Abstract
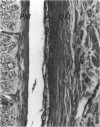
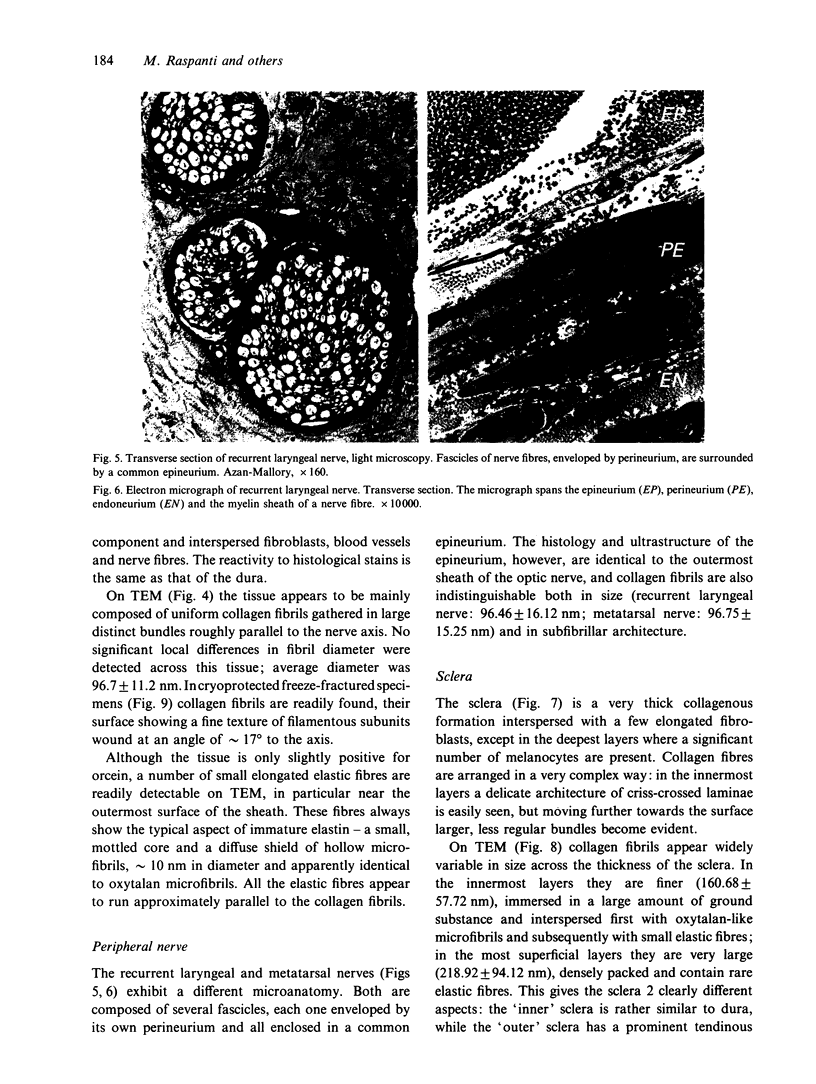
The sclera, the outermost sheath of the optic nerve and the dura mater have been investigated histologically and ultrastructurally. Although these tissues appear very similar under the light microscope, being dense connective tissues mainly composed of collagen bundles and a limited amount of cells and elastic fibres, they exhibit subtle differences on electron microscopy. In the dura and sclera collagen appears in the form of large, nonuniform fibrils, similar to those commonly found in tendons, while in the optic nerve sheath the fibrils appear smaller and uniform, similar to those commonly observed in reticular tissues, vessel walls and skin. Freeze-fracture also reveals these fibrils to have different subfibrillar architectures, straight or helical, which correspond to 2 distinct forms of collagen fibril previously described (Raspanti et al. 1989). The other extracellular matrix components also vary with the particular collagen fibril structure. Despite their common embryological derivation, the dura mater, optic nerve sheath and sclera exhibit diversification of their extracellular matrix consistent with the mechanical loads to which these tissues are subjected. Our observations indicate that the outermost sheath of the optic nerve resembles the epineurium of peripheral nerves rather than the dura to which it is commonly likened.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunn M. G., Silver F. H. Viscoelastic behavior of human connective tissues: relative contribution of viscous and elastic components. Connect Tissue Res. 1983;12(1):59–70. doi: 10.3109/03008208309005612. [DOI] [PubMed] [Google Scholar]
- GAMBLE H. J., EAMES R. A. AN ELECTRON MICROSCOPE STUDY OF THE CONNECTIVE TISSUES OF HUMAN PERIPHERAL NERVE. J Anat. 1964 Oct;98:655–663. [PMC free article] [PubMed] [Google Scholar]
- Gabriel G., Thomas P. K., King R. H., Stolinski C., Breathnach A. S. Freeze-fracture observations on human peripheral nerve. J Anat. 1986 Jun;146:153–166. [PMC free article] [PubMed] [Google Scholar]
- Junqueira L. C., Montes G. S., Krisztán R. M. The collagen of the vertebrate peripheral nervous system. Cell Tissue Res. 1979 Nov;202(3):453–460. doi: 10.1007/BF00220437. [DOI] [PubMed] [Google Scholar]
- Lieberman A. R. The connective tissue elements of the mammalian nodose ganglion. An electron microscope study. Z Zellforsch Mikrosk Anat. 1968;89(1):95–111. doi: 10.1007/BF00332655. [DOI] [PubMed] [Google Scholar]
