-induced filaments (Figure 5). These results provide striking evidence that each plasmid is targeted to a separate location within the cell independently of cell division.
Fig. 5. Percentage of coincident plasmid foci in cephalexin-induced filaments. Cultures grown in LB at 30°C (for F and P1 in strain JP821; left half) or minimal glucose at 30°C (for RK2 and P1 in strain JP869; right half) were treated with cephalexin for 2–3 generations prior to collecting samples for FISH. The percentage of foci that were overlapping (white bars) or not overlapping (black bars) is plotted for each pair. For F and P1, 39 filaments containing a total of 444 foci were scored (n = 274 for F, n = 170 for P1). For RK2 and P1, a total of 342 foci were scored (n = 209 for RK2, n = 133 for P1).
Localization of RK2 is conserved in Pseudomonas aeruginosa and Vibrio cholerae
Studies of plasmid localization have focused primarily on E.coli (Gordon and Wright, 2000), raising the question as to the generality of the results. While F and P1 only replicate in E.coli and very closely related species, RK2 is capable of replicating in >30 Gram-negative bacteria (Thomas and Helinski, 1989). To determine if plasmid dynamics are conserved among Gram-negative bacteria, we localized a GFP-tagged version of RK2 in two other species: P.aeruginosa and V.cholerae. We chose P.aeruginosa because of its evolutionary distance from E.coli. Vibrio cholerae was chosen because it contains two separate circular chromosomes rather than one (Trucksis et al., 1998; Heidelberg et al., 2000). Since these two chromosomes differ in size by 2 Mb, the timing of replication initiation, termination or segregation is likely to be regulated differentially. Therefore, V.cholerae potentially represents a very different way of orchestrating DNA dynamics in bacteria.
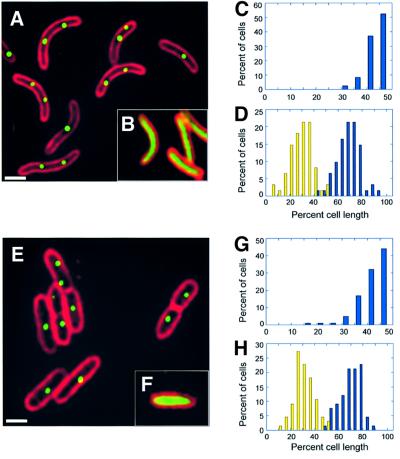
We constructed a derivative of RK2 (pZZ15) that contains a 10 kb lacO array and expresses GFP–LacI from the arabinose promoter of E.coli. Binding of GFP–LacI to the lacO array produces green fluorescent foci, indicating the subcellular position of the RK2 plasmid (Straight et al., 1996; Gordon et al., 1997; Belmont, 2001). When GFP–LacI is induced by the addition of arabinose to a culture of the V.cholerae strain JP909 (O395N1/pZZ15), green fluorescent foci are observed in 99% of the cells containing pZZ15 (Figure 6A), while green fluorescence fills the cytoplasm of the cells containing the control plasmid pZZ16, which expresses GFP–LacI in the absence of lacO sequences (Figure 6B). When grown in minimal glucose media, 95% of the cells contained from one to three foci, with a maximum of five or six foci occurring in only a small (1%) percentage of cells (Figure 6). In E.coli, the average number of foci per cell increases substantially with growth rate; however, in V.cholerae, the number of foci per cell changes only slightly between growth in LB glucose and minimal glucose (Figure 7). We quantitated the subcellular distribution of the foci by measuring the length of the cell and the position of the foci with respect to one cell pole. A single focus occurred primarily near midcell (Figure 6C), while two foci occurred primarily near the one-quarter and three-quarters cell positions (Figure 6D).
Fig. 6. Localization of GFP-tagged RK2 in Vibrio cholerae (A–D) and Pseudomonas. aeruginosa (E–H). Fluorescence micrographs showing a field of V.cholerae (A) or P.aeruginosa (E) in which the cell membranes were stained red with FM 4-64, and GFP–LacI was expressed from plasmid pZZ15 by induction with 0.2% l-arabinose as described in Materials and methods. Cells containing the control plasmid pZZ16 are shown in (B) and (F). White bars = 1 µm. Subcellular distribution of RK2 in V.cholerae for cells (n = 144) containing one focus (C) or two foci (D) and in P.aeruginosa for cells (n = 191) containing one focus (G) or two foci (H). the positions of GFP foci were measured with respect to one end of the cell (reported as a percentage of cell length) and plotted versus the percentage of total cells.
