Abstract
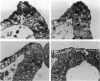
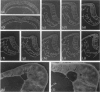
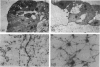
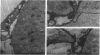
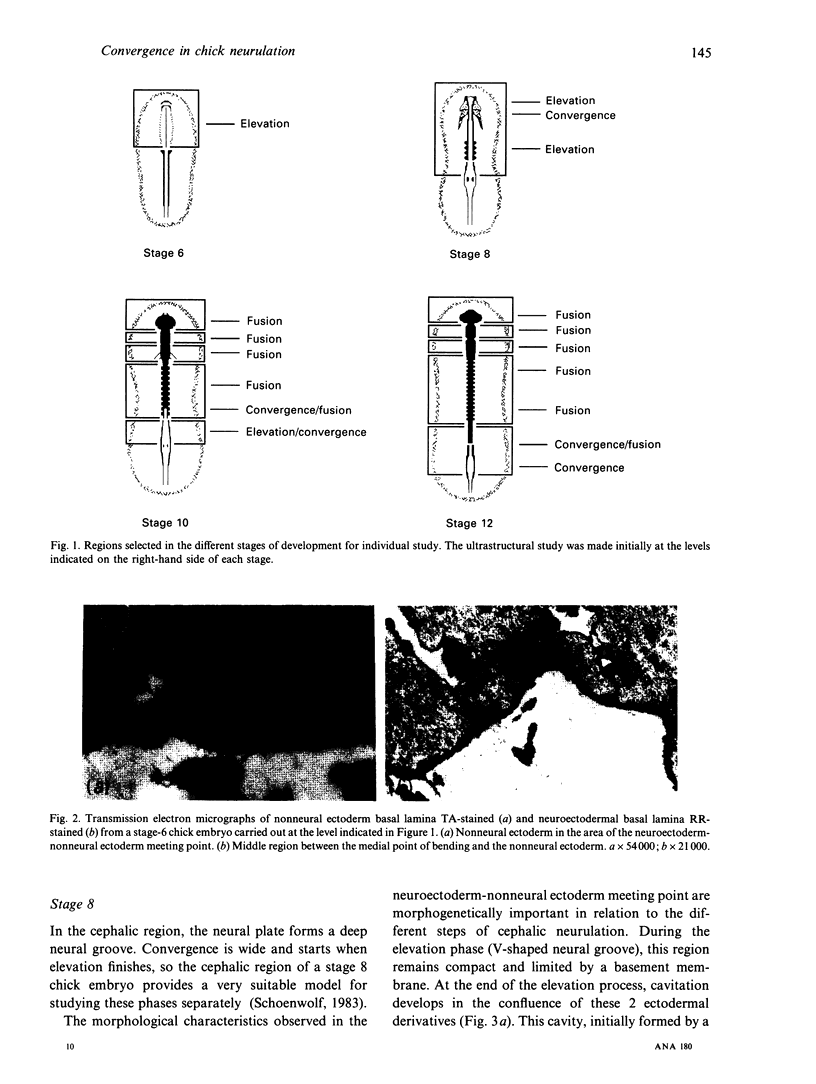
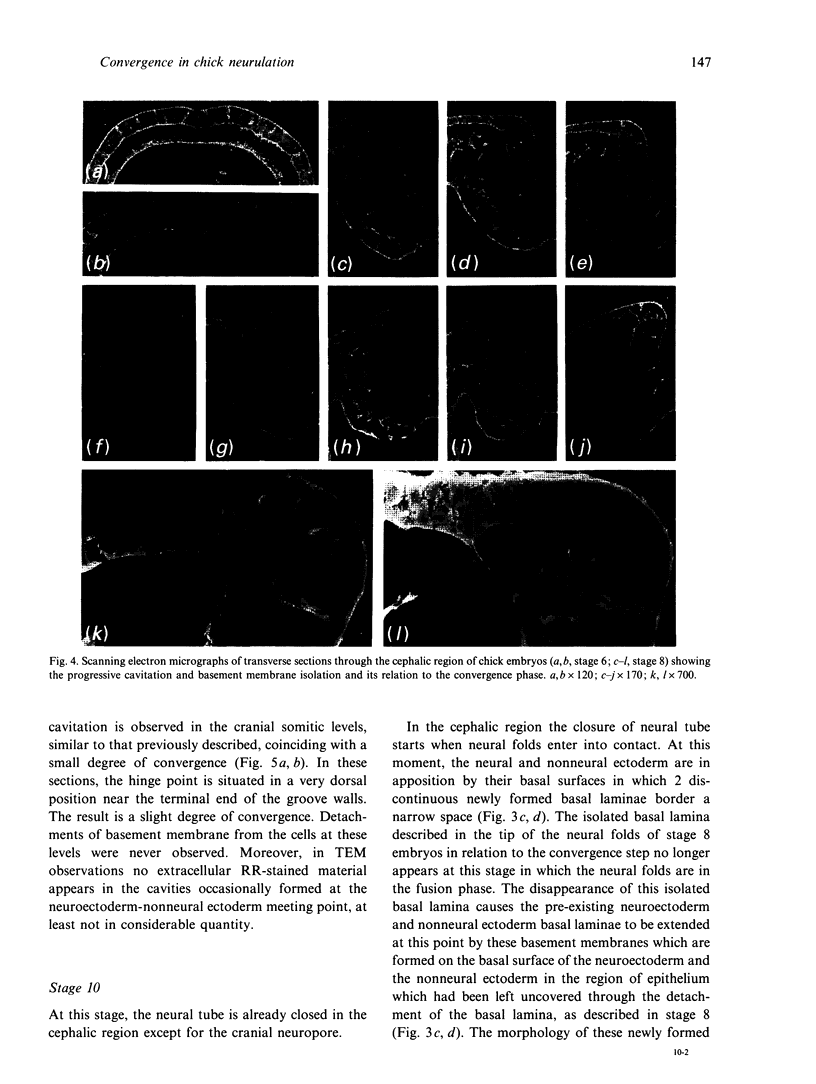
We have analysed the characteristics of the neuroectoderm-nonneural ectoderm meeting point at several axial levels in relation to the mechanics of neurulation in each level. The results show wide differences at cephalic and somitic levels. At cephalic levels, where convergence plays an important role, the delamination process appears at the beginning of the convergence step. This phenomenon produces a major isolation of the basal lamina, forming a space between this structure and the epithelial sheet in whose basal surface a new basal lamina begins to form. This cavity contains abundant extracellular matrix stained with ruthenium red (RR) and tannic acid (TA), and its increase in volume correlates with the progressive convergence of neural folds. At somitic levels, where the convergence is not important, delamination involves the progressive formation of a half-moon-shaped cavity. This structure appears between a dorsal attachment point, in the tip of neuroectodermal wall, and a ventral attachment point which coincides with the point of bending that determines the bilateral furrow, if it exists. In this small cavity, delamination is not related to an isolation of basal lamina. The RR-staining of the extracellular matrix in this cavity is scarce and the volume increase is smaller than in the cephalic region. These results are discussed in terms of neural fold convergence and neural tube closure.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong P. B., Parenti D. Scanning electron microscopy of the chick embryo. Dev Biol. 1973 Aug;33(2):457–462. doi: 10.1016/0012-1606(73)90150-4. [DOI] [PubMed] [Google Scholar]
- Brauer P. R., Markwald R. R. Specific configurations of fibronectin-containing particles correlate with pathways taken by neural crest cells at two axial levels. Anat Rec. 1988 Sep;222(1):69–82. doi: 10.1002/ar.1092220111. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M., Lallier T. A monoclonal antibody against a laminin-heparan sulfate proteoglycan complex perturbs cranial neural crest migration in vivo. J Cell Biol. 1988 Apr;106(4):1321–1329. doi: 10.1083/jcb.106.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp A. J., Bernfield M. Accumulation of basement membrane-associated hyaluronate is reduced in the posterior neuropore region of mutant (curly tail) mouse embryos developing spinal neural tube defects. Dev Biol. 1988 Dec;130(2):583–590. doi: 10.1016/0012-1606(88)90353-3. [DOI] [PubMed] [Google Scholar]
- Copp A. J., Bernfield M. Glycosaminoglycans vary in accumulation along the neuraxis during spinal neurulation in the mouse embryo. Dev Biol. 1988 Dec;130(2):573–582. doi: 10.1016/0012-1606(88)90352-1. [DOI] [PubMed] [Google Scholar]
- Csato W., Merker H. J. Production and formation of the basement membrane in embryonic tissues of the mouse. An electron-microscopic study. Cell Tissue Res. 1983;228(1):85–98. doi: 10.1007/BF00206267. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Thiery J. P. Distribution of laminin and collagens during avian neural crest development. Development. 1987 Nov;101(3):461–478. doi: 10.1242/dev.101.3.461. [DOI] [PubMed] [Google Scholar]
- Fernandez J. G., de Paz P., Chamorro C. A. Effects of colchicine on the shape of chick neuroepithelial cells during neurulation. Anat Rec. 1987 Nov;219(3):296–303. doi: 10.1002/ar.1092190310. [DOI] [PubMed] [Google Scholar]
- Gordon R. A review of the theories of vertebrate neurulation and their relationship to the mechanics of neural tube birth defects. J Embryol Exp Morphol. 1985 Nov;89 (Suppl):229–255. [PubMed] [Google Scholar]
- Karfunkel P. The mechanisms of neural tube formation. Int Rev Cytol. 1974;38(0):245–271. doi: 10.1016/s0074-7696(08)60927-4. [DOI] [PubMed] [Google Scholar]
- Lee H. Y., Nagele R. G. Studies on the mechanisms of neurulation in the chick: interrelationship of contractile proteins, microfilaments, and the shape of neuroepithelial cells. J Exp Zool. 1985 Aug;235(2):205–215. doi: 10.1002/jez.1402350207. [DOI] [PubMed] [Google Scholar]
- Lee H., Nagele R. G. Intrinsic forces alone are sufficient to cause closure of the neural tube in the chick. Experientia. 1988 Jan 15;44(1):60–61. doi: 10.1007/BF01960246. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Martins-Green M., Erickson C. A. Basal lamina is not a barrier to neural crest cell emigration: documentation by TEM and by immunofluorescent and immunogold labelling. Development. 1987 Nov;101(3):517–533. doi: 10.1242/dev.101.3.517. [DOI] [PubMed] [Google Scholar]
