Abstract
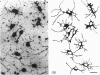
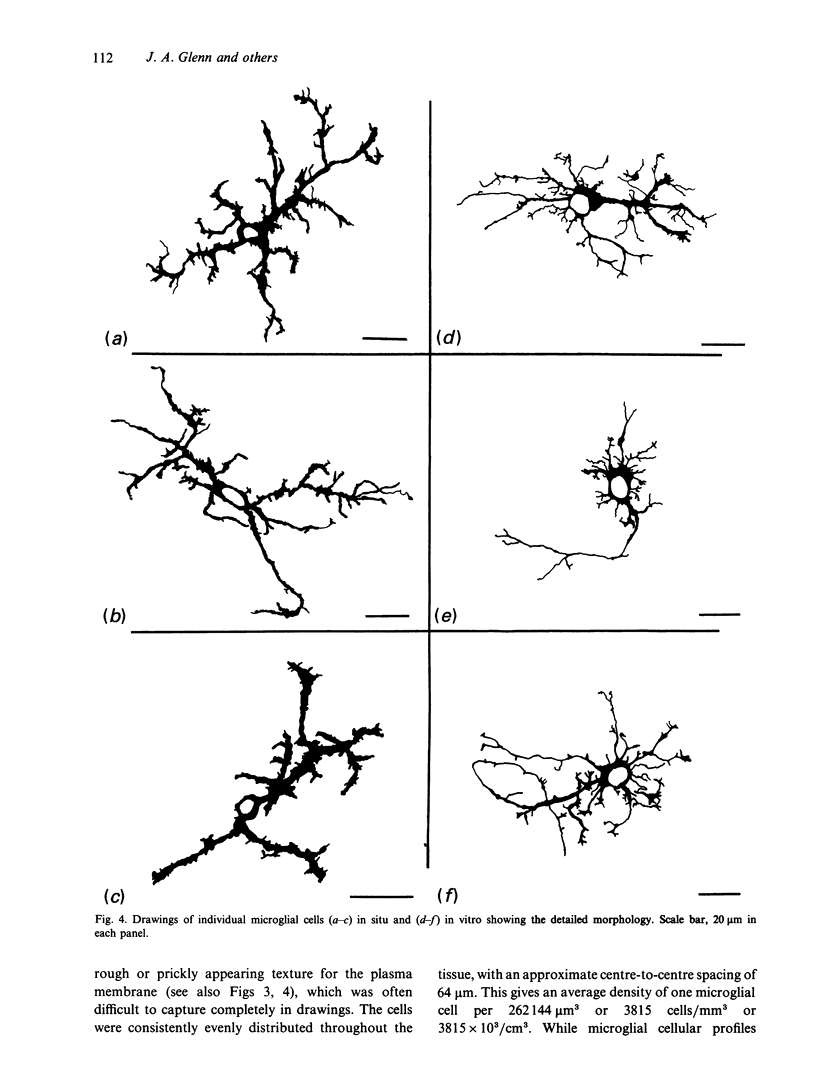
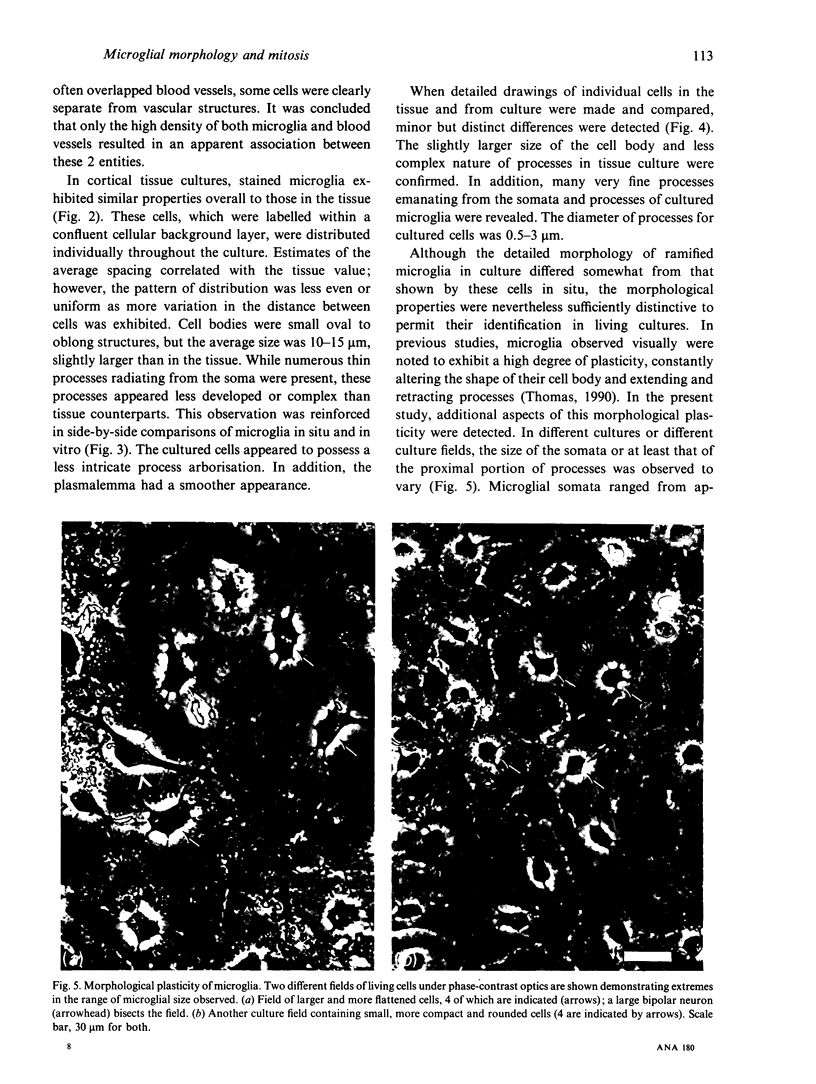
Several cellular properties of brain microglia in the rat were investigated using both whole tissue and cultures of dissociated cerebral cortical cells. As revealed by thiamine pyrophosphatase histochemistry, tissue microglia possessed a highly distinctive cellular morphology. Stained microglia showed similar overall features of morphology and distribution in both preparations; however, the cells in culture displayed some slight differences from those of the tissue, including larger somata and less developed processes. Through studying living ramified cells in culture, both morphological plasticity as evidenced by patterned variations in soma size and mitotic activity were directly confirmed. It was concluded that ramified microglia definitely possess proliferative capability, and this may reduce the need for blood cell recruitment in brain immune responses. In addition, cultured microglia exist in a somewhat more activated state than those in normal tissue, and in some instances undergo further activation as macrophages. This cortical tissue culture system should provide an amenable preparation for investigating the regulation of microglial function.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama H., Itagaki S., McGeer P. L. Major histocompatibility complex antigen expression on rat microglia following epidural kainic acid lesions. J Neurosci Res. 1988;20(2):147–157. doi: 10.1002/jnr.490200202. [DOI] [PubMed] [Google Scholar]
- Akiyama H., McGeer P. L. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990 Nov;30(1):81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter P. J., Bauer J., Schobert A., Northoff H. Lipopolysaccharide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. J Neurosci. 1989 Jan;9(1):183–194. doi: 10.1523/JNEUROSCI.09-01-00183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. A., Jordan F. L., Thomas W. E. Further studies on the identification of microglia in mixed brain cell cultures. Brain Res Bull. 1989 Jun;22(6):1049–1052. doi: 10.1016/0361-9230(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Banati R. B., Streit W. J., Kreutzberg G. W. Immunophenotypic characterization of rat brain macrophages in culture. Neurosci Lett. 1989 Sep 11;103(3):241–246. doi: 10.1016/0304-3940(89)90106-7. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kreutzberg G. W. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res. 1988 Sep;21(1):18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Tetzlaff W., Streit W. J., Kreutzberg G. W. Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neurosci Lett. 1988 Mar 10;85(3):317–321. doi: 10.1016/0304-3940(88)90585-x. [DOI] [PubMed] [Google Scholar]