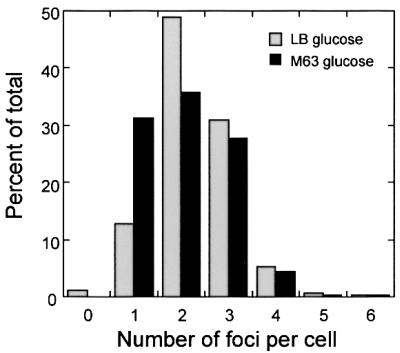
Fig. 7. Histogram showing the number of GFP-tagged RK2 foci per cell in V.cholerae strain JP909 in different growth conditions. The percentage of total cells is plotted versus the number of foci per cell during growth in M63 glucose at 30°C (black bars, 470 total cells) and LB glucose at 30°C (gray bars, 374 total cells).
Similar results were found when we localized RK2 in P.aeruginosa strains JP977 (PAO1/pZZ15) and JP978 (PAO1/pZZ16) (Figure 6). When these strains were grown in the presence of arabinose, green fluorescent foci only occurred in cells of the strain (PAO1/pZZ15) containing the lacO array (Figure 6E). In LB glucose media, most of these cells (78%) contained between one and three foci, with a maximum number of four foci per cell. A single focus occurred primarily near midcell (Figure 6G), while two foci occurred preferentially near the one-quarter and three-quarters cell positions (Figure 6H). These results are strikingly similar to those obtained in V.cholerae and to those previously reported in E.coli (Pogliano et al., 2001), suggesting that RK2 localization and the host receptors involved in RK2 targeting are conserved.
Discussion
For years, many plasmids were assumed to be diffusing randomly inside bacterial cells. We now know that a number of plasmids are tethered near the midcell or quarter cell positions in E.coli (Gordon et al., 1997; Niki and Hiraga, 1997; Pogliano et al., 2001). Here we expand this view of plasmid organization by showing that three plasmids (F, P1 and RK2) are targeted separately to different positions in the vicinity of the cell midpoints or cell quarters. At these positions, multicopy plasmids form clusters composed of many molecules (Eliasson et al., 1992; Reich et al., 1994; Weitao et al., 2000; Pogliano et al., 2001). Previous studies showed that plasmid R1 is targeted near the cell midpoint or cell poles, rather than near the cell quarters (Jensen and Gerdes, 1999; Weitao et al., 2000). Therefore, R1 is also likely to be spatially separated from F, P1 and RK2 in E.coli. Taken together, these results imply that plasmids belonging to different incompatibility groups are effectively separated from one another (or compartmentalized) during much of the E.coli cell cycle.
Our results support a model in which different plasmids interact with different subcellular structures whose numbers and positions are regulated separately within the host cell. This model potentially provides another mechanistic explanation of plasmid incompatibility in which two plasmids that are differentially targeted are compatible and stably co-inherited, whereas incompatible plasmids compete with one another for occupying a limited number of sites important for replication and/or segregation. Targeting of incompatible plasmids to the same structure might also result in the formation of mixed pairs, which could lead to unstable inheritance. Thus, some types of incompatibility may be influenced or determined by the targeting activities encoded by each plasmid.
RK2 can replicate in representatives from the α, β and γ subdivisions of Proteobacteria, including such diverse organisms as P.aeruginosa, V.cholerae, C.crescentus, Hyphomicrobium, Azotobacter vinlandii and Agro bacterium tumefaciens (Thomas and Helinski, 1989). Here we show that RK2 localization is remarkably well conserved between P.aeruginosa, V.cholerae and E.coli. The fact that a single plasmid can be stably maintained in so many distantly related species and display a similar localization pattern in at least three of them suggests that the host cell components with which RK2 interacts may be conserved. These results suggest that, like E.coli, other Gram-negative bacteria may also contain organized, dynamic and highly regulated assemblages of functionally independent plasmid complexes.
Substantial effort has been applied toward elucidating when during the bacterial cell cycle individual plasmids are replicated (Cooper and Keasling, 1998; Bogan et al., 2001), but less is known about the timing of plasmid segregation in relation to each other or other host cell cycle events. The results presented here, taken together with those of previous studies (Niki and Hiraga, 1997; Jensen and Shapiro, 1999b; Gordon and Wright, 2000; Hiraga, 2000; Pogliano et al., 2001), indicate that different plasmids are not only spatially separated in the cell, but they segregate at different times relative to one another. Previously, F and P1 were shown to display dramatically different localizations relative to the E.coli cell cycle (Gordon et al., 1997). In our comparisons, P1 was typically the last to segregate relative to F or RK2. Although segregation of F and RK2 from midcell to quartercell is coordinated similarly with the E.coli cell cycle, segregation of these two plasmids is not strictly coordinated with one another. For example, F segregated before RK2 62% of the time. How the timing of segregation is regulated by the different plasmids is unknown, but it could involve both replicative and post-replicative events. For example, the resolution of plasmid dimers could play an important role in the timing of their separation (Austin et al., 1981). Additionally, the relative timing of segregation might also be substantially different if replication of one plasmid occurs much later in the cell cycle relative to that of another.
The host cell structures with which plasmids interact remain unidentified. The fact that several different plasmids (RK2, F and P1) can localize independently to the midcell and quartercell positions in E.coli, and that plasmid RK2 can localize to these same positions in different bacteria (P.aeruginosa, V.cholerae and E.coli) suggests that the mechanisms involved in plasmid targeting are conserved in both the bacteria and the plasmids. One possible candidate is the cellular replisome, which is located similarly and might interact with plasmids as part of their DNA replication cycle. Consistent with this idea, Miller and Cohen (1999) suggested that DNA replication proteins contribute to the segregation of plasmid pSC101 separately from their role in DNA replication. However, the relationship between plasmids and replisomes currently is unclear. If plasmids were permanently attached to the cellular replisomes involved in chromosome replication then, under the growth conditions used here, many plasmids would co-localize, which was not the case. Alternatively, each plasmid could recruit the assembly of a new complex of replication proteins at the site of the plasmid origin. In C.crescentus, the number of replisomes per cell increased in a strain containing a multicopy plasmid (Jensen et al., 2001), suggesting that new replisomes are assembled when plasmids are present. However, given the speed of DNA polymerization (∼1 kb/s), even relatively large plasmids such as RK2 (60 kb) require only 1 min to replicate. Therefore, plasmid–replisome complexes could be either relatively short-lived or permanent, depending on the particular plasmid, the mode of replication (theta versus rolling circle) and the mechanism of replication initiation. If this speculative model turns out to be relevant, then the data presented here suggest that many plasmids may be tethered to their own unique replisomes inside the cell.
The role of cell division in plasmid localization has been examined previously for F and P1 (Gordon et al., 1997; Erdmann et al., 1999). While segregation of plasmid F was shown to be unaffected, the results for P1 segregation were conflicting. One study found P1 segregation to be dramatically affected by cephalexin (Gordon et al., 1997), while another found that localization of the P1 ParB protein (whose position probably indicates the location of P1) was unaffected (Erdmann et al., 1999). In our experiments, F and RK2 formed equally distributed foci in filaments formed by inactivating cell division with cephalexin. When P1 was localized under identical conditions, foci were distributed regularly in ∼90% of the filaments. This suggests that the receptors with which plasmids interact are not dependent upon the cell division machinery. However, P1 localization was complicated by the fact that segregation appeared to be affected in a low but significant (10%) number of filaments. Taken together with previous studies, these results suggest that different mini-P1 plasmids vary in their sensitivity to the effects of inhibiting cell division with cephalexin. These differences may provide clues as to the plasmid-encoded genes involved in P1 localization.
Materials and methods
Strains, media and growth conditions
Strains were grown at 30°C in M63 0.2% glucose for minimal media or LB for rich media. Ampicillin (50 µg/ml), kanamycin (40 µg/ml), chloramphenicol (10 µg/ml) and tetracycline (20 µg/ml) were used to select for plasmids in E.coli and V.cholerae, and tetracycline (100 µg/ml) to select for plasmids in P.aeruginosa. Cephalexin (10 µg/ml) was added to inhibit cell division 2–3 generations prior to collecting samples for microscopy (Pogliano et al., 1997). A λ-P1 hybrid plasmid, designated λ-P1:5R, was used as a representative P1 replicon (Sternberg and Austin, 1983), and a kanamycin-resistant derivative of pOX38 (O’Conner and Malamy, 1984), designated pOX38Kan (Anthony et al., 1996), was used as a representative F replicon. The native 60 kb RK2 plasmid (Ingram et al., 1973; Pansegrau et al., 1994) was used for co-localization with P1 replicons, while pRK290 (Ditta et al., 1980), a 20 kb RK2 derivative that lacks the kanamycin-resistant determinant, was used for co-localization with pOX38Kan. All plasmids are Par+. λ-P1:5R plasmids were introduced into the strain JP313 [araΔ714, Δ(argF-lac)U169, rpsL150, relA1, thi, flb5301, deoC1, ptsF25, rbsR] (Economou et al., 1995) by infection with a λ-P1:5R lysate (Sternberg and Austin, 1983), followed by selection for chloramphenicol-resistant prophage at 30°C to create strain JP857 (JP313/λ-P1:5R). RK2 and F plasmids were introduced by electroporation to create JP704 (JP313/RK2) (Pogliano et al., 2001), JP869 (λ-P1:5R/RK2), JP911 (JP313/pOX38Kan), JP821 (JP313/λ-P1:5R/pOX38Kan) and JP941 (JP313/pRK290/pOX38Kan). To localize RK2 by GFP–LacI tagging in organisms besides E.coli, we constructed plasmids pZZ15 and pZZ16, two derivatives of whole RK2 plasmid that express GFP–LacI from the arabinose promoter of E.coli. Plasmid pZZ15 contains the lacO array (Straight et al., 1996; Gordon et al., 1997; Belmont, 2001), while pZZ16 does not. pZZ15 was constructed by ligating the HindIII–NsiI fragment from plasmid pGAP60 to plasmid pZZ6 (Pogliano et al., 2001). Plasmid pZZ16 was constructed by creating a blunt NsiI end with T4 DNA polymerase on the HindIII–NsiI fragment of plasmid pGAP60, and then ligating this fragment to the 57.8 kb HpaI–HindIII fragment of RK2. RK2 plasmids were introduced into the P.aeruginosa strain PAO1 (Holloway et al., 1979) by conjugation with E.coli strains to create JP977 (PAO1/pZZ15) and JP978 (PAO1/pZZ16). RK2 plasmids were introduced into the V.cholerae strain 0395N1 (Mekalanos, 1983) by electroporation to create strains JP908 (0395N1/pZZ16) and JP909 (0395N1/pZZ15).
Fluorescence in situ hybridization
Cells were fixed directly in growth media with 0.5% paraformaldehyde and processed for FISH as described (Jensen and Shapiro, 1999a; Pogliano et al., 2001). Probes were created by labeling 20 µg of DNA with Cy3-dCTP (Molecular Probes) and Cy5-dCTP (Molecular Probes) using terminal deoxynucleotidyl transferase (Promega). λ DNA was used to prepare probes specific for λ-P1:5R plasmids. pOX38Kan and RK2 DNA were isolated by CsCl density gradient centrifugation and used to prepare probes specific for each plasmid. Labeling efficiency varied for each probe between experiments, from 90 to 95%. Cell membranes were visualized by staining with MTG (Molecular Probes) (Sharp and Pogliano, 1999; Pogliano et al., 2001). Chromosomal DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI; 0.5 µg/ml).
Microscopy
For GFP tagging, strains were grown in LB at 30°C overnight, and diluted 1:100 into fresh media. When cultures reached an OD600 = 0.1, GFP–LacI expression was induced with 0.2% l-arabinose and, after 1 h, 0.2% glucose was added to inhibit further expression of the fusion protein. Cells were stained with FM 4-64 (0.2 µg/ml). For P.aeruginosa, cells were concentrated by centrifugation and 3 µl of cells were placed on an agarose slab (1% agarose, 10% LB, 0.2% glucose). A coverslip was added after the cells soaked into the agarose pad. For V.cholerae, cells were concentrated 10-fold by centrifugation, and 3 µl of cells were placed on an untreated coverslip. Images from eight optical sections spaced 0.15 µm apart were captured using an Applied Precision Deconvolution microscope as described (Pogliano et al., 1999). Images were deconvolved using 10 iterations of DeltaVision v2.1 software. Deconvolution improved the visual clarity of the results but did not affect the number of foci observed or their extent of overlap. For scoring the number and extent of foci overlap, we scanned all eight optical sections, which allowed foci from different focal planes along the Z-axis to be scored and distinguished from one another. Two foci were scored as coincident if at least half of each focus was overlapping.
Acknowledgments
Acknowledgements
We are grateful to Donald Helinski, Aresa Toukdarian, Kit Pogliano, Marc Sharp and Igor Konieczny for helpful discussions, to Stuart Austin for providing λ-P1:5R, and to Virginia Waters for providing plasmid pOX38Kan. This work was supported by National Institutes of Health Research Grant AI-07194.
References
- Anthony K.G., Kathir,P., Moore,D., Ippen-Ihler,K. and Frost,L. (1996) Analysis of the traLEKBP sequence and the TraP protein from three F-like plasmids: F, R100-1 and ColB2. J. Bacteriol., 178, 3194–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S. and Nordstrom,K. (1990) Partition-mediated incompatibility of bacterial plasmids. Cell, 60, 351–354. [DOI] [PubMed] [Google Scholar]
- Austin S., Ziese,M. and Sternberg,N. (1981) A novel role for site-specific recombination in maintenance of bacterial replicons. Cell, 25, 729–736. [DOI] [PubMed] [Google Scholar]
- Belmont A.S. (2001) Visualizing chromosome dynamics with GFP. Trends Cell Biol., 11, 250–257. [DOI] [PubMed] [Google Scholar]
- Bignell C.R., Haines,A.S., Khare,D. and Thomas,C.M. (1999) Effect of growth rate and incC mutation on symmetric plasmid distribution by the IncP-1 partitioning apparatus. Mol. Microbiol., 34, 205–216. [DOI] [PubMed] [Google Scholar]
- Bingle L.E. and Thomas,C.M. (2001) Regulatory circuits for plasmid survival. Curr. Opin. Microbiol., 4, 194–200. [DOI] [PubMed] [Google Scholar]
- Bogan J.A., Grimwade,J.E., Thornton,M., Zhou,P., Denning,G.D. and Helmstetter,C.E. (2001) P1 and NR1 plasmid replication during the cell cycle of Escherichia coli. Plasmid, 45, 200–208. [DOI] [PubMed] [Google Scholar]
- Brendler T., Sawitzke,J., Sergueev,K. and Austin,S. (2000) A case for sliding SeqA tracts at anchored replication forks during Escherichia coli chromosome replication and segregation. EMBO J., 19, 6249–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P.R. (1999) The organization of replication and transcription. Science, 284, 1790–1795. [DOI] [PubMed] [Google Scholar]
- Cooper S. and Keasling,J.D. (1998) Cycle-specific replication of chromosomal and F-plasmid origins. FEMS Microbiol. Lett., 163, 217–222. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Maisnier-Patin,S. and Nordstrom,K. (2000) New genes with old modus operandi. The connection between supercoiling and partitioning of DNA in Escherichia coli. EMBO Rep., 1, 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman C.W. (1974) Bidirectional chromosome replication: some topological considerations. J. Theor. Biol., 43, 187–195. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield,S., Corbin,D. and Helinski,D.R. (1980) Broad host range DNA cloning system for Gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl Acad. Sci. USA, 77, 7347–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W.D. (2001) Co-ordinate regulation of the Escherichia coli cell cycle or the cloud of unknowing. Mol. Microbiol., 40, 779–785. [DOI] [PubMed] [Google Scholar]
- Economou A., Pogliano,J.A., Beckwith,J., Oliver,D.B. and Wickner,W. (1995) SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell, 83, 1171–1181. [DOI] [PubMed] [Google Scholar]
- Eliasson A., Bernander,R., Dasgupta,S. and Nordstrom,K. (1992) Direct visualization of plasmid DNA in bacterial cells. Mol. Microbiol., 6, 165–170. [DOI] [PubMed] [Google Scholar]
- Erdmann N., Petroff,T. and Funnell,B. (1999) Intracellular localization of P1 ParB protein depends on ParA and parS. Proc. Natl Acad. Sci. USA, 96, 14905–14910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G.S. and Wright,A. (2000) DNA segregation in bacteria. Annu. Rev. Microbiol., 54, 681–708. [DOI] [PubMed] [Google Scholar]
- Gordon G.S., Sitnikov,D., Webb,C.D., Teleman,A., Straight,A., Losick,R., Murray,A.W. and Wright,A. (1997) Chromosome and low copy plasmid segregation in E.coli: visual evidence for distinct mechanisms. Cell, 90, 1113–1121. [DOI] [PubMed] [Google Scholar]
- Heidelberg J.F. et al. (2000) DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature, 406, 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S. (2000) Dynamic localization of bacterial and plasmid chromosomes. Annu. Rev. Genet., 34, 21–59. [DOI] [PubMed] [Google Scholar]
- Holloway B.W., Krishnapillai,V. and Morgan,A.F. (1979) Chromosomal genetics of Pseudomonas. Microbiol. Rev., 43, 73–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes V.F. and Cozzarelli,N.R. (2000) Closing the ring: links between SMC proteins and chromosome partitioning, condensation and supercoiling. Proc. Natl Acad. Sci. USA, 97, 1322–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L.C., Richmond,M.H. and Sykes,R.B. (1973) Molecular characterization of the R factors implicated in the carbenicillin resistance of a sequence of Pseudomonas aeruginosa strains isolated from burns. Antimicrob. Agents Chemother., 3, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R.B. and Gerdes,K. (1999) Mechanism of DNA segregation in prokaryotes: ParM partitioning protein of plasmid R1 co-localizes with its replicon during the cell cycle. EMBO J., 18, 4076–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R.B. and Shapiro,L. (1999a) The Caulobacter crescentus smc gene is required for cell cycle progression and chromosome segregation. Proc. Natl Acad. Sci. USA, 96, 10661–10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R.B. and Shapiro,L. (1999b) Chromosome segregation during the prokaryotic cell cycle. Curr. Opin. Cell Biol., 11, 726–731. [DOI] [PubMed] [Google Scholar]
- Jensen R.B., Wang,S.C. and Shapiro,L. (2001) A moving replication factory in Caulobacter crescentus. EMBO J., 20, 4952–4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L.J., Woldringh,C.L. and Nanninga,N. (1999) Escherichia coli contains a DNA replication compartment in the cell center. Biochimie, 81, 803–810. [DOI] [PubMed] [Google Scholar]
- Lemon K.P. and Grossman,A.D. (1998) Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science, 282, 1516–1519. [DOI] [PubMed] [Google Scholar]
- Lemon K.P. and Grossman,A.D. (2000) Movement of replicating DNA through a stationary replisome. Mol. Cell, 6, 1321–1330. [DOI] [PubMed] [Google Scholar]
- Lemon K.P. and Grossman,A.D. (2001) The extrusion–capture model for chromosome partitioning in bacteria. Genes Dev., 15, 2031–2041. [DOI] [PubMed] [Google Scholar]
- Lewis P.J. and Errington,J. (1997) Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localization with the SpoOJ partitioning protein. Mol. Microbiol., 25, 945–954. [DOI] [PubMed] [Google Scholar]
- Losick R. and Shapiro,L. (1998) Bringing the mountain to Mohammed. Science, 282, 1430–1431. [DOI] [PubMed] [Google Scholar]
- Mekalanos J.J. (1983) Duplication and amplification of toxin genes in Vibrio cholerae. Cell, 35, 253–263. [DOI] [PubMed] [Google Scholar]
- Miller C. and Cohen,S.N. (1999) Separate roles of Escherichia coli replication proteins in synthesis and partitioning of pSC101 plasmid DNA. J. Bacteriol., 181, 7552–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H. and Hiraga,S. (1997) Subcellular distribution of actively partitioning F plasmid during the cell division cycle of E.coli. Cell, 90, 951–957. [DOI] [PubMed] [Google Scholar]
- Niki H. and Hiraga,S. (1998) Polar localization of the replication origin and terminus in Escherichia coli nucleoids during chromosome partitioning. Genes Dev., 12, 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom K. and Austin,S.J. (1989) Mechanisms that contribute to the stable segregation of plasmids. Annu. Rev. Genet., 23, 37–69. [DOI] [PubMed] [Google Scholar]
- Novick R.P. (1987) Plasmid incompatibility. Microbiol. Rev., 51, 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Conner M.B. and Malamy,M.H. (1984) Role of the F factor oriV1 region in recA-independent illegitimate recombination. J. Mol. Biol., 175, 263–284. [DOI] [PubMed] [Google Scholar]
