Abstract
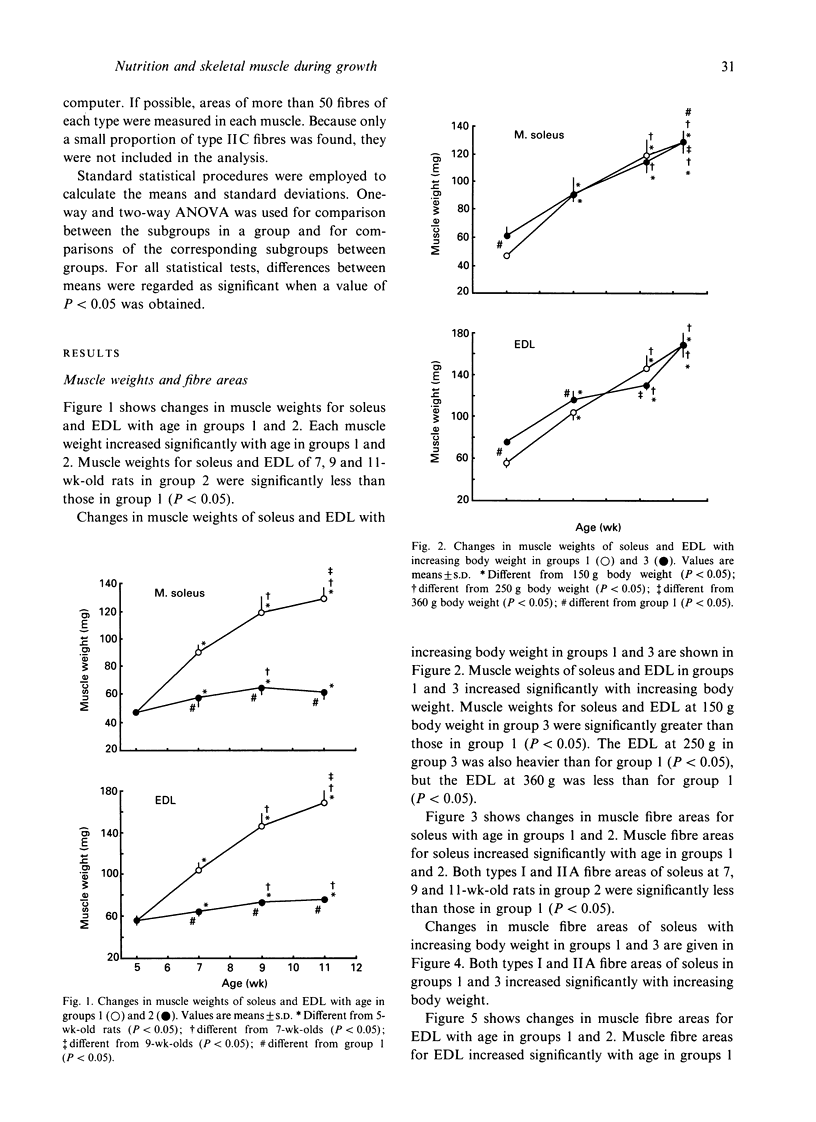
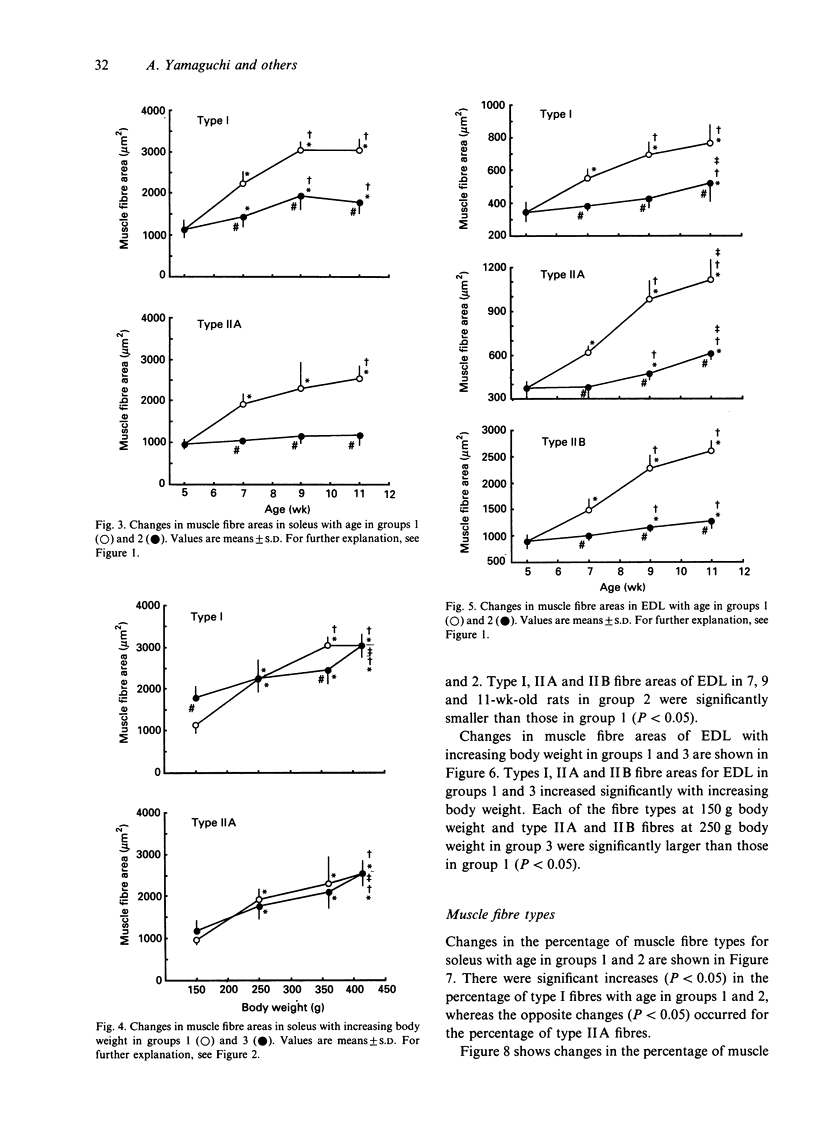
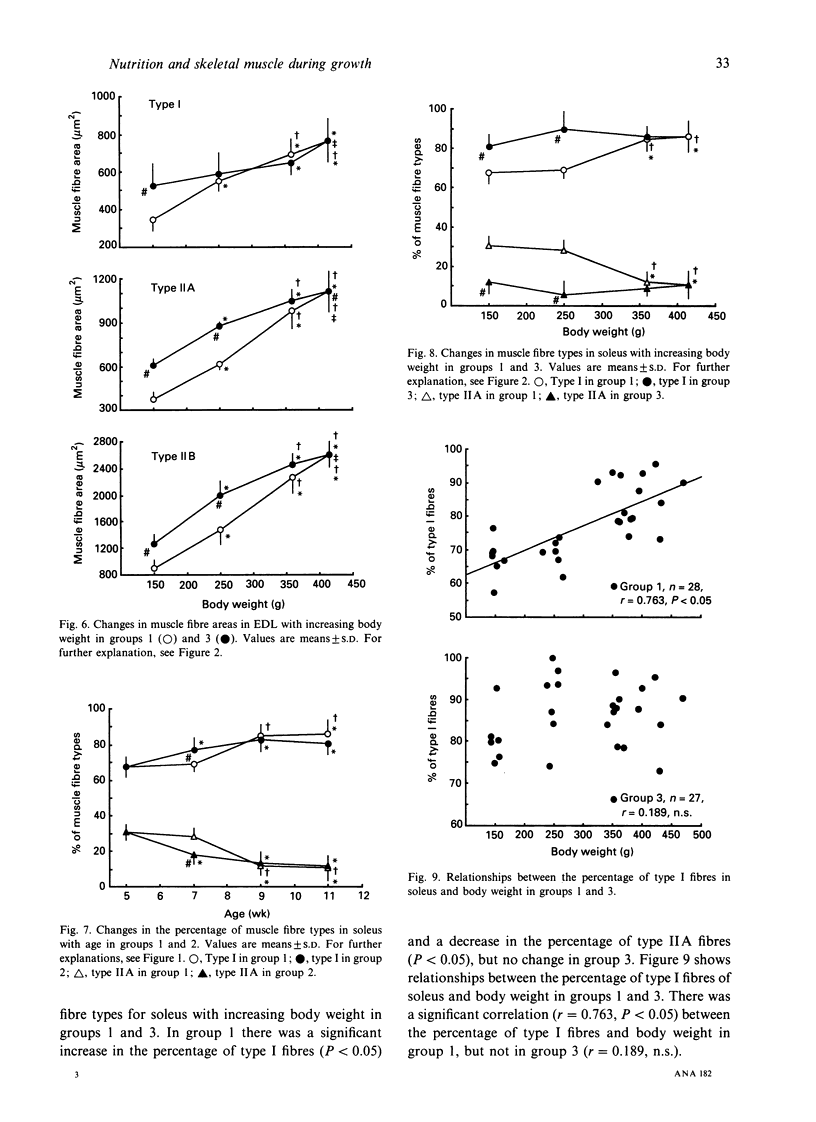
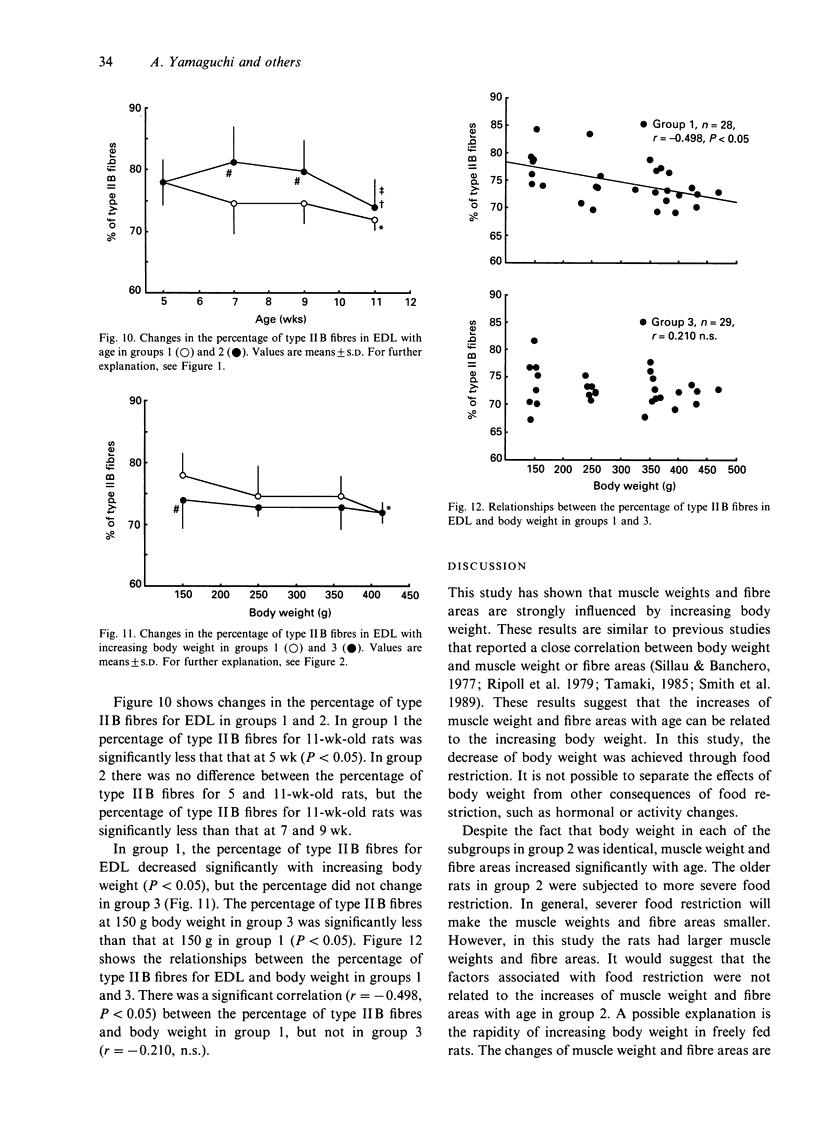
To investigate whether the retardation in the increase of body weight produced by reduced food intake could influence the transformation of muscle fibre types in soleus and extensor digitorum longus (EDL) during growth, rats were divided into 3 groups at 3 wk of age. Each group was subjected to food restriction from 3 wk of age to the following ages. Group 1 comprised 5 (148.7 +/- 7.3 g), 7 (250.7 +/- 11.1 g), 9 (362.9 +/- 19.3 g) and 11-wk-old rats (414.9 +/- 35.2 g) fed ad libitum. Group 2 comprised 5 (148.7 +/- 7.3 g), 7 (148.6 +/- 7.7 g), 9 (147.7 +/- 6.0 g) and 11-wk-old rats (148.8 +/- 5.7 g) fed a restricted diet; these animals were similar in weight to the 5-wk-old rats in group 1. Group 