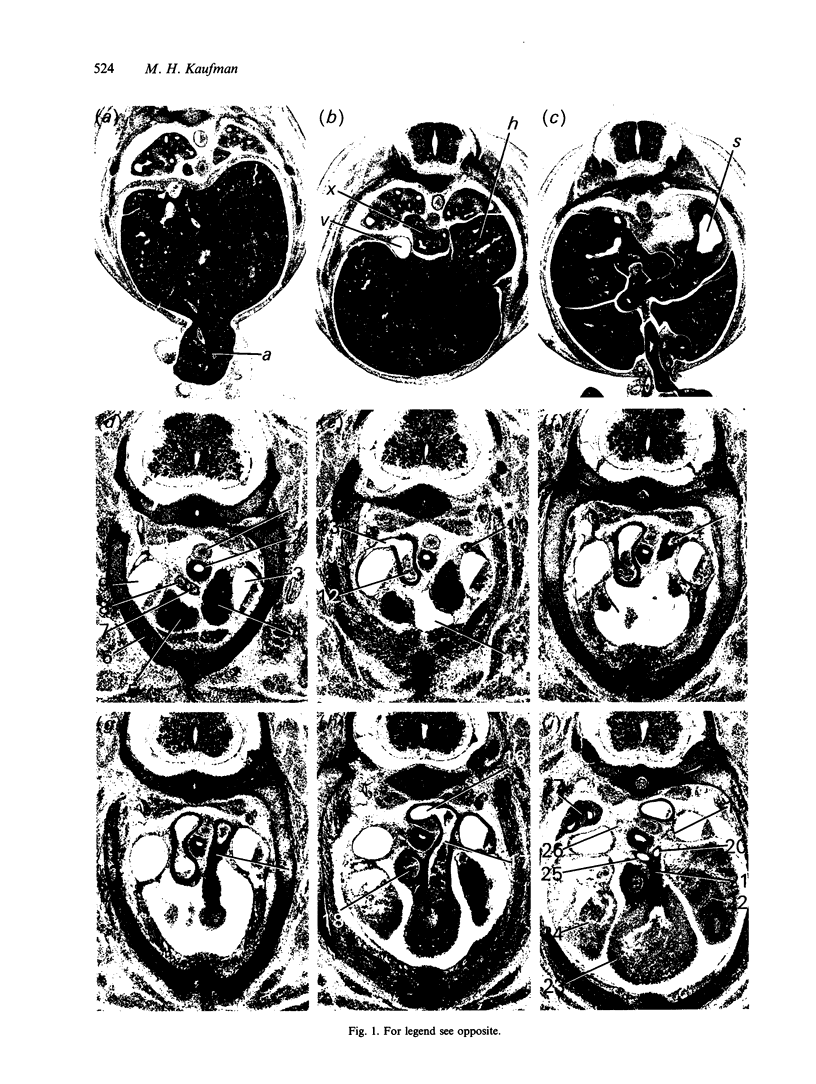
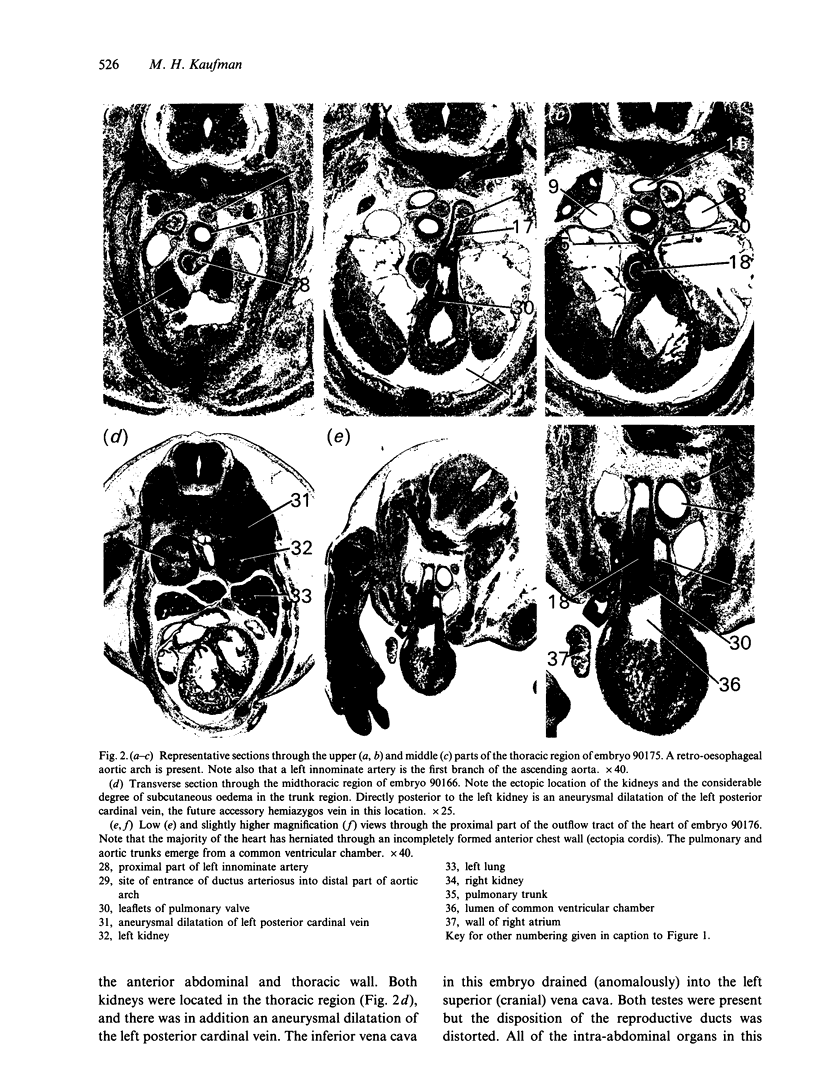
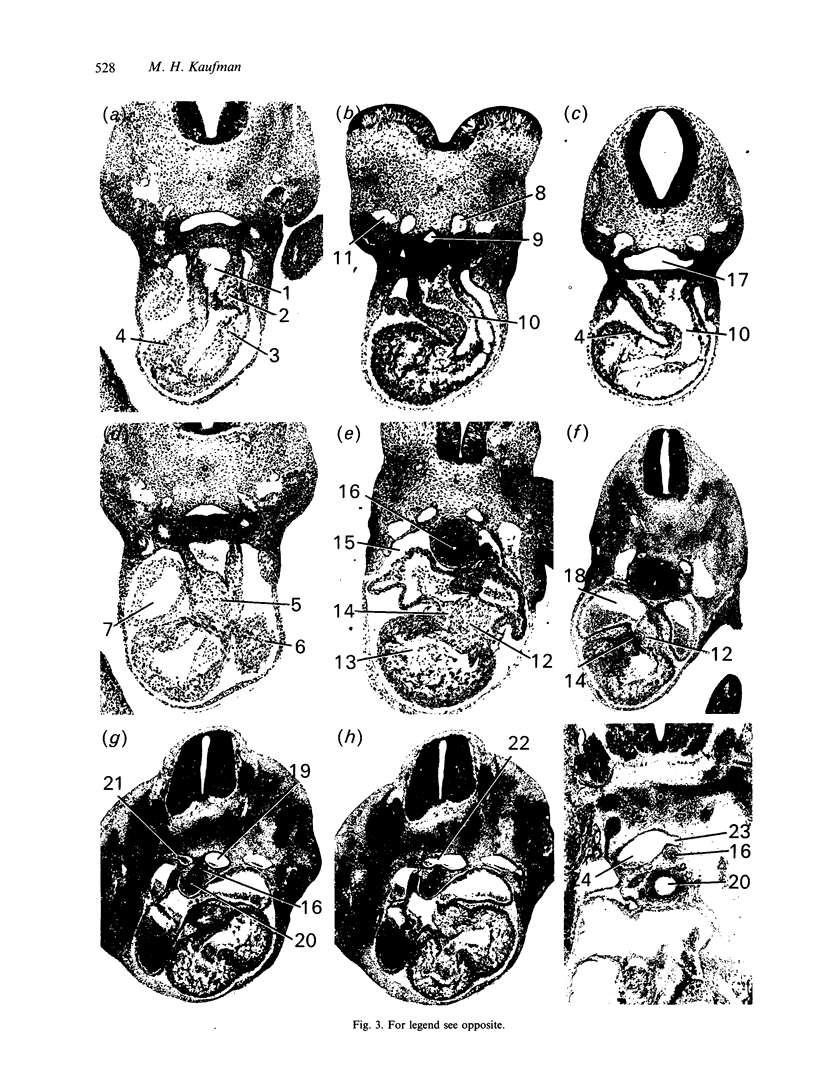
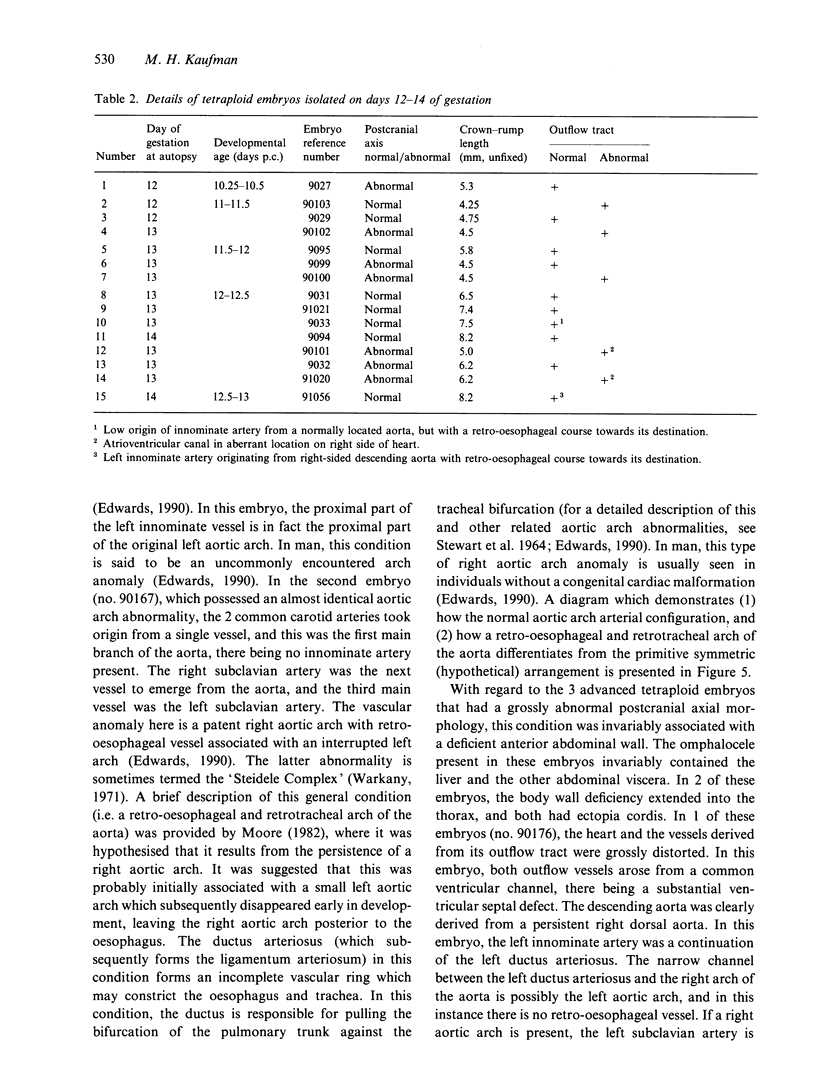
Abstract
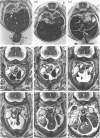
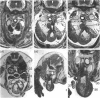
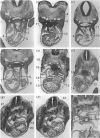
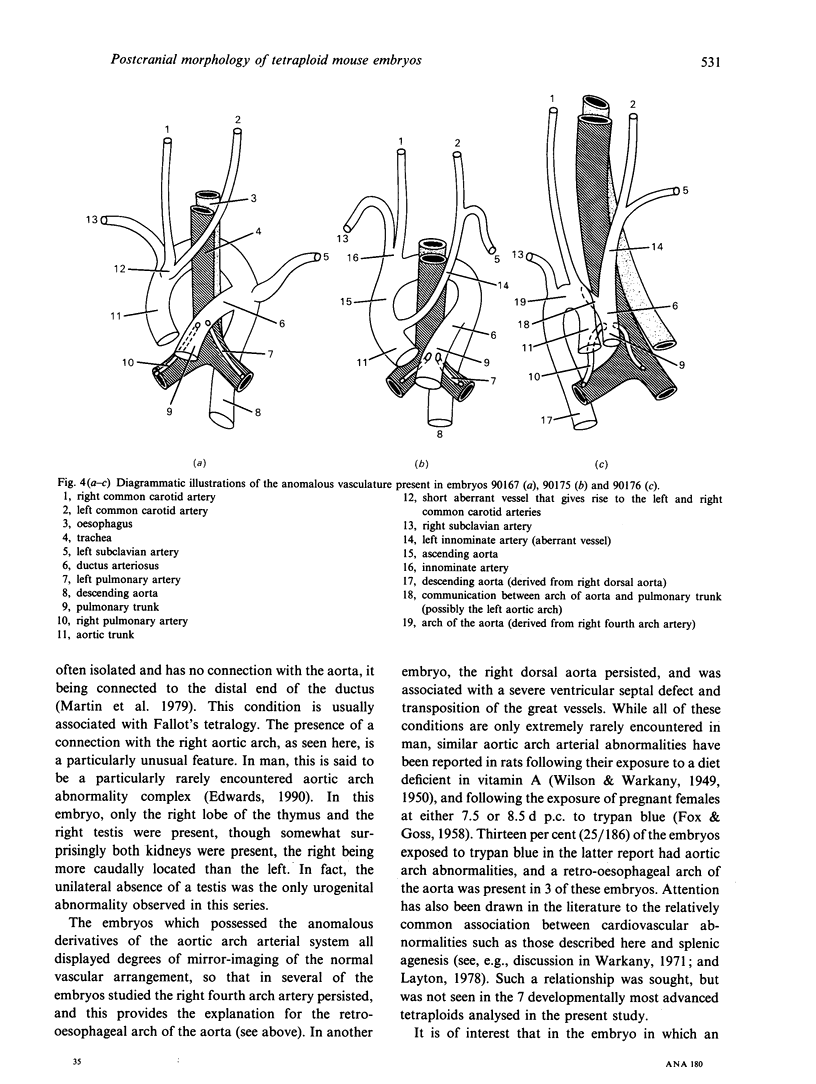
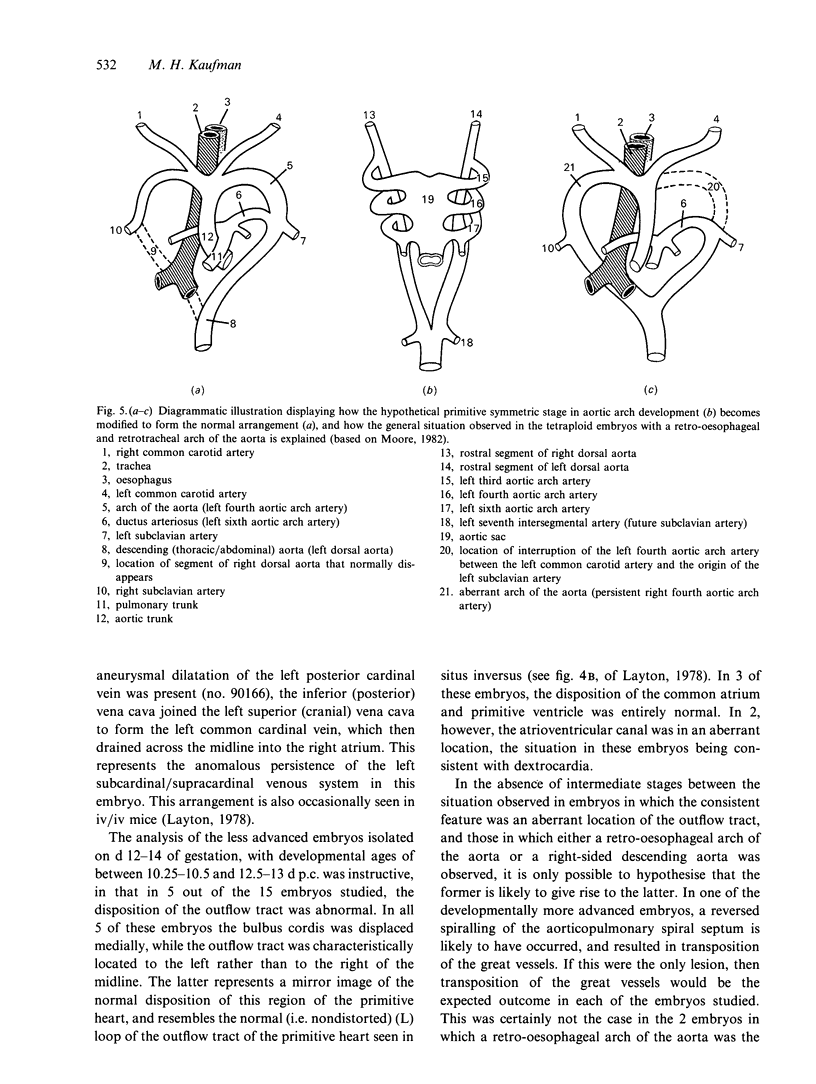
The postcranial morphological features of 22 homozygous tetraploid mouse embryos were studied. The latter were produced by electrofusion of blastomeres at the 2-cell stage in vitro, and were transferred to appropriate recipients that were autopsied on d 12-16 of gestation. Seven embryos were isolated on d 15 or d 16 of gestation and were developmentally equivalent to control diploid embryos of 13-14.5 d p.c. In each of these embryos, their sex could be determined from the histological analysis of their gonads. A further 15 embryos were isolated on d 12-14 of gestation and most were developmentally equivalent to controls of between 11-11.5 and 12-12.5 d p.c. The sex of these embryos could not be determined from the analysis of their gonads, as these were all at the 'indifferent' stage. Twelve of the 22 embryos had a