- Martins-Green M., Erickson C. A. Development of neural tube basal lamina during neurulation and neural crest cell emigration in the trunk of the mouse embryo. J Embryol Exp Morphol. 1986 Nov;98:219–236. [PubMed] [Google Scholar]
- Martins-Green M. Origin of the dorsal surface of the neural tube by progressive delamination of epidermal ectoderm and neuroepithelium: implications for neurulation and neural tube defects. Development. 1988 Aug;103(4):687–706. doi: 10.1242/dev.103.4.687. [DOI] [PubMed] [Google Scholar]
- Mayer B. W., Jr, Hay E. D., Hynes R. O. Immunocytochemical localization of fibronectin in embryonic chick trunk and area vasculosa. Dev Biol. 1981 Mar;82(2):267–286. doi: 10.1016/0012-1606(81)90451-6. [DOI] [PubMed] [Google Scholar]
- Morriss G. M., Solursh M. Regional differences in mesenchymal cell morphology and glycosaminoglycans in early neural-fold stage rat embryos. J Embryol Exp Morphol. 1978 Aug;46:37–52. [PubMed] [Google Scholar]
- Moury J. D., Jacobson A. G. Neural fold formation at newly created boundaries between neural plate and epidermis in the axolotl. Dev Biol. 1989 May;133(1):44–57. doi: 10.1016/0012-1606(89)90295-9. [DOI] [PubMed] [Google Scholar]
- Nagele R. G., Doane K. J., Lee H., Wilson F. J., Roisen F. J. A method for exposing the internal anatomy of small and delicate tissues for correlated SEM/TEM studies using polyethylene glycol embedding. J Microsc. 1984 Feb;133(Pt 2):177–183. doi: 10.1111/j.1365-2818.1984.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Nagele R. G., Hunter E., Bush K., Lee H. Y. Studies on the mechanisms of neurulation in the chick: morphometric analysis of force distribution within the neuroepithelium during neural tube formation. J Exp Zool. 1987 Dec;244(3):425–436. doi: 10.1002/jez.1402440309. [DOI] [PubMed] [Google Scholar]
- Nagele R. G., Lee H. Y. Studies on the mechanisms of neurulation in the chick: morphometric analysis of the relationship between regional variations in cell shape and sites of motive force generation. J Exp Zool. 1987 Feb;241(2):197–205. doi: 10.1002/jez.1402410206. [DOI] [PubMed] [Google Scholar]
- Nagele R. G., Lee H. Y. Ultrastructural changes in cells associated with interkinetic nuclear migration in the developing chick neuroepithelium. J Exp Zool. 1979 Oct;210(1):89–106. doi: 10.1002/jez.1402100110. [DOI] [PubMed] [Google Scholar]
- Newgreen D. F. Physical influences on neural crest cell migration in avian embryos: contact guidance and spatial restriction. Dev Biol. 1989 Jan;131(1):136–148. doi: 10.1016/s0012-1606(89)80045-4. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C., Fisher M. Analysis of the effects of Streptomyces hyaluronidase on formation of the neural tube. J Embryol Exp Morphol. 1983 Feb;73:1–15. [PubMed] [Google Scholar]
- Schoenwolf G. C., Folsom D., Moe A. A reexamination of the role of microfilaments in neurulation in the chick embryo. Anat Rec. 1988 Jan;220(1):87–102. doi: 10.1002/ar.1092200111. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C., Franks M. V. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev Biol. 1984 Oct;105(2):257–272. doi: 10.1016/0012-1606(84)90284-7. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C. On the morphogenesis of the early rudiments of the developing central nervous system. Scan Electron Microsc. 1982;(Pt 1):289–308. [PubMed] [Google Scholar]
- Schoenwolf G. C. Shaping and bending of the avian neuroepithelium: morphometric analyses. Dev Biol. 1985 May;109(1):127–139. doi: 10.1016/0012-1606(85)90353-7. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C., Smith J. L. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990 Jun;109(2):243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C. The chick epiblast: a model for examining epithelial morphogenesis. Scan Electron Microsc. 1983;(Pt 3):1371–1385. [PubMed] [Google Scholar]
- Smith J. L., Schoenwolf G. C. Notochordal induction of cell wedging in the chick neural plate and its role in neural tube formation. J Exp Zool. 1989 Apr;250(1):49–62. doi: 10.1002/jez.1402500107. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Schoenwolf G. C. Role of cell-cycle in regulating neuroepithelial cell shape during bending of the chick neural plate. Cell Tissue Res. 1988 Jun;252(3):491–500. doi: 10.1007/BF00216636. [DOI] [PubMed] [Google Scholar]
- Tosney K. W. The segregation and early migration of cranial neural crest cells in the avian embryo. Dev Biol. 1982 Jan;89(1):13–24. doi: 10.1016/0012-1606(82)90289-5. [DOI] [PubMed] [Google Scholar]
- Tuckett F., Morriss-Kay G. M. Heparitinase treatment of rat embryos during cranial neurulation. Anat Embryol (Berl) 1989;180(4):393–400. doi: 10.1007/BF00311170. [DOI] [PubMed] [Google Scholar]