- Onogi T., Niki,H., Yamazoe,M. and Hiraga,S. (1999) The assembly and migration of SeqA–GFP fusion in living cells of Escherichia coli. Mol. Microbiol., 31, 1775–1782. [DOI] [PubMed] [Google Scholar]
- Pansegrau W. et al. (1994) Complete nucleotide sequence of Birmingham IncPα plasmids. Compilation and comparative analysis. J. Mol. Biol., 239, 623–663. [DOI] [PubMed] [Google Scholar]
- Pogliano J., Pogliano,K., Weiss,D., Losick,R. and Beckwith,J. (1997) Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites. Proc. Natl Acad. Sci. USA, 94, 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J., Osborne,N., Sharp,M.D., Abanes-DeMello,A., Perez,A., Sun,Y.-L. and Pogliano,K. (1999) A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol., 31, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J., Ho,T.Q., Zhong,Z. and Helinski,D.R. (2001) Multicopy plasmids are clustered and localized in Escherichia coli. Proc. Natl Acad. Sci. USA, 98, 4486–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich Z., Wachtel,E.J. and Minsky,A. (1994) Liquid-crystalline mesophases of plasmid DNA in bacteria. Science, 264, 1460–1463. [DOI] [PubMed] [Google Scholar]
- Roos M., van Geel,A.B., Aarsman,M.E., Veuskens,J.T., Woldringh,C.L. and Nanninga,N. (2001) The replicated ftsQAZ and minB chromosomal regions of Escherichia coli segregate on average in line with nucleoid movement. Mol. Microbiol., 39, 633–640. [DOI] [PubMed] [Google Scholar]
- Sawitzke J. and Austin,S. (2000) Suppression of chromosome segregation defects of Escherichia coli muk mutants by mutations in topoisomerase I. Proc. Natl Acad. Sci. USA, 97, 1671–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzke J. and Austin,S. (2001) An analysis of the factory model for chromosome replication and segregation in bacteria. Mol. Microbiol., 40, 786–794. [DOI] [PubMed] [Google Scholar]
- Shapiro L. and Losick,R. (2000) Dynamic spatial regulation in the bacterial cell. Cell, 100, 89–98. [DOI] [PubMed] [Google Scholar]
- Sharp M.D. and Pogliano,K. (1999) An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc. Natl Acad. Sci. USA, 96, 14553–14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M.E. and Errington,J. (1998) A fixed distance for separation of newly replicated copies of oriC in Bacillus subtilis: implications for co-ordination of chromosome segregation and cell division. Mol. Microbiol., 28, 981–990. [DOI] [PubMed] [Google Scholar]
- Sharpe M.E. and Errington,J. (1999) Upheaval in the bacterial nucleoid. An active chromosome segregation mechanism. Trends Genet., 15, 70–74. [DOI] [PubMed] [Google Scholar]
- Sternberg N. and Austin,S. (1983) Isolation and characterization of P1 minireplicons, λ-P1:5R and λ-P1:5L. J. Bacteriol., 153, 800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A.F., Belmont,A.S., Robinett,C.C. and Murray,A.W. (1996) GFP tagging of budding yeast chromosomes reveals that protein–protein interactions can mediate sister chromatid cohesion. Curr. Biol., 6, 1599–1608. [DOI] [PubMed] [Google Scholar]
- Thomas C.M. and Helinski,D.R. (eds) (1989) Vegetative Replication and Stable Inheritance of IncP Plasmids. Academic Press, San Diego, CA.
- Trucksis A., Michalski,J., Deng,Y.K. and Kaper,J.B. (1998) The Vibrio cholerae genome contains two unique circular chromosomes. Proc. Natl Acad. Sci. USA, 95, 14464–14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C.D., Teleman,A., Gordon,S., Straight,A., Belmont,A., Lin,D.C.-H., Grossman,A.D., Wright,A. and Losick,R. (1997) Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B.subtilis. Cell, 88, 667–674. [DOI] [PubMed] [Google Scholar]
- Webb C.D., Graumann,P.L., Kahana,J.A., Teleman,A.A., Silver,P.A. and Losick,R. (1998) Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol. Microbiol., 28, 883–892. [DOI] [PubMed] [Google Scholar]
- Weitao T., Dasgupta,S. and Nordstrom,K. (2000) Plasmid R1 is present as clusters in the cells of Escherichia coli. Plasmid, 43, 200–204. [DOI] [PubMed] [Google Scholar]