normal postcranial axial morphology, while 10 had an abnormal postcranial axial morphology associated with an enormous omphalocele which contained most of the abdominal viscera and often, particularly in the more advanced specimens, the heart as well. In each of the 7 developmentally most advanced embryos studied, a major congenital abnormality involving at least one organ system was present, but none of these was believed to be life-threatening. The most interesting finding was that 3 of these embryos had abnormalities of the aortic arch arterial system; in 2 embryos the arch of the aorta was retro-oesophageal and retrotracheal, while a 3rd embryo had transposition of the great vessels associated with a right-sided descending aorta. In a 4th embryo, an aneurysmal dilatation of the posterior cardinal vein was present, and this was associated with the anomalous persistence of the left subcardinal/supracardinal venous system. These abnormalities represent a degree of mirror imaging of the normal vascular arrangement. Analysis of the gonads revealed that all of the embryos with vascular abnormalities in this group were male (XXYY), and no vascular abnormalities were present in the females (XXXX). The histological features of the viscera were normal. In 5 of the 15 developmentally less advanced embryos, the distal part of the bulbus cordis region of the heart was displaced medially, and consequently the outflow tract was located on the left part of the pericardial cavity rather than on the right where it is normally found. This may represent an early stage in the differentiation of the aortic arch arterial vascular anomalies indicated above. In 2 additional embryos, abnormally located (i.e. retro-oesophageal) innominate vessels were present.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N. A., McCarthy A., Wolpert L. The development of handed asymmetry in aggregation chimeras of situs inversus mutant and wild-type mouse embryo. Development. 1990 Nov;110(3):949–954. doi: 10.1242/dev.110.3.949. [DOI] [PubMed] [Google Scholar]
- Brown N. A., Wolpert L. The development of handedness in left/right asymmetry. Development. 1990 May;109(1):1–9. doi: 10.1242/dev.109.1.1. [DOI] [PubMed] [Google Scholar]
- Evans E. P., Burtenshaw M. D., Ford C. E. Chromosomes of mouse embryos and newborn young: preparations from membranes and tail tips. Stain Technol. 1972 Sep;47(5):229–234. doi: 10.3109/10520297209116541. [DOI] [PubMed] [Google Scholar]