3 comprised 4 subgroups of 11-wk-old rats (148.7 +/- 5.7 g, 247.6 +/- 6.8 g, 354.4 +/- 8.6 g, 414.4 +/- 35.2 g); their body weights were adjusted to the weights of 5, 7, 9 and 11-wk-old rats in group 1 by restriction of food intake. Muscle weights and fibre areas in soleus and EDL significantly increased with growth. The muscle weights and fibre areas in group 2 in which body weights were equal increased significantly with age, but the increases were significantly less than for group 1. The muscle weights and fibre areas in group 3 in which ages were equal increased significantly with increasing body weight; the increases were the same as those in group 1.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquin L., Sillau A. H., Lechner A. J., Banchero N. Growth and skeletal muscle microvascularity in the guinea pig. Microvasc Res. 1980 Jul;20(1):41–50. doi: 10.1016/0026-2862(80)90018-7. [DOI] [PubMed] [Google Scholar]
- Ayling C. M., Moreland B. H., Zanelli J. M., Schulster D. Human growth hormone treatment of hypophysectomized rats increases the proportion of type-1 fibres in skeletal muscle. J Endocrinol. 1989 Dec;123(3):429–435. doi: 10.1677/joe.0.1230429. [DOI] [PubMed] [Google Scholar]
- Boreham C. A., Watt P. W., Williams P. E., Merry B. J., Goldspink G., Goldspink D. F. Effects of ageing and chronic dietary restriction on the morphology of fast and slow muscles of the rat. J Anat. 1988 Apr;157:111–125. [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Williamson E., Kaiser K. K. The behavior of four fiber types in developing and reinnervated muscle. Arch Neurol. 1971 Oct;25(4):360–366. doi: 10.1001/archneur.1971.00490040086010. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Browne G. S., Herlicoviez D., Whalen R. G. Effects of hypothyroidism on myosin isozyme transitions in developing rat muscle. FEBS Lett. 1984 Jan 23;166(1):71–75. doi: 10.1016/0014-5793(84)80047-2. [DOI] [PubMed] [Google Scholar]
- Curless R. G., Nelson M. B. Developmental patterns of rat muscle histochemistry. J Embryol Exp Morphol. 1976 Oct;36(2):355–362. [PubMed] [Google Scholar]
- Elder G. C., McComas A. J. Development of rat muscle during short- and long-term hindlimb suspension. J Appl Physiol (1985) 1987 May;62(5):1917–1923. doi: 10.1152/jappl.1987.62.5.1917. [DOI] [PubMed] [Google Scholar]
- Gambke B., Lyons G. E., Haselgrove J., Kelly A. M., Rubinstein N. A. Thyroidal and neural control of myosin transitions during development of rat fast and slow muscles. FEBS Lett. 1983 Jun 13;156(2):335–339. doi: 10.1016/0014-5793(83)80524-9. [DOI] [PubMed] [Google Scholar]
- Goldspink G., Ward P. S. