Abstract
The human pathogen Neisseria gonorrhoeae induces host cell apoptosis during infection by delivering the outer membrane protein PorB to the host cell’s mitochondria. PorB is a pore-forming β-barrel protein sharing several features with the mitochondrial voltage-dependent anion channel (VDAC), which is involved in the regulation of apoptosis. Here we show that PorB of pathogenic Neisseria species produced by host cells is efficiently targeted to mitochondria. Imported PorB resides in the mitochondrial outer membrane and forms multimers with similar sizes as in the outer bacterial membrane. The mitochondria completely lose their membrane potential, a characteristic previously observed in cells infected with gonococci or treated with purified PorB. Closely related bacterial porins of non-pathogenic Neisseria mucosa or Escherichia coli remain in the cytosol. Import of PorB into mitochondria in vivo is independent of a linear signal sequence. Insertion of PorB into the mitochondrial outer membrane in vitro depends on the activity of Tom5, Tom20 and Tom40, but is independent of Tom70. Our data show that human VDAC and bacterial PorB are imported into mitochondria by a similar mechanism.
Keywords: import pathways/mitochondria/Neisseria gonorrhoeae/PorB/VDAC
Introduction
Many bacterial and viral pathogens manipulate induction of host cell apoptosis to their own advantage. Some pathogens, such as Chlamydia species and many viruses, prevent apoptosis and benefit from prolonged host cell survival because it enables them to replicate and produce viable progeny (Fan et al., 1998; Meinl et al., 1998; Tschopp et al., 1998; Rajalingam et al., 2001). Others, like the enteropathogenic bacterial species Salmonella, Shigella and Yersinia, escape the attack of the host’s immune system by efficiently killing key cells involved in the immune response (Zychlinsky et al., 1992; L.M.Chen et al., 1996; Y.Chen et al., 1996; Monack et al., 1996, 1997; Ruckdeschel et al., 1997).
Only recently, mitochondria emerged as targets for bacterial and viral manipulation of apoptotic pathways (Müller and Rudel, 2001). These organelles irreversibly trigger cell death upon receiving an apoptotic signal by increasing the permeability of both mitochondrial membranes (Green and Reed, 1998; Kroemer and Reed, 2000). This leads to a breakdown of the inner membrane potential and leakiness for intermembrane space proteins such as cytochrome c, AIF and others (Kroemer and Reed, 2000). One of the bacterial pathogens acting on mitochondria to modulate host cell apoptosis is Neisseria gonorrhoeae (Müller et al., 2000). The genus Neisseria comprises the human pathogenic species N.gonorrhoeae and N.menin gitidis, which cause gonorrhoea and meningitis, respectively, and several commensal, non-infectious species such as N.mucosa. The first contact takes place at epithelial surfaces, where tight association of the pathogen to the cells is brought about by means of pili (Swanson et al., 1987) and Opa proteins, which interact with specific receptors on the cell surface (Makino et al., 1991; Chen and Gotschlich, 1996; Gray-Owen et al., 1997; Dehio et al., 1998). Attachment to epithelia initiates the transfer of the outer membrane porin PorB to host cell membranes (Weel and van Putten, 1991; Müller et al., 1999).
Both our group and others (Massari et al., 2000; Müller et al., 2000) have demonstrated that PorB is subsequently targeted to mitochondria. This occurs in infected epithelial cells (Müller et al., 2000), but also in cultured lymphocytes that have been treated with purified porin in vitro (Massari et al., 2000). The outcome of PorB targeting to these organelles is a matter of debate and seems to depend on the Neisseria species, the purification procedure and/or the cell type. In cultured epithelial cells infected with N.gonorrhoeae, PorB triggers apoptosis by inducing the release of cytochrome c from mitochondria, a process that is accompanied by a complete breakdown of the membrane potential, matrix swelling, and the activation of caspases (Müller et al., 1999, 2000).
PorB resembles the mitochondrial porin or voltage-dependent anion channel (VDAC) with respect to several features. Both proteins are ATP-regulated trimeric β-barrel proteins, forming voltage-gated pores of similar size (Rudel et al., 1996). Interestingly, the VDAC along with other components appears to constitute the permeability transition pore complex (PTPC; Zoratti and Szabo, 1995), which has been implicated in many forms of cell death (Kroemer and Reed, 2000). The fact that both porins play a role in apoptosis regulation invites speculations regarding a common ancestor (Rudel et al., 1996; Frade and Michaelidis, 1997; Kroemer, 1997). This hypothesis is addressed in this study with respect to possible similarities in the targeting to mitochondria.
Nearly all mitochondrial proteins are encoded by the nuclear genome. They are synthesized in the cytosol and post-translationally imported into the mitochondria (Pfanner, 2000). Targeting and import are mediated by distinct segments of the newly synthesized proteins. Many preproteins contain a positively charged presequence at the N-terminus. Other mitochondrial proteins including the ANT (Brix et al., 1999), the cytochrome c haem lyase (Diekert et al., 1999), the BCS1 protein (Fölsch et al., 1996) and Tom70 (McBride et al., 1992) contain internal or C-terminal targeting signals. Preproteins bind to the import receptors Tom20 or Tom70 at the mitochondrial surface and are subsequently inserted into a general import pore, GIP, which is essentially formed by Tom40. In addition to these components, the TOM complex (translocase of the outer membrane) includes the import receptor Tom22 and the small subunits Tom5, 6 and 7. The import pathway of VDAC into mitochondria has recently been elucidated in more detail. Whereas there is no doubt that import of VDAC requires the surface receptor Tom20, the involvement of the GIP complex is a subject of debate (Pfaller and Neupert, 1987; Schleiff et al., 1997, 1999; Krimmer et al., 2001). As for other β-barrel proteins of the mitochondrial outer membrane, the targeting signal of VDAC is enigmatic (Krimmer et al., 2001).
In this study we compared the import mechanisms of the porins human VDAC and bacterial PorB both in vitro and in whole cells. We found that PorB is specifically inserted into the mitochondrial outer membrane. Although bacteria and mitochondria are related in evolution, their requirements for the insertion of membrane proteins are different. To reach the outer membrane of N.gonorrhoeae, PorB initially has to traverse the bacterial inner membrane and react with the bacterial Sec machinery. For import into mitochondria, PorB may insert spontaneously or it may interact with the TOM complex, although this has no bacterial counterpart. Endogenous mitochondrial homologues of bacterial proteins usually contain additional targeting signals (Hartl and Neupert, 1990). However, PorB is apparently devoid of such sequences. Moreover, the outer membranes of bacteria and mitochondria have a completely different lipid composition. Surprisingly, we found that PorB follows a similar import pathway as VDAC, including Tom20-dependent targeting and an involvement of the GIP. Productive targeting and insertion of PorB into the outer membrane were found to be strictly dependent on a cooperative effect of discontinuous parts of PorB that are distributed in the sequence. Truncated parts that could act as autonomous modules were not observed. These characteristics are different both from other Tom20-dependent precursor proteins and from Tom70-dependent preproteins, thus defining a unique and possibly primordial system of mitochondrial protein targeting.
Results
Endogenously expressed neisserial porin targets mitochondria
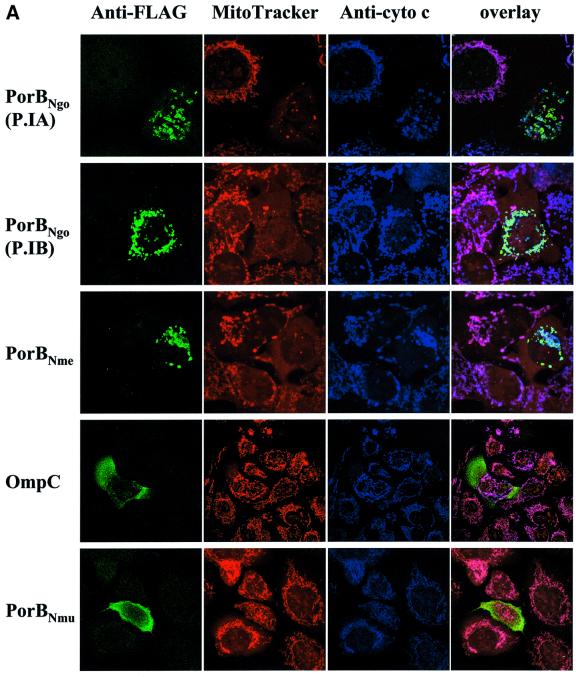
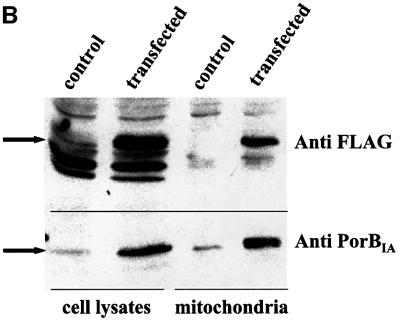
Porin genes from N.gonorrhoeae, N.meningitidis, the commensal strain N.mucosa as well as from Escherichia coli were amplified from genomic DNA, cloned into the mammalian expression vector pCMV-Tag-1 and transiently transfected into HeLa cells. Co-staining of transfected cells with the potential-sensitive dye Mitotracker and a specific antibody against the FLAG tag revealed a complete loss of mitochondrial inner membrane potential in cells transfected with porins from the two pathogenic Neisseria species N.gonorrhoeae and N.meningitidis, whereas cells expressing either porin from N.mucosa or E.coli did not differ from neighbouring non-transfected cells with respect to their Mitotracker staining (Figure 1A). Whereas the two latter porins display a diffuse staining pattern, which probably reflects a cytosolic localization, the porins of pathogenic Neisseria are distributed in a granular compartment resembling mitochondria. Counter staining with antibodies against mitochondrial antigens such as cytochrome c (Figure 1A), cytochrome c oxidase and Hsp60 (not shown) did show an almost complete overlap, thereby demonstrating that endogenously synthesized porin targets these organelles just like its bacterial counterpart. This could be confirmed by isolating mitochondria from transiently transfected HeLa cells and subsequent detection of porin in lysates and the mitochondrial preparation by western blotting (Figure 1B). Interestingly, the mitochondria of porin-transfected cells take on the peculiar swollen shape also seen in infected cells (Müller et al., 2000). However, although the targeting to mitochondria of endogenously made porin is more efficient than during an infection, where only up to 50% of all porin in the cell is found in this compartment, most of the mitochondria of porin-transfected cells, in contrast to infected cells, retain cytochrome c (Müller et al., 2000). Furthermore, porin-transfected cells do not undergo apoptosis in the time frame of the experiment. Whereas transfection of eukaryotic cells with porin is, therefore, not suitable for investigating the mechanism of apoptosis induction by neisserial porin at the mitochondrial level, it does represent an ideal model for studying the targeting process.