- FOX M. H., GOSS C. M. Experimentally produced malformations of the heart and great vessels in rat fetuses; transposition complexes and aortic arch abnormalities. Am J Anat. 1958 Jan;102(1):65–91. doi: 10.1002/aja.1001020104. [DOI] [PubMed] [Google Scholar]
- Fananapazir K., Kaufman M. H. Observations on the development of the aortico-pulmonary spiral septum in the mouse. J Anat. 1988 Jun;158:157–172. [PMC free article] [PubMed] [Google Scholar]
- Jones K. W., Singh L., Edwards R. G. The use of probes for the Y chromosome in preimplantation embryo cells. Hum Reprod. 1987 Jul;2(5):439–445. doi: 10.1093/oxfordjournals.humrep.a136565. [DOI] [PubMed] [Google Scholar]
- Kaufman M. H. Histochemical identification of primordial germ cells and differentiation of the gonads in homozygous tetraploid mouse embryos. J Anat. 1991 Dec;179:169–181. [PMC free article] [PubMed] [Google Scholar]
- Kaufman M. H., Lee K. K., Speirs S. Influence of diandric and digynic triploid genotypes on early mouse embryogenesis. Development. 1989 Jan;105(1):137–145. doi: 10.1242/dev.105.1.137. [DOI] [PubMed] [Google Scholar]