- Mechanic G. L., Katz E. P., Henmi M., Noyes C., Yamauchi M. Locus of a histidine-based, stable trifunctional, helix to helix collagen cross-link: stereospecific collagen structure of type I skin fibrils. Biochemistry. 1987 Jun 16;26(12):3500–3509. doi: 10.1021/bi00386a038. [DOI] [PubMed] [Google Scholar]
- Raspanti M., Ottani V., Ruggeri A. Different architectures of the collagen fibril: morphological aspects and functional implications. Int J Biol Macromol. 1989 Dec;11(6):367–371. doi: 10.1016/0141-8130(89)90009-3. [DOI] [PubMed] [Google Scholar]
- Raspanti M., Ottani V., Ruggeri A. Subfibrillar architecture and functional properties of collagen: a comparative study in rat tendons. J Anat. 1990 Oct;172:157–164. [PMC free article] [PubMed] [Google Scholar]
- Reale E., Benazzo F., Ruggeri A. Differences in the microfibrillar arrangement of collagen fibrils. Distribution and possible significance. J Submicrosc Cytol. 1981 Apr;13(2):135–143. [PubMed] [Google Scholar]
- Reale E., Luciano L., Spitznas M. Freeze-fracture faces of the perineurial sheath of the rabbit sciatic nerve. J Neurocytol. 1975 Jun;4(3):261–270. doi: 10.1007/BF01102112. [DOI] [PubMed] [Google Scholar]
- Ruggeri A., Benazzo F., Reale E. Collagen fibrils with straight and helicoidal microfibrils: a freeze-fracture and thin-section study. J Ultrastruct Res. 1979 Jul;68(1):101–108. doi: 10.1016/s0022-5320(79)90146-1. [DOI] [PubMed] [Google Scholar]
- Stolinski C., Breathnach A. S. Freeze-fracture replication of mammalian peripheral nerve--a review. J Neurol Sci. 1982 Nov-Dec;57(1):1–28. doi: 10.1016/0022-510x(82)90107-1. [DOI] [PubMed] [Google Scholar]
- THOMAS P. K. The connective tissue of peripheral nerve: an electron microscope study. J Anat. 1963 Jan;97:35–44. [PMC free article] [PubMed] [Google Scholar]
- Ushiki T., Ide C. Three-dimensional architecture of the endoneurium with special reference to the collagen fibril arrangement in relation to nerve fibers. Arch Histol Jpn. 1986 Dec;49(5):553–563. doi: 10.1679/aohc.49.553. [DOI] [PubMed] [Google Scholar]
- Ushiki T., Ide C. Three-dimensional organization of the collagen fibrils in the rat sciatic nerve as revealed by transmission- and scanning electron microscopy. Cell Tissue Res. 1990 Apr;260(1):175–184. doi: 10.1007/BF00297503. [DOI] [PubMed] [Google Scholar]
- Viidik A. Functional properties of collagenous tissues. Int Rev Connect Tissue Res. 1973;6:127–215. doi: 10.1016/b978-0-12-363706-2.50010-6. [DOI] [PubMed] [Google Scholar]