Fig. 1. Transient transfection of epithelial cells with bacterial porins reveals mitochondrial localization of porins from pathogenic but not commensal species. (A) HeLa cells were transfected with constructs encoding the indicated FLAG-tagged bacterial porins. After an expression time of 24 h, the cells were stained with Mitotracker, fixed, and co-stained with specific antibodies against cytochrome c (Anti- cyto c) and the FLAG tag. Confocal images of single colours and an overlay of all three colours are shown. PorBNgo (P.IA), porB gene isolated from N.gonorrhoeae strain VPI; PorBNgo (P.IB), porB gene isolated from N.gonorrhoeae strain MS11; PorBNme, porB gene from N.meningitidis; OmpC, ompC gene from E.coli; PorBNmu, porB gene from N.mucosa. (B) Cell lysates and mitochondria were isolated from transiently transfected cells according to the protocol detailed in Materials and methods, separated by SDS–PAGE and blotted onto PVDF membrane. The blots were probed with specific antibodies against the FLAG tag and PorBIA. Detection was performed using the ECL system (Amersham) according to the manufacturer’s instructions.
Porin is an integral protein of the mitochondrial outer membrane and forms multimers
In order to elucidate further the conformation of porin in the cell, cross-linking experiments were performed on isolated mitochondria from transfected cells (Figure 2A). Surprisingly, porin in transfected cells adopts the same multimeric conformation as in the bacterial outer membrane (Figure 2A) and in mitochondria from infected cells (not shown). The sizes of the detected multimers correspond to porin dimers, trimers and a high-molecular-weight band that probably represents a hexamer. This argues in favour of an intrinsic ‘multimerization signal’ in the primary porin sequence and suggests that porin might form a membrane channel in either mitochondrial membrane.
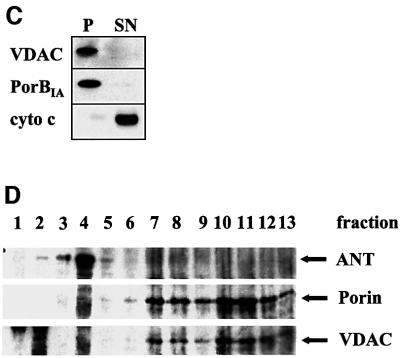
Fig. 2. Endogenously expressed PorBIA forms multimers and is an integral protein of the mitochondrial outer membrane. (A) HeLa cells were transfected with a construct encoding PorBIA, the mitochondria were isolated, and both purified mitochondria and cultured gonococci were subjected to chemical cross-linking. Samples were separated by SDS–PAGE in the absence or presence of β-mercaptoethanol as indicated. The blot was probed with a specific antibody against PorBIA. (B) Mitochondria from transfected or infected cells as well as cultured gonococci were subjected to sodium carbonate extraction. After 30 min of incubation in 0.1 M sodium carbonate, the membranes were pelleted by ultracentrifugation. Pellets (P) and supernatants (SN) were separated by SDS–PAGE, blotted, and probed with antibodies against the marker proteins Hsp60, VDAC, cytochrome c oxidase (cyto c ox) and cytochrome c (cyto c) (left panel). Immunoblots of PorBIA extracted from mitochondria of transfected (transfected) or infected cells (Ngo infected) or of intact bacteria (bacteria) are shown in the right panel. (C) Mitochondria were isolated from S.cerevisiae expressing human VDAC or gonococcal PorBIA from a plasmid under an inducible promoter according to a protocol detailed in Materials and methods and subjected to carbonate extraction as described in (B). Shown are immunoblots of pellets (P) and supernatants (SN) of mitochondria containing human VDAC (VDAC) or gonococcal PorBIA (PorBIA). Yeast cytochrome c (cyto c) was detected as a soluble marker protein. (D) Purified mitochondria were sonicated and the resulting vesicles were separated by centrifugation over a linear sucrose gradient for 16 h. Fractions of 0.8 ml were harvested, TCA precipitated and separated by SDS–PAGE. After blotting onto PVDF membrane, bound proteins were detected with specific antibodies against VDAC, ANT and PorBIA. (E) HeLa cells were transfected with constructs encoding mitochondrial matrix (MiMat)-targeted RFP and PorBIA or human VDAC, respectively, or with MiMat–RFP alone. After 24 h of expression, cells were fixed and stained with an anti-FLAG antibody followed by an Alexa 488-coupled secondary antibody. Confocal images of single colours and an overlay of both colours are shown.
Sodium carbonate extraction of mitochondria was performed to investigate whether porin is an integral membrane protein under these circumstances or only associates with mitochondria loosely. In the latter case, the interaction should be sensitive to the basic pH of sodium carbonate. The fate of marker proteins revealed the principal usefulness of the method (Figure 2B), as soluble proteins such as cytochrome c and the matrix protein Hsp60 were found in the supernatant, whereas integral membrane proteins such as VDAC and cytochrome c oxidase were detected in the pellet of carbonate-extracted mitochondria. The method further revealed that neisserial porin, regardless of whether it was endogenously synthesized or delivered to mitochondria during an infection, is always resistant to carbonate extraction (Figure 2B). Porin in its natural environment, the bacterial outer membrane, served as an additional control (Figure 2B).
The mitochondrial porin VDAC is an integral protein of the outer mitochondrial membrane. Owing to the overall similarities between mitochondrial and neisserial porin, it was expected that the latter would also reside in the outer rather than the inner membrane. This was examined with the help of a technique that allows the density gradient separation of both membranes on the basis of their different protein contents. This method requires large amounts of mitochondria to start with, a demand not met by the transfection system. For the purpose of collecting large quantities of mitochondrial material, recombinant yeast strains were generated expressing either neisserial porin or human VDAC under an inducible (glucose-repressed and galactose-induced) promoter. The structural and functional homology between human and yeast VDAC has been demonstrated earlier (Shimizu et al., 1999). Indeed, the correct targeting of both porins to mitochondria could be confirmed in this biological system (Figure 2C). By digesting purified mitochondria from the recombinant strains with proteinase K (PK), it could further be shown that both proteins are integral components (and therefore PK resistant) of the membrane rather than merely associated (data not shown). This was confirmed by sodium carbonate extraction of isolated mitochondria (Figure 2C), which revealed the localization of VDAC and PorBIA in the membrane (pellet) and cytochrome c in the supernatant. Separation of the two membranes by sucrose density gradient centrifugation revealed that both recombinant proteins appear in the same gradient fractions (Figure 2D), which also contained the yeast VDAC (not shown). In contrast, a typical inner membrane protein, the ANT, is exclusively found in a remote fraction (Figure 2D). There is no overlap of both membranes, as judged by the markers.
An additional experimental design directed towards determining the sub-mitochondrial localization of both porins involved co-expressing them with a variant of the red-fluorescing protein (RFP), which is artificially targeted to the mitochondrial matrix by a typical cleavable localization signal (Figure 2E). The shape of mitochondria containing only the RFP did not differ from that of normal mitochondria in non-transfected cells (upper panel); neither did those co-expressing RFP and VDAC (lower panel). These mitochondria revealed a complete overlap of both antigens. In the case of mitochondria containing both RFP and porin, however, the typical swollen morphology was observed (central panel), which can also be triggered by addition of porin to mitochondria in vitro (Müller et al., 2000). Interestingly, hardly any overlap could be observed in these mitochondria; instead, rings of porin seem to surround sphere-shaped matrices. All these data taken together clearly indicate that neisserial porin as well as mitochondrial porin, when expressed by either human or yeast cells, specifically inserts into the mitochondrial outer membrane, where it acquires its typical multimeric conformation and presumably forms functional channels.
Targeting of neisserial porin to the mitochondrial outer membrane is independent of inner membrane potential
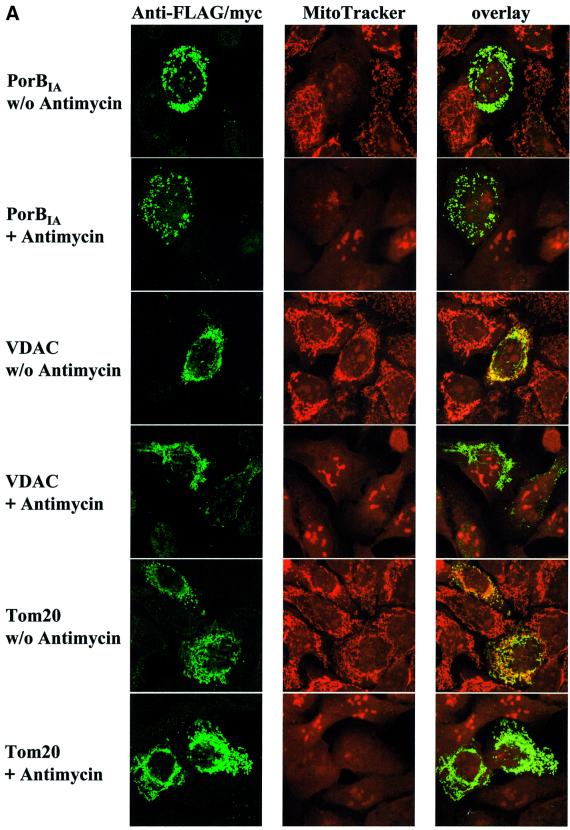
The import of many mitochondrial proteins requires an intact inner membrane potential, which provides the driving force necessary for protein translocation across one or both membranes. In the case of PorB import, the role of the membrane potential for import seems especially intriguing, since breakdown of the membrane potential is one of the earliest features observed both during an infection and upon porin treatment of isolated mitochondria (Müller et al., 2000). In order to evaluate the importance of an intact membrane potential for PorB import, cells were treated with the uncoupler antimycin prior to transfection. Antimycin binds to and irreversibly inactivates complex III of the electron transport chain in the inner membrane. Its action was confirmed by counterstaining cells with the potential-sensitive Mitotracker. Neisserial porin, just like the outer membrane proteins VDAC and Tom20, clearly targets mitochondria independently of an intact membrane potential (Figure 3A).
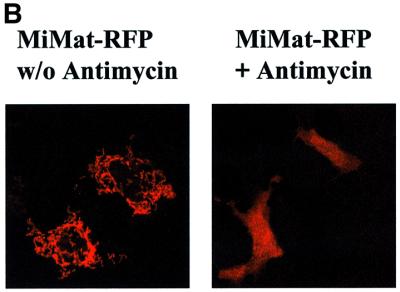
Fig. 3. Import of PorBIA does not depend on an intact mitochondrial membrane potential. (A and B) HeLa cells were transfected with constructs encoding the indicated proteins, stained with Mitotracker, fixed and co-stained with antibodies against the FLAG (PorBIA, VDAC) or Myc (Tom20) tags. Where indicated, the cells were treated with the uncoupling agent antimycin at 100 µM prior to transfection. Both single colours and overlays are shown of confocal sections. Note that in the case of MiMat–RFP, no double staining with Mitotracker could be performed, as both dyes emit light at similar wavelengths.
On the other hand, protein import of an RFP fused to the N-terminal signal sequence of the cytochrome c oxidase subunit Va localized to the inner mitochondrial membrane (MiMat–RFP) is completely blocked in the presence of antimycin (Figure 3B), demonstrating a clear dependence of translocation across the inner membrane on an intact membrane potential.
The primary sequence of neisserial porin does not code for a linear mitochondrial targeting signal
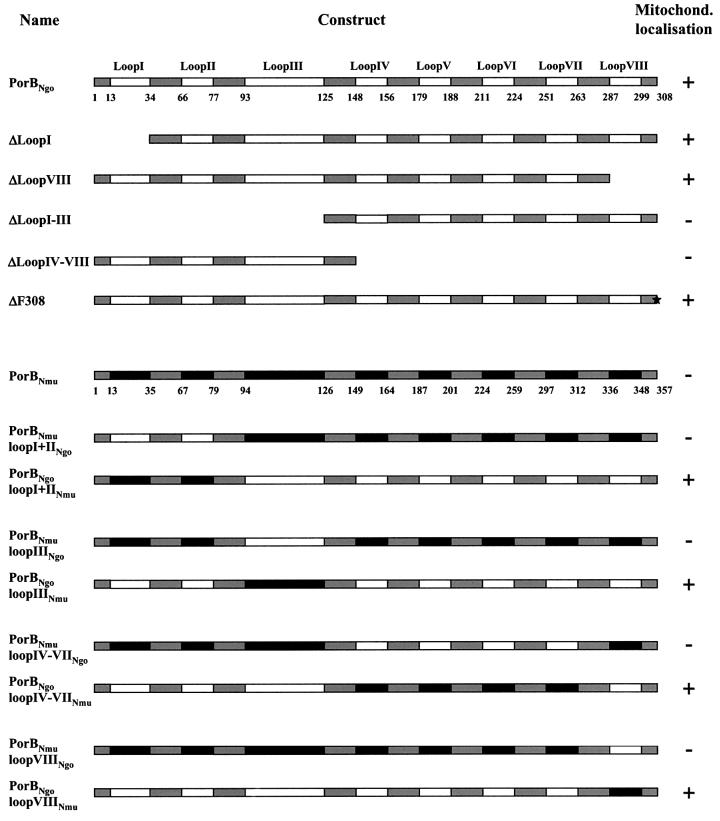
Two different strategies were employed to characterize a possible mitochondrial targeting signal: one involved the construction of deletion mutants; the other exploited the fact that the very similar N.mucosa porin is not imported by mitochondria of transfected cells (Figure 1A). Consequently, hybrid molecules were generated by fusing the two genes at the highly conserved membrane-spanning regions, thereby exchanging only the variable loop domains. All constructs were used for transfection experiments in HeLa cells followed by immunostaining and confocal microscopy (Figure 4).
Fig. 4. The primary sequence of PorBIA does not contain a linear mitochondrial targeting signal. PorBIA mutants carrying deletions in one or several loop domains, as well as hybrid molecules consisting of PorBIA and PorBNmu sequences, were constructed by standard PCR techniques. All proteins were expressed in HeLa cells and analysed with respect to their subcellular distribution as described in Figure 1A. Conserved membrane-spanning regions are schematically depicted in grey, whereas the variable loop domains are depicted in white (PorBIA sequences) or black (PorBNmu sequences). The amino acid positions of the junctions between the conserved membrane-spanning regions and the variable loop domains are indicated below the wild-type PorBNgo and PorBNmu. ΔF308 describes a mutant that lacks the terminal phenylalanine residue.
A well-studied prerequisite for the correct assembly of porins into the outer bacterial membrane is the presence of a phenylalanine at the extreme C-terminus of the porin sequence (de Cock et al., 1997). In order to dissect whether a mechanistic parallel can be established between folding of porin into the outer membrane of bacteria and of mitochondria, the terminal phenylalanine was deleted and the resulting construct was expressed in HeLa cells (Figure 4). Since this mutant targeted mitochondria (Figure 4) and induced the breakdown of the mitochondrial membrane potential just like the wild-type protein (not shown), it can be concluded that the terminal phenylalanine of PorB is dispensable for mitochondrial membrane integration.
Most targeting sequences identified to date are located at either terminus of the preprotein, which is why the two terminal PorB loops seemed the most likely candidates. However, truncations at either end affecting loop I and loop VIII, respectively, did not lead to a loss of mitochondrial targeting, thereby ruling out the existence of a targeting signal as known from other preproteins. Truncation of loops I–III or IV–VIII, on the other hand, generated mutants that were no longer transported to mitochondria. It must be assumed, however, that these short forms no longer retain the ability to form β-barrel structures, raising doubts about the reliability of a simple deletion approach and stressing the necessity of an alternative strategy. The generation of chimeric molecules provides the opportunity to exchange one loop after the other without interfering with the tertiary structure of the pore.
Interestingly, N.mucosa porins engineered to carry either gonococcal loops I and II, loop III, loops IV–VII or loop VIII showed a cytoplasmic localization indistinguishable from the wild type (Figure 4). Consistent with this observation, constructs of gonococcal PorB carrying the respective mucosal sequences did not fail to target mitochondria. In all these cases, the distribution of the porins in the cell follows an all-or-none principle. Partial effects of mitochondrial targeting with low efficiency were not observed. These data strongly argue against the existence of a linear targeting signal and provide evidence in favour of a targeting mechanism that requires the cooperation of discontinuous parts of the sequence. Moreover, it is remarkable that all constructs that co-localized with mitochondria also caused the complete dissipation of the membrane potential that is characteristic for the original PorB, demonstrating not only targeting but functional interactions with the mitochondria.
Protein–protein interactions with potential binding partners which could contribute to the ‘trapping’ of PorB in the mitochondrial membranes include VDAC and ANT. Although PorB clearly co-purified with both of these factors when mitochondria were lysed mildly and subjected to non-denaturing purification techniques such as gel filtration or anion-exchange chromatography, no direct interaction was detected by co-immunoprecipitation experiments (data not shown).