- Kaufman M. H., Speirs S., Lee K. K. The sex-chromosome constitution and early postimplantation development of diandric triploid mouse embryos. Cytogenet Cell Genet. 1989;50(2-3):98–101. doi: 10.1159/000132732. [DOI] [PubMed] [Google Scholar]
- Kaufman M. H., Webb S. Postimplantation development of tetraploid mouse embryos produced by electrofusion. Development. 1990 Dec;110(4):1121–1132. doi: 10.1242/dev.110.4.1121. [DOI] [PubMed] [Google Scholar]
- Layton W. M., Jr Heart malformations in mice homozygous for a gene causing situs inversus. Birth Defects Orig Artic Ser. 1978;14(7):277–293. [PubMed] [Google Scholar]
- López Pajares I., Delicado A., Diaz de Bustamante A., Pellicer A., Pinel I., Pardo M., Martin M. Tetraploidy in a liveborn infant. J Med Genet. 1990 Dec;27(12):782–783. doi: 10.1136/jmg.27.12.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E. C., Mesko Z. G., Griepp R. B., Haller J. O., Gordon D. H. Isolation of the left innominate artery, a right arch, and a left patent ductus arteriosus. AJR Am J Roentgenol. 1979 May;132(5):833–835. doi: 10.2214/ajr.132.5.833. [DOI] [PubMed] [Google Scholar]
- O'Neill G. T., Speirs S., Kaufman M. H. Sex-chromosome constitution of postimplantation tetraploid mouse embryos. Cytogenet Cell Genet. 1990;53(4):191–195. doi: 10.1159/000132928. [DOI] [PubMed] [Google Scholar]