- Jordan F. L., Rieke G. K., Thomas W. E. Presence and development of ependymal cells in primary tissue cultures derived from embryonic rat cerebral cortex. Brain Res. 1987 Sep;432(1):97–110. doi: 10.1016/0165-3806(87)90012-5. [DOI] [PubMed] [Google Scholar]
- Jordan F. L., Thomas W. E. Brain macrophages: questions of origin and interrelationship. Brain Res. 1988 Apr-Jun;472(2):165–178. doi: 10.1016/0165-0173(88)90019-7. [DOI] [PubMed] [Google Scholar]
- Jordan F. L., Thomas W. E. Identification of somatostatin-containing neurons in primary cultures of rat cerebral cortex. Neurosci Lett. 1987 Jun 26;77(3):249–254. doi: 10.1016/0304-3940(87)90507-6. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Morphological studies on neuroglia. V. Microglial cells in the cerebral cortex of the rat, with special reference to their possible involvement in synaptic function. Cell Tissue Res. 1982;223(3):493–506. doi: 10.1007/BF00218471. [DOI] [PubMed] [Google Scholar]
- Murabe Y., Sano Y. Thiaminepyrophosphatase activity in the plasma membrane of microglia. Histochemistry. 1981;71(1):45–52. doi: 10.1007/BF00592569. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and microglia in the nervous system. Trends Neurosci. 1988 Jun;11(6):273–277. doi: 10.1016/0166-2236(88)90110-5. [DOI] [PubMed] [Google Scholar]
- Ranson P. A., Thomas W. E. Pinocytosis as a select marker of ramified microglia in vivo and in vitro. J Histochem Cytochem. 1991 Jun;39(6):853–858. doi: 10.1177/39.6.2033242. [DOI] [PubMed] [Google Scholar]
- Rieske E., Graeber M. B., Tetzlaff W., Czlonkowska A., Streit W. J., Kreutzberg G. W. Microglia and microglia-derived brain macrophages in culture: generation from axotomized rat facial nuclei, identification and characterization in vitro. Brain Res. 1989 Jul 17;492(1-2):1–14. doi: 10.1016/0006-8993(89)90883-4. [DOI] [PubMed] [Google Scholar]
- Sawada M., Suzumura A., Yamamoto H., Marunouchi T. Activation and proliferation of the isolated microglia by colony stimulating factor-1 and possible involvement of protein kinase C. Brain Res. 1990 Feb 12;509(1):119–124. doi: 10.1016/0006-8993(90)90317-5. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Response of endogenous glial cells to motor neuron degeneration induced by toxic ricin. J Comp Neurol. 1988 Feb 8;268(2):248–263. doi: 10.1002/cne.902680209. [DOI] [PubMed] [Google Scholar]
- Thomas W. E. Characterization of the dynamic nature of microglial cells. Brain Res Bull. 1990 Aug;25(2):351–354. doi: 10.1016/0361-9230(90)90083-c. [DOI] [PubMed] [Google Scholar]