Gonococcal porin is imported into isolated mitochondria in vitro
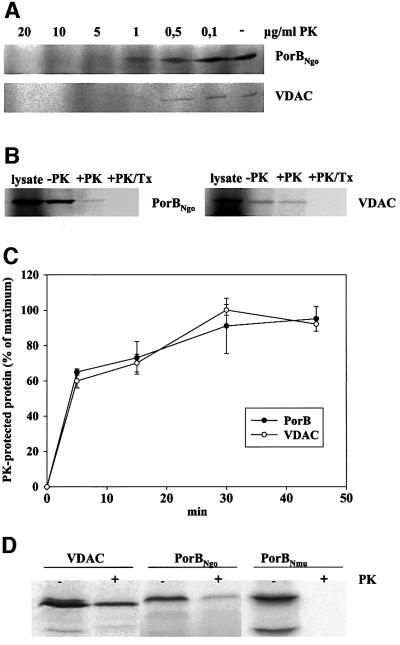
Isolated mitochondria from Saccharomyces cerevisiae have been used extensively for in vitro import studies. This requires the presence of ATP and an electron source such as NADH. Proteins to be imported are synthesized in vitro using a coupled transcription/translation system and radiolabelled simultaneously with [35S]methionine. Since proteins tend to associate with mitochondria unspecifically, the import reaction is followed by PK digestion. Only the PK-resistant population can truly be classified as ‘imported’. As shown in Figure 5A, in vitro translated gonococcal porin as well as mitochondrial VDAC are readily digested by PK; a 20-fold amount (100 µM) of the PK concentration shown to digest both proteins completely (5 µM) was used in all subsequent assays. One obstacle preventing the in vitro approach, the lack of sulfur-containing amino acids in the PorB sequence, was overcome by the generation of a hybrid containing the N-terminal portion of gonococcal porin and the C-terminal loop VIII of homologous N.mucosa porin (Figure 4). This mutant retains the ability to target mitochondria in transfected cells, but has gained an additional methionine in the last membrane-spanning region. It is, therefore, more readily labelled than the wild type.
Fig. 5. PorBIA is imported by isolated yeast mitochondria in vitro with lower efficiency but similar kinetics as human VDAC. (A) In vitro translated PorBIA and human VDAC were digested with increasing amounts of PK as indicated for 15 min on ice, the reaction was stopped by addition of 1 mM PMSF and the samples were run on an SDS gel. Autoradiographs are shown. (B) In order to assess and compare the import efficiency of both porins, 50 µg of mitochondria were mixed with 1 µl of in vitro translatate (lysate) of the respective preprotein and incubated at 25°C for 25 min. One half of the sample was then subjected to a PK digestion (+PK) in the presence or absence of 0.5% Triton X-100. The bands resulting from SDS–PAGE were quantified by phosphoimager and compared with an equal amount (1 µl) of in vitro translatate. A representative experiment is shown. (C) In order to compare the import kinetics of PorBIA and human VDAC, import reactions were performed for the indicated times for both proteins in parallel and PK protected protein was quantified by phosphoimager after SDS–PAGE. Mean values and standard deviations calculated from three independent experiments are shown. The highest value in every experiment was set as 100% and all other values were calculated with respect to the highest one. (D) Human VDAC, PorBIA and PorBNmu were imported under standard conditions as described in Materials and methods. An autoradiograph is shown for samples treated with PK and left untreated, repectively.
When radiolabelled human VDAC or gonococcal PorB was incubated with yeast mitochondria for 25 min, ∼60% of PorB porin and 45% of VDAC associated with mitochondria, whereas 10% of PorB porin and 25% of VDAC became PK resistant (Figure 5B). Addition of 0.5% Triton X-100 leads to a complete digestion of preprotein. The import of VDAC therefore seems to be a more efficient process. Interestingly, however, the import kinetics are comparable (Figure 5C): the majority of all PK-resistant molecules are imported in the first 5 min; the import reaction eventually reaches a plateau between 15 and 30 min.
Neisseria mucosa porin carrying the C-terminal loop of gonococcal porin, which is the reciprocal construct of the 35S-labelled gonococcal PorB used for import studies, also clearly associated with mitochondria, but failed to acquire PK resistance (Figure 5D). Active import of this construct could, therefore, not be observed. This confirms the data obtained with the same construct in living cells.
Import of gonococcal porin and VDAC requires both the Tom20 receptor and the general import pore
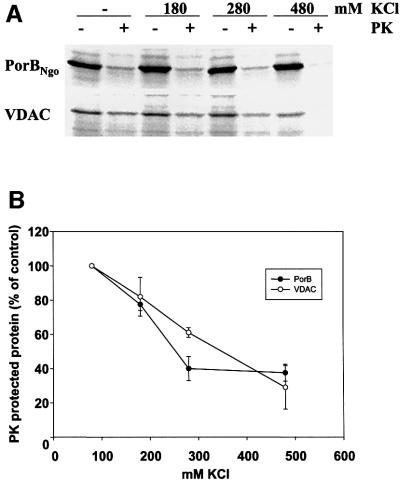
In order to elucidate and compare the import path ways exploited by gonococcal porin and VDAC, two approaches were chosen. First, import assays were performed in the presence of potassium chloride, which at concentrations up to 250 mM reportedly blocks import via Tom20, but not the Tom70 receptor (Pfaller et al., 1989). These experiments revealed a clear inhibition of import of both gonococcal porin and VDAC at KCl concentrations as low as 280 mM (Figure 6). These results point to Tom20 as the relevant import receptor and confirm previous findings made for VDAC (Schleiff et al., 1997, 1999; Krimmer et al., 2001). To test this hypothesis in a more defined setting, tom20-deficient yeast mutant mitochondria were compared with mitochondria from the parental wild-type strain with respect to their import capacity for PorB and VDAC. Lack of Tom20 resulted in a clear fall in the amount of imported VDAC (42%; p = 0.00006) and PorB (60%; p = 0.015) (Figure 7). In contrast, mitochondria deficient in Tom70 imported both proteins with an only slightly reduced efficiency, compared with the respective wild type, of 82 and 83% (Figure 7).
Fig. 6. Import of human VDAC and PorBIA is blocked at high salt concentrations. Both proteins were imported under standard conditions in the absence or presence of increasing concentrations of KCl as indicated. (A) A representative autoradiograph is shown for import of both proteins. (B) Quantification was performed by phosphoimager. Mean values and standard deviations were calculated from three independent experiments. The numbers are given as % of control, which reflects the sample not supplemented with additional KCl. Note that the import buffer used under standard conditions already contains 80 mM KCl.
Fig. 7. Import of human VDAC and PorBIA is dependent on Tom5, 20 and 40, but not on Tom70. Both proteins were imported under standard conditions. Mitochondria purified from the indicated yeast mutants (tomΔ) were used and compared with the respective wild types (WT). (A) A representative autoradiograph is shown for import of both proteins into the indicated mutant mitochondria. (B) Quantification was performed by phosphoimager. Mean values and standard deviations were calculated from at least three and up to eight independent experiments. The p-values of the respective experiments are depicted over the standard deviation bars in the figure. Import into WT mitochondria was set as 100% and all other values were calculated accordingly.
To shed light on the possible downstream events following preprotein–receptor interaction, mutants with defects in the general import pore were used (Figure 7). These included a strain with a deletion in the tom5 gene as well as a temperature-sensitive mutant of Tom40, tom40-4 (Krimmer et al., 2001). Both proteins are components of the general import channel: whereas Tom40 is a β-barrel protein forming the channel in the membrane, Tom5 is a small protein located close to the entry of the channel. Mitochondria purified from these mutants revealed a decrease in import efficiency comparable to the tom20 mutant. The amount of PK-resistant protein in tom5 mutant mitochondria fell to 27% (p = 0.082) and 61% (p = 0.033) of the wild-type VDAC and PorB, respectively, and to 60% (p = 0.000023) and 66% (p = 0.027) of the wild-type VDAC and PorB in the case of the tom40-4 mutant. These results confirm the conclusions drawn by Krimmer et al. (2001) with respect to the function of the general import complex in VDAC import. Furthermore, despite differences in the overall efficiency of import and in the contribution of the various components of the import machinery, the results imply the utilization of a common import pathway by both the gonococcal and the mitochondrial porin protein.
Discussion
The porin PorB of N.gonorrhoeae is a remarkable bacterial virulence factor because of its unusual ability to translocate from the bacterial outer membrane to host cells, followed by rapid transfer to the mitochondria, where it induces a pro-apoptotic transition of these organelles (Rudel et al., 1996; Müller et al., 2000). In this study we characterized the structural requirements for assembly of this bacterial porin in the membranes of mitochondria. We expressed PorB lacking the bacterial leader sequence in HeLa cells and monitored the distribution of the protein in the cell. PorB targeted mitochondria in an extremely effective way and caused dissipation of the mitochondrial membrane potential and swelling of the mitochondria, closely resembling the effects observed in cells infected with N.gonorrhoeae. Within the time frame of these experiments, release of cytochrome c and activation of caspases were not observed, suggesting that additional signals are required to trigger the apoptotic programme. However, these conditions allowed a detailed analysis of the interactions of PorB with the mitochondria.
Mitochondria containing PorB were isolated and tested for assembly of oligomeric PorB by chemical cross-linking. The pattern of reaction products indicated that in mitochondria PorB adopted the same oligomeric state as in the outer membrane of the bacteria. Sucrose gradient fractionations of mitochondrial membranes demonstrated that PorB accumulated exclusively in the outer membrane. PorB is thus able to assemble in two different biological membranes, although the lipid composition is very different. In particular, it should be noted that mitochondria are lacking the lipopolysaccharides that form the outer layer of the outer membrane of Gram-negative bacteria (de Cock et al., 2001). Moreover, it is surprising that PorB, while located in the outer membrane, promoted the rapid dissipation of the membrane potential that is established across the mitochondrial inner membrane. Dissipation of the membrane potential was not due simply to overexpression of a channel-forming protein, as loss of membrane potential was not observed in cells transfected with VDAC or with the porin of N.mucosa. Different from the observations of Massari et al. (2000), no direct interaction could be detected between the neisserial porin and VDAC by immunoprecipitation, even when both proteins were overexpressed at high levels (not shown). A two-hybrid screen did not reveal a direct interaction of the two porins either. However, both porins co-purified with the PTPC component ANT from mitochondria of infected cells under native conditions, indicating that a complex may exist containing this inner membrane channel, the VDAC and the neisserial porin (not shown).
PorB resembles the endogenous mitochondrial proteins VDAC and Tom40 in being a β-barrel protein lacking a positively charged N-terminal presequence. This similarity raised the question of whether there are similarities in the import pathways. VDAC and Tom40 interact with Tom20 as an import receptor, import of Tom40 is additionally facilitated by Tom70 (Söllner et al., 1989; Keil et al., 1993). As observed with other precursor proteins, bypass of the import receptors is possible for import at a reduced rate (Pfaller et al., 1989). Porin can insert into membranes spontaneously (Schleiff et al., 1999); however, assembly into authentic mitochondrial membranes is significantly facilitated by the TOM complex and involves the Tom40 channel (Krimmer et al., 2001). Interestingly, the bacterial porin PorB appears to follow essentially the same import pathway as the mitochondrial porin (VDAC), including binding to Tom20 and subsequent insertion into the GIP, as demonstrated by the participation of Tom5 and Tom40. Surprisingly, an original bacterial porin can thus assemble specifically into the mitochondrial outer membrane, although no obvious mitochondrial targeting signals are adopted. We conclude that in the evolution of the mitochondrial β-barrel proteins, limited changes in the structure of these proteins may have been sufficient to retain efficient targeting and assembly under the changing conditions of endosymbiosis.
Previous data have indicated that residues at the N- and C-terminus seem to be relevant for the import of mitochondrial porins (Hamajima et al., 1988; Smith et al., 1995; Court et al., 1996). We therefore decided to determine the targeting signal of PorB under authentic in vivo conditions using a set of constructs for expression in HeLa cells. We found that no part of PorB could act as an autonomous signal sequence on its own. Both the N- and the C-terminal thirds of PorB were necessary but not sufficient to direct mitochondrial targeting. Strikingly, all constructs that were efficiently targeted to the mitochondria also caused rapid dissipation of the mitochondrial membrane potential. Expression of the other constructs retained the membrane potential intact. Targeting of the PorB chimeras was obviously independent of a linear signal sequence but dependent on structures that are discontinuous and distributed within the sequence of PorB. Previous studies have established that unfolding of mitochondrial porin is a prerequisite for import into the outer membrane (Pfanner et al., 1988). However, the extreme sensitivity of PorB import to minor changes in any internal parts of PorB (together with the strict relationship of mitochondrial targeting versus function in dissipation of the membrane potential) indicates that the protein must have the capability to coordinate several parts of the sequence into a larger tertiary structure to allow insertion into the mitochondrial outer membrane.
Several previous observations are in agreement with this concept, suggesting that bacterial β-barrel proteins may have established a peculiar mechanism of membrane insertion that is retained in the mitochondria of eukaryotic cells. (i) To our knowledge, no mitochondrial β-barrel protein has revealed any localized and continuous targeting sequence. (ii) The porin PhoE of E.coli can assemble into the bacterial outer membrane after intramolecular cross-linking in the periplasm (Eppens et al., 1997). (iii) Prior to assembly of Tom40 into the TOM complex of the mitochondrial outer membrane, Tom40 accumulates in a 250 kDa complex in the intermembrane space (Model et al., 2001).
While most mitochondrial proteins that have been characterized so far are imported with the help of N- or C-terminal signal sequences (Pfanner, 2000; Wattenberg and Lithgow, 2001), the import of the inner membrane carrier proteins shows some similarity to the biogenesis of PorB. In particular, the biogenesis of the ANT (also named ADP/ATP carrier, AAC) was analysed in detail (Pfanner et al., 1987; Smagula and Douglas, 1988; Endres et al., 1999; Wiedemann et al., 2001). The ANT and related proteins are composed of three modules that cooperate in import. Similar to PorB, the ANT is devoid of an N- or C-terminal signal sequence. To insert into the Tom40 channel, the ANT adopts a loop structure (Wiedemann et al., 2001). However, in contrast to PorB and different from the mitochondrial β-barrel proteins, the individual modules can act as autonomous preproteins that are efficiently imported (Pfanner et al., 1987; Brix et al., 1999). PorB therefore appears to represent a unique type of preproteins, defined by an essential dependence on the cooperation of discontinuous parts of the sequence that cannot act as independent units.
How does PorB reach the mitochondrial membranes if provided directly by the bacteria? Inhibitors of the actin cytoskeleton, as well as the microtubule network and of receptor-mediated endocytosis, were not able to block the targeting of PorB to mitochondria in infected cells (not shown). This argues against a trafficking process involving endosomes or other membrane-bound compartments and rather suggests a soluble intermediate mobile form of PorB. However, the precise pathway that is used by PorB from the bacterial outer membrane to reach the mitochondria of the host cells remains to be established. Interestingly, although VDAC is generally regarded as residing exclusively in mitochondria, a subtype of this porin has also been found in other cellular membranes, including the plasma membrane, raising the possibility that at least some proteins of this class may have some mobility in the cell (Reymann et al., 1994).
Only recently, an increasing number of proteins were described that are provided by several different pathogens to interfere with apoptosis by targeting the mitochondria of host cells. VacA is secreted by Helicobacter pylori and was shown to target mitochondria and trigger apoptosis (Galmiche et al., 2000). Other examples include the apoptogenic HIV protein Vpr (Jacotot et al., 2000, 2001), and the hepatitis B virus protein X, which interacts with a new human isoform of VDAC, hVDAC3, inducing an alteration in transmembrane potential (Rahmani et al., 2000). A viral protein that acts in an anti- rather than a pro-apoptotic fashion, but also targets mitochondria, is the M11L protein of myxoma virus (Everett et al., 2000). M11L is actively taken up by the mitochondria via a C-terminal targeting signal that conforms to a newly identified consensus sequence for directing proteins to mitochondria which is also present in Bcl-2 and Bcl-XL (Isenmann et al., 1998; Everett et al., 2000). The definition of this consensus targeting signal seemed promising with respect to neisserial PorB, since a similar sequence exists in the second to last membrane-spanning region of this protein. However, deletion of this C-terminal portion of the molecule sequence did not affect mitochondrial targeting. Tagging of EGFP with the 40 C-terminal amino acids of PorB did not direct EGFP to the mitochondria either (not shown), again confirming the peculiar type of mitochondrial targeting that is applied by PorB.
In summary, we found that the bacterial porin PorB is imported into mitochondria via Tom20 and the GIP, following the same pattern of interactions as the mitochondrial porin. PorB is assembled into oligomers of similar composition as in the bacterial outer membrane. Imported PorB is functional with respect to rapid dissipation of the membrane potential and induction of swelling of the mitochondria. Our data on PorB indicate a unique type of mitochondrial targeting, defined by a lack of autonomous signal sequences at either end of the imported protein, and a strict dependence on a cooperational effect of discontinuous parts of the sequence.
Our findings are intriguing for several reasons. First, they reveal a functional parallel between the mitochondrial import of VDAC and the bacterial PorB porin, thereby reflecting an evolutionary link. Secondly, they represent another astonishing example of the sophisticated strategies that pathogens have evolved to exploit their host’s cellular functions. Thirdly, they demonstrate that the subtle differences in protein factors of pathogenic and commensal origin, despite similar functions in the bacterial context, can lead to a completely different outcome.
Materials and methods
DNA constructs
Porin genes from N.gonorrhoeae MS11 (P.IB) and VP1 (P.IA), N.meningitidis (Z2491), N.mucosa and E.coli (DH5α) were amplified from genomic DNA without their leader sequences using the primers specified in Table I. PCR products were cloned into the BglII and HindIII restriction sites of the expression vector pCMV-Tag-1 (Stratagene Corp.) in-frame with the FLAG tag. The cDNA coding for the human VDAC1 was amplified from a pBluescript plasmid [generously provided by Dr Friedrich Thinnes, Max Planck Institute (MPI) for Experimental Medicine, Göttingen, Germany]. The cDNA coding for full-length Tom20 was amplified from genomic DNA from ME180 cells. Both genes were cloned into pCMV-Tag-1 as described for the porins. The constructs for mammalian expression of mitochondrial matrix-targeted RFP are described elsewhere (T.Rudel, K.Lättig, N.Machuy, T.Manke, A.Müller and O.Thieck, in preparation).
Table I. Oligonucleotides.
| Name | 5′–3′ sequence |
|---|---|
| Ngo PorBIA 5′ | TCGACGTTACCCTGTACGGCACC |
| Ngo PorBIA3′ | CTGCTAGAATTTTTCTGCGCCTTT |
| Ngo PorBIB 5′ | TCGATGTCACCCTGTACGGTGCCA |
| Ngo PorBIB 3′ | CTGTTAGAATTTGTGGCGCAGAAC |
| Nme PorB 5′ | TCGACGTTACCCTGTACGGCACC |
| Nme PorB 3′ | CTGTTAGAATTTGTGGCGCAGACCGA |
| Nmu PorB 5′ | TCGATGTAACTCTGTACGGCCAAA |
| Nmu PorB 3′ | CTGTTAGAATTTGTGACGCAGACCA |
| Eco OmpC 5′ | TCGCTGAAGTTTACAACAAAGACGGC |
| Eco OmpC 3′ | CTGTTAGAACTGGTAAACCAGACC |
| hVDAC1 5′ | TCGCTGTGCCACCCACGTATGCCGA |
| hVDAC1 3′ | CTGTTATGCTTGAAATTCCAGTCCT |
| hTOM20 5′ | TCGCCACCATGGTGGGTCGGAACAGCGCCATCGCC |
| hTOM20 3′ | CTGTCATTCCACATCATCTTCAGCCA |
| Ngo PorBIA 5′m2 | TCGGTACTGACACAGGCTGGGGCAA |
| Ngo PorBIA 5′m4-1 | TCGACAATTCGGGCAAAAAtTCGCA |
| Ngo PorBIA 3′m4-1 | CTGCTAGTTAGGCACGTATTGTACGCT |
| Ngo PorBIA 3′m8-1 | CTGCTAGACAACCACTTGGTCGTA |
| Ngo PorBIA 3′ΔF308 | CTGCTATTTTTCTGCGCCTTTGCC |
| Ngo PorBIA 5′m3 | GGTGGCTTCGGTAAAGTGCGCGT |
| Nmu PorB 5′m3 | GGTGGTTTTGGTAAAGTACGT |
| Ngo PorBIA 3′m3 | CCTTTCAAACCGATGAAGAA |
| Nmu PorB 3′m3 | CCTTCCAAACCAACGAAAGATTCA |
| Ngo PorBIA 5′m4-2 | GGTTTCAGCGGCAGCGTACAATACGT |
| Nmu PorB 5′m4 | GGTTTTAGCGCAAACGTTCAATTTACT |
| Ngo PorBIA 3′m4-2 | CCTGCAAATTCGGGAGAATCGTAGCGT |
| Nmu PorB 3′m4 | CCTGCAAATACTGGGGAATCATAA |
| Ngo PorBIA 5′m8-2 | GGTGCGGAATACGACTTCTCC |
| Nmu PorB 5′m8 | GGTGCTGATTACGACTTCTCCAA |
| Ngo PorBIA 3′m8-2 | CCAACAACCACTTGGTCGTAAGTATT |
| Nmu PorB 3′m8 | CCTACTACAACTTGGTTGTACTCA |
Deletion constructs of PorBIA of N.gonorrhoeae were made with additional primers, which bind in the membrane-spanning regions of the porin (designated m1, m2, m3, etc.). All of these fragments were also cloned into pCMV-Tag-1. Hybrid porins consisting of PorBIA and PorBNmu sequences were constructed by standard PCR techniques. PorBIA of N.gonorrhoeae and human VDAC were also cloned into the EcoRI and BamHI sites of the vector pEYPD for expression in yeast. PorBIA of N.gonorrhoeae containing the loop VIII sequence of N.mucosa was additionally cloned into the NcoI and XhoI restriction sites of pET28a (Novagen) for improved in vitro transcription and translation. The integrity of all constructs was confirmed by sequence analysis. See Table II for a description of the constructs and the oligonucleotides used for PCR.
Table II. Constructs.
| Strain/construct | Description | Oligonucleotides used for PCR |
|---|---|---|
| H3073/pCMV-Tag:PorBIA-Ngo | pCMV-Tag-1 containing the porBIA gene of N.gonorrhoeae strain N242 | Ngo PorBIA 5′ and Ngo PorBIA 3′ |
| H3096/pCMV-Tag:PorBIB-Ngo | pCMV-Tag-1 containing the porBIB gene of N.gonorrhoeae strain N138 | Ngo PorBIB 5′ and Ngo PorBIB 3′ |
| H3098/pCMV-Tag:PorBNme | pCMV-Tag-1 containing the porB gene of N.meningitidis strain Z2491 | Nme PorB 5′ and Nme PorB 3′ |
| H3079/pCMV-Tag:PorBNmu | pCMV-Tag-1 containing the porB gene of N.mucosa | Nmu PorB 5′ and Nmu PorB 3′ |
| H3078/pCMV-Tag:OmpCEco | pCMV-Tag-1 containing the ompC gene of E.coli | Eco OmpC 5′ and Eco OmpC 3′ |
| H3106/pCMV-Tag:hVDAC1 | pCMV-Tag-1 containing the human vdac1 gene | hVDAC1 5′ and hVDAC1 3′ |
| H3085/pCMV-Tag:hTom20 | pCMV-Tag-1 containing the human tom20 gene | hTOM20 5′ and hTOM20 3′ |
| H3075/pCMV-Tag:PorBΔloopI | pCMV-Tag-1 containing a truncated version of the porBIA gene of N.gonorrhoeae lacking loop I | Ngo PorBIA 5′m2 and Ngo PorBIA 3′ |
| H3076/pCMV-Tag:PorBNgoΔloopI–III | pCMV-Tag-1 containing a truncated version of the porBIA gene of N.gonorrhoeae lacking loops I–III | Ngo PorBIA 5′m4-1 and Ngo PorBIA 3′ |
| H3077/pCMV-Tag:PorBNgoΔloopIV–VIII | pCMV-Tag-1 containing a truncated version of the porBIA gene of N.gonorrhoeae lacking loops IV–VIII | Ngo PorBIA 5′ and Ngo PorBIA 3′m4-1 |
| H3105/pCMV-Tag:PorBΔloopVIII | pCMV-Tag-1 containing a truncated version of the porBIA gene of N.gonorrhoeae lacking loop VIII | Ngo PorBIA 5′ and Ngo PorBIA 3′m8-1 |
| H3104/pCMV-Tag:PorBΔF308 | pCMV-Tag-1 containing a truncated version of the porBIA gene of N.gonorrhoeae lacking the last amino acid (F308) | Ngo PorBIA 5′ and Ngo PorBIA 3′ΔF308 |
| H3094/pCMV-Tag:PorBNmuloopI–II Ngo | pCMV-Tag-1 containing a gene fusion of loops I and II of the porBIA gene of N.gonorrhoeae with loops III–VIII of the porB gene of N.mucosa | Nmu PorB 5′m3 and Ngo PorBIA 3′m3 |
| H3095/pCMV-Tag:PorBNgoloopI–II Nmu | pCMV-Tag-1 containing a gene fusion of loops I and II of the porB gene of N.mucosa with loops III–VIII of the porBIA gene of N.gonorrhoeae | Ngo PorBIA 5′m3 and Nmu PorB 3′m3 |
| H3133/pCMV-Tag:PorBNmuloopIII Ngo | pCMV-Tag-1 containing a gene fusion of loops I and II and IV–VIII of the porB gene of N.mucosa with loop III of the porBIA gene of N.gonorrhoeae | Ngo PorBIA 5′m3 and Ngo PorBIA 3′m4-2 |
| H3134/pCMV-Tag:PorBNgoloopIII Nmu | pCMV-Tag-1 containing a gene fusion of loops I and II and IV–VIII of the porBIA gene of N.gonorrhoeae with loop III of the porB gene of N.mucosa | Nmu PorB 5′m3 and Nmu PorB 3′m4 |
| H3135/pCMV-Tag:PorBNmuloopIV–VII Ngo | pCMV-Tag-1 containing a gene fusion of loops I–III and VIII of the porB gene of N.mucosa with loops IV–VII of the porBIA gene of N.gonorrhoeae | Ngo PorBIA 5′m4-2 and Nmu PorB 3′m4 |
| H3136/pCMV-Tag:PorBNgoloopIV–VII Nmu | pCMV-Tag-1 containing a gene fusion of loops I–III and VIII of the porBIA gene of N.gonorrhoeae with loops IV–VII of the porB gene of N.mucosa | Nmu PorB 5′m4 and Ngo PorBIA 3′m4-2 |
| H3099/pCMV-Tag:PorBNmuloopVIII Ngo | pCMV-Tag-1 containing a gene fusion of loops I–VII of the porB gene of N.mucosa with loop VIII of the porBIA gene of N.gonorrhoeae | Ngo PorBIA 5′m8-2 and Nmu PorB 3′m8 |
Yeast strains and mammalian cell cultures
The yeast tom mutants and respective wild-type strains used in this study have been described elsewhere: tom5Δ (Dietmeier et al., 1997); tom20Δ (Moczko et al., 1994; Honlinger et al., 1996); tom40-4 (Krimmer et al., 2001); tom70Δ (Moczko et al., 1994). HeLa cells were cultured in RPMI supplemented with 10% FCS.
Immunofluorescence microscopy
Cells were seeded on cover slips, transfected with the mammalian expression vector pCMV-Tag encoding either bacterial or mammalian proteins with the Lipofectamine 2000 lipofection reagent (Gibco) according to the manufacturer’s instructions and fixed in 3% paraformaldehyde 24 h after transfection. Fixed cells were permeabilized using 0.2% Triton X-100, unspecific binding was blocked by 30 min of incubation in goat serum, and staining was performed using specific antibodies against the FLAG and Myc tags (Chemicon) as well as a monoclonal antibody against native cytochrome c (PharMingen, San Diego, CA), followed by detection with fluorochrome-coupled secondary antibodies. Co-localization experiments required additional staining of live mitochondria with 150 nM Mitotracker (Molecular Probes, Eugene, OR) for 30 min at 37°C prior to fixation. When indicated, cells were treated with 100 µM antimycin (Sigma) prior to transfection in order to dissipate the mitochondrial membrane potential. Samples were analysed on a Leica confocal microscope using TCS software.
Purification of mammalian mitochondria, cross-linking and sodium carbonate extraction
Mitochondria were purified as described previously (Müller et al., 2000). For extraction of membrane-associated but not integral proteins from mitochondrial membranes, purified mitochondria were resuspended in freshly made 0.1 M Na2CO3 and incubated for 30 min on ice. Membranes were pelleted for 15 min at 100 000 g in a fixed angle rotor, and both pellet and supernatant were subjected to SDS–PAGE followed by western blotting using antibodies against Hsp60 (U.Steinhoff, MPI for Infection Biology, Berlin, Germany) and cytochrome c (PharMingen) as soluble marker proteins, and antibodies against VDAC (F.Thinnes, MPI for Experimental Medicine, Göttingen, Germany) and cytochrome c oxidase (Molecular Probes) as insoluble marker proteins. Cross-linking was performed with the chemical cross-linker DSP (Pierce Chemical Co.) according to the manufacturer’s instructions for 30 min at 0°C. The reaction was stopped by addition of Tris buffer. Cross-linked proteins were solubilized in sample buffer with or without β-mercaptoethanol, thereby destroying or protecting the amide bond, respectively. Samples were run on an SDS gel followed by western blotting and detection with an antibody directed against gonococcal porin.
Sucrose gradient separation of mitochondrial inner and outer membranes
The protocol was modified from Pon et al. (1989). In brief, purified mitochondria were resuspended in buffer A (2.5 mM EDTA, 100 mM KCl, 20 mM MOPS–KOH pH 7.2) supplemented with 3 µg/ml pepstatin and 1 mM PMSF, and sonicated six times for 5 s at intervals of 15 s with continuous cooling on ice. Intact mitochondria were removed by centrifugation at 16 000 g for 10 min (4°C). Membrane vesicles were pelleted by centrifuging the supernatant for 30 min at 100 000 g in a fixed angle rotor (Beckman table top ultracentrifuge). The pellet was resuspended in buffer A and layered onto a 55–20% sucrose gradient (overall volume 10 ml). The gradients were centrifuged for 16 h at 100 000 g (4°C) in a swing-out rotor. Fractions of 800 µl were collected, the proteins were precipitated with 12% TCA final concentration and washed once in acetone. Proteins were analysed by SDS–PAGE and western blotting using monoclonal antibodies against VDAC and gonococcal porin or a polyclonal serum directed against ANT.
Isolation of yeast mitochondria
The method was modified from Daum et al. (1982). In brief, an overnight culture of S.cerevisiae in YP medium was harvested, resuspended in 30 ml of TD buffer (100 mM Tris-SO4 pH 9.4, 10 mM DTT) and incubated for 5 min at 30°C. After one wash in SP buffer (1.2 M sorbitol, 20 mM potassium phosphate pH 7.4), the cells were resuspended in 40 ml of SP containing 1.5 mg of zymolyase 100T (Seikagaku Corp., Japan) per gram of yeast wet weight. Enzymatic breakdown of the cell wall was performed for 30 min with gentle shaking. The resulting spheroplasts were collected by centrifugation and washed twice in ice-cold SP. They were then lysed in SHP buffer (0.6 M sorbitol, 20 mM HEPES–KOH pH 7.4, 1 mM PMSF) by 20 strokes of a glass homogenizer. Unbroken cells were removed by centrifugation at 2500 g before the mitochondria were harvested at 12 000 g for 10 min. The pellet was resuspended in SH and layered onto a 14.5%/18.5% Nycodenz gradient. The gradient was then centrifuged for 30 min at 270 000 g in a swing-out rotor. Purified mitochondria were harvested from the interface and centrifuged at 12 000 g prior to aliquoting in SH and freezing in liquid nitrogen.
Protein import into isolated mitochondria
In vitro translated proteins were imported into isolated mitochondria according to standard protocols (Krimmer et al., 2001). Both S.cerevisiae and mammalian mitochondria (isolated from Jurkat T cells) were used. The in vitro transcription and translation reaction was performed according to the manufacturer’s instructions (TnT coupled reticulocyte lysate system; Promega) in the presence of [35S]methionine (Amersham Pharmacia Biotech). Five microlitres of preprotein were mixed with 50 µg of mitochondria, 2 mM ATP, 1 mM NADH (yeast mitochondria) or 10 mM sodium succinate (mammalian mitochondria) in import buffer (3% BSA, 250 mM sucrose, 80 mM KCl, 5 mM MgCl2, 5 mM methionine, 10 mM MOPS–KOH pH 7.2) and incubated at 30°C for 5–20 min. The import reaction was stopped on ice. One half of every sample was subjected to PK digestion on ice (10 min, 50 µg/ml) followed by inhibition of PK with 1 mM PMSF for 10 min on ice. Import samples were centrifuged (12 000 g, 10 min) and mitochondrial pellets were washed once in SEM buffer (250 mM sucrose, 1 mM EDTA, 10 mM MOPS–KOH pH 7.2) prior to analysis by SDS–PAGE. Autoradiography was performed to visualize radiolabelled proteins.
Acknowledgments
Acknowledgements
We would like to thank Drs M.Klingenberg (University of Munich, Institute for Physiological Chemistry, Munich, Germany) for the ANT antiserum, F.Thinnes (MPI for Experimental Medicine, Department of Immunochemistry, Göttingen, Germany) and U.Steinhoff (MPI for Infection Biology, Department of Immunology, Berlin, Germany) for the Hsp60 antiserum. O.Thieck is thanked for constructing MiMat–RFP. We are grateful to Drs T.Krimmer, K.Diekert and R.Lill for valuable technical advice and helpful discussions.
References
- Brix J., Rudiger,S., Bukau,B., Schneider-Mergener,J. and Pfanner,N. (1999) Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, and Tom70 in a presequence-carrying preprotein and a non-cleavable preprotein. J. Biol. Chem., 274, 16522–16530. [DOI] [PubMed] [Google Scholar]
- Chen T. and Gotschlich,E.C. (1996) CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl Acad. Sci. USA, 93, 14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.M., Kaniga,K. and Galan,J.E. (1996) Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol., 21, 1101–1115. [DOI] [PubMed] [Google Scholar]
- Chen Y., Smith,M.R., Thirumalai,K. and Zychlinsky,A. (1996) A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J., 15, 3853–3860. [PMC free article] [PubMed] [Google Scholar]
- Court D.A., Kleene,R., Neupert,W. and Lill,R. (1996) Role of the N- and C-termini of porin in import into the outer membrane of Neurospora mitochondria. FEBS Lett., 390, 73–77. [DOI] [PubMed] [Google Scholar]
- Daum G., Bohni,P.C. and Schatz,G. (1982) Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem., 257, 13028–13033. [PubMed] [Google Scholar]
- de Cock H., Struyve,M., Kleerebezem,M., van der Krift,T. and Tommassen,J. (1997) Role of the carboxy-terminal phenylalanine in the biogenesis of outer membrane protein PhoE of Escherichia coli K-12. J. Mol. Biol., 269, 473–478. [DOI] [PubMed] [Google Scholar]
- de Cock H., Pasveer,M., Tommassen,J. and Bouveret,E. (2001) Identification of phospholipids as new components that assist in the in vitro trimerization of a bacterial pore protein. Eur. J. Biochem., 268, 865–875. [DOI] [PubMed] [Google Scholar]
- Dehio M., Gomez-Duarte,O.G., Dehio,C. and Meyer,T.F. (1998) Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves αv integrin receptors. FEBS Lett., 424, 84–88. [DOI] [PubMed] [Google Scholar]
- Diekert K., Kispal,G., Guiard,B. and Lill,R. (1999) An internal targeting signal directing proteins into the mitochondrial intermembrane space. Proc. Natl Acad. Sci. USA, 96, 11752–11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietmeier K., Honlinger,A., Bomer,U., Dekker,P.J., Eckerskorn,C., Lottspeich,F., Kubrich,M. and Pfanner,N. (1997) Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature, 388, 195–200. [DOI] [PubMed] [Google Scholar]
- Endres M., Neupert,W. and Brunner,M. (1999) Transport of the ADP/ATP carrier of mitochondria from the TOM complex to the TIM22·54 complex. EMBO J., 18, 3214–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppens E.F., Nouwen,N. and Tommassen,J. (1997) Folding of a bacterial outer membrane protein during passage through the periplasm. EMBO J., 16, 4295–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett H., Barry,M., Lee,S.F., Sun,X., Graham,K., Stone,J., Bleackley,R.C. and McFadden,G. (2000) M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med., 191, 1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan T., Lu,H., Hu,H., Shi,L., Mcclarty,G.A., Nance,D.M., Greenberg,A.H. and Zhong,G. (1998) Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med., 187, 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch H., Guiard,B., Neupert,W. and Stuart,R.A. (1996) Internal targeting signal of the BCS1 protein: a novel mechanism of import into mitochondria. EMBO J., 15, 479–487. [PMC free article] [PubMed] [Google Scholar]
- Frade J.M. and Michaelidis,T.M. (1997) Origin of eukaryotic programmed cell-death—a consequence of aerobic metabolism. BioEssays, 19, 827–832. [DOI] [PubMed] [Google Scholar]
- Galmiche A. et al. (2000) The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J., 19, 6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Owen S.D., Dehio,C., Haude,A., Grunert,F. and Meyer,T.F. (1997) CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J., 16, 3435–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R. and Reed,J.C. (1998) Mitochondria and apoptosis. Science, 281, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Hamajima S., Sakaguchi,M., Mihara,K., Ono,S. and Sato,R. (1988) Both amino- and carboxy-terminal portions are required for insertion of yeast porin into the outer mitochondrial membrane. J. Biochem., 104, 362–367. [DOI] [PubMed] [Google Scholar]
- Hartl F.U. and Neupert,W. (1990) Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science, 247, 930–938. [DOI] [PubMed] [Google Scholar]
- Honlinger A., Bomer,U., Alconada,A., Eckerskorn,C., Lottspeich,F., Dietmeier,K. and Pfanner,N. (1996) Tom7 modulates the dynamics of the mitochondrial outer membrane translocase and plays a pathway-related role in protein import. EMBO J., 15, 2125–2137. [PMC free article] [PubMed] [Google Scholar]
- Isenmann S., Khewgoodall,Y., Gamble,J., Vadas,M. and Wattenberg,B.W. (1998) A splice-isoform of vesicle-associated membrane protein-1 (VAMP-1) contains a mitochondrial targeting signal. Mol. Biol. Cell, 9, 1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E. et al. (2000) The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med., 191, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E. et al. (2001) Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein R and Bcl-2. J. Exp. Med., 193, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil P., Weinzierl,A., Kiebler,M., Dietmeier,K., Sollner,T. and Pfanner,N. (1993) Biogenesis of the mitochondrial receptor complex. Two receptors are required for binding of MOM38 to the outer membrane surface. J. Biol. Chem., 268, 19177–19180. [PubMed] [Google Scholar]
- Krimmer T. et al. (2001) Biogenesis of porin of the outer mitochondrial membrane involves an import pathway via receptors and the general import pore of the TOM complex. J. Cell Biol., 152, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G. (1997) Mitochondrial implication in apoptosis—towards an endosymbiont hypothesis of apoptosis evolution. Cell Death Diff., 4, 443–456. [DOI] [PubMed] [Google Scholar]
- Kroemer G. and Reed,J.C. (2000) Mitrochondrial control of cell death. Nature Med., 6, 513–519. [DOI] [PubMed] [Google Scholar]
- Makino S., van Putten,J.P. and Meyer,T.F. (1991) Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J., 10, 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari P., Ho,Y. and Wetzler,L.M. (2000) Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl Acad. Sci. USA, 97, 9070–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride H.M., Millar,D.G., Li,J.M. and Shore,G.C. (1992) A signal-anchor sequence selective for the mitochondrial outer membrane. J. Cell Biol., 119, 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinl E., Fickenscher,H., Thome,M., Tschopp,J. and Fleckenstein,B. (1998) Anti-apoptotic strategies of lymphotropic viruses. Immunol. Today, 19, 474–479. [DOI] [PubMed] [Google Scholar]
- Moczko M., Ehmann,B., Gartner,F., Honlinger,A., Schafer,E. and Pfanner,N. (1994) Deletion of the receptor MOM19 strongly impairs import of cleavable preproteins into Saccharomyces cerevisiae mitochondria. J. Biol. Chem., 269, 9045–9051. [PubMed] [Google Scholar]
- Model K., Meisinger,C., Prinz,T., Wiedemann,N., Truscott,K.N., Pfanner,N. and Ryan,M.T. (2001) Multistep assembly of the protein import channel of the mitochondrial outer membrane. Nature Struct. Biol., 8, 361–370. [DOI] [PubMed] [Google Scholar]
- Monack D.M., Raupach,B., Hromockyj,A.E. and Falkow,S. (1996) Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl Acad. Sci. USA, 93, 9833–9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack D.M., Mecsas,J., Ghori,N. and Falkow,S. (1997) Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl Acad. Sci. USA, 94, 10385–10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A. and Rudel,T. (2001) Modification of host cell apoptosis by viral and bacterial pathogens. Int. J. Med. Microbiol., 291, 197–207. [DOI] [PubMed] [Google Scholar]
- Müller A., Gunther,D., Dux,F., Naumann,M., Meyer,T.F. and Rudel,T. (1999) Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J., 18, 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A., Gunther,D., Brinkmann,V., Hurwitz,R., Meyer,T.F. and Rudel,T. (2000) Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J., 19, 5332–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller R. and Neupert,W. (1987) High-affinity binding sites involved in the import of porin into mitochondria. EMBO J., 6, 2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller R., Pfanner,N. and Neupert,W. (1989) Mitochondrial protein import. Bypass of proteinaceous surface receptors can occur with low specificity and efficiency. J. Biol. Chem., 264, 34–39. [PubMed] [Google Scholar]
- Pfanner N. (2000) Protein sorting: recognizing mitochondrial presequences. Curr. Biol., 10, R412–R415. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Hoeben,P., Tropschug,M. and Neupert,W. (1987) The carboxyl-terminal two-thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria. J. Biol. Chem., 262, 14851–14854. [PubMed] [Google Scholar]
- Pfanner N., Pfaller,R., Kleene,R., Ito,M., Tropschug,M. and Neupert,W. (1988) Role of ATP in mitochondrial protein import. Conformational alteration of a precursor protein can substitute for ATP requirement. J. Biol. Chem., 263, 4049–4051. [PubMed] [Google Scholar]
- Pon L., Moll,T., Vestweber,D., Marshallsay,B. and Schatz,G. (1989) Protein import into mitochondria: ATP-dependent protein translocation activity in a submitochondrial fraction enriched in membrane contact sites and specific proteins. J. Cell Biol., 109, 2603–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Z., Huh,K.W., Lasher,R. and Siddiqui,A. (2000) Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J. Virol., 74, 2840–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalingam K., Al Younes,H., Müller,A., Meyer,T.F., Szczepek,A.J. and Rudel,T. (2001) Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect. Immun., 69, 7880–7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymann S., Florke,H., Heiden,M., Jacob,C., Stadtmüller,U., Steinacker,P., Lalk,V.E., Pardowitz,I. and Thinnes,F.P. (1994) Further evidence for multitopological localization of mammalian porin (VDAC) in the plasmalemma forming part of a chloride channel complex affected in cystic fibrosis and encephalomyopathy. Biochem. Mol. Med., 54, 75–87. [DOI] [PubMed] [Google Scholar]
- Ruckdeschel K., Roggenkamp,A., Lafont,V., Mangeat,P., Heesemann,J. and Rouot,B. (1997) Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell-death through apoptosis. Infect. Immun., 65, 4813–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel T., Schmid,A., Benz,R., Kolb,H.A., Lang,F. and Meyer,T.F. (1996) Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell, 85, 391–402. [DOI] [PubMed] [Google Scholar]
- Schleiff E., Shore,G.C. and Goping,I.S. (1997) Interactions of the human mitochondrial protein import receptor, hTom 20, with the precursor proteins in vitro reveal pleiotropic specificities and different receptor domain requirements. J. Biol. Chem., 272, 17784–17789. [DOI] [PubMed] [Google Scholar]
- Schleiff E., Silvius,J.R. and Shore,G.C. (1999) Direct membrane insertion of voltage-dependent anion-selective channel protein catalyzed by mitochondrial Tom20. J. Cell Biol., 145, 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Narita,M. and Tsujimoto,Y. (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature, 399, 483–487. [DOI] [PubMed] [Google Scholar]
- Smagula C. and Douglas,M.G. (1988) Mitochondrial import of the ADP/ATP carrier protein in Saccharomyces cerevisiae. Sequences required for receptor binding and membrane translocation. J. Biol. Chem., 263, 6783–6790. [PubMed] [Google Scholar]
- Smith M.D. et al. (1995) Lysine residues at positions 234 and 236 in yeast porin are involved in its assembly into the mitochondrial outer membrane. J. Biol. Chem., 270, 28331–28336. [DOI] [PubMed] [Google Scholar]
- Söllner T., Griffiths,G., Pfaller,R., Pfanner,N. and Neupert,W. (1989) MOM19, an import receptor for mitochondrial precursor proteins. Cell, 59, 1061–1070. [DOI] [PubMed] [Google Scholar]
- Swanson J., Robbins,K., Barrera,O., Corwin,D., Boslego,J., Ciak,J., Blake,M.S. and Koomey,J.M. (1987) Gonococcal pilin variants in experimental gonorrhoea. J. Exp. Med., 165, 1344–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Thome,M., Hofmann,K. and Meinl,E. (1998) The fight of viruses against apoptosis. Curr. Opin. Genet. Dev., 8, 82–87. [DOI] [PubMed] [Google Scholar]
- Wattenberg B. and Lithgow,T. (2001) Targeting of C-terminal (tail)-anchored proteins: understanding how cytoplasmic activities are anchored to intracellular membranes. Traffic, 2, 66–71. [DOI] [PubMed] [Google Scholar]
- Weel J.F. and van Putten,J.P. (1991) Fate of the major outer membrane protein P.IA in early and late events of gonococcal infection of epithelial cells. Res. Microbiol., 142, 985–993. [DOI] [PubMed] [Google Scholar]
- Wiedemann N., Pfanner,N. and Ryan,M.T. (2001) The three modules of ADP/ATP carrier cooperate in receptor recruitment and translocation into mitochondria. EMBO J., 20, 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratti M. and Szabo,I. (1995) The mitochondrial permeability transition. Biochim. Biophys. Acta, 1241, 139–176. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Prevost,M.C. and Sansonetti,P.J. (1992) Shigella flexneri induces apoptosis in infected macrophages. Nature, 358, 167–169. [DOI] [PubMed] [Google Scholar]