- Sheppard D. M., Fisher R. A., Lawler S. D., Povey S. Tetraploid conceptus with three paternal contributions. Hum Genet. 1982;62(4):371–374. doi: 10.1007/BF00304561. [DOI] [PubMed] [Google Scholar]
- Speirs S., Kaufman M. H. Analysis of the sex-chromosome constitution of digynic triploid mouse embryos. Cytogenet Cell Genet. 1989;52(3-4):151–153. doi: 10.1159/000132866. [DOI] [PubMed] [Google Scholar]
- Stalsberg H. Development and ultrastructure of the embryonic heart. II. Mechanism of dextral looping of the embryonic heart. Am J Cardiol. 1970 Mar;25(3):265–271. doi: 10.1016/s0002-9149(70)80002-9. [DOI] [PubMed] [Google Scholar]
- Surti U., Szulman A. E., Wagner K., Leppert M., O'Brien S. J. Tetraploid partial hydatidiform moles: two cases with a triple paternal contribution and a 92,XXXY karyotype. Hum Genet. 1986 Jan;72(1):15–21. doi: 10.1007/BF00278810. [DOI] [PubMed] [Google Scholar]
- WILSON J. G., WARKANY J. Cardiac and aortic arch anomalies in the offspring of vitamin A deficient rats correlated with similar human anomalies. Pediatrics. 1950 Apr;5(4):708–725. [PubMed] [Google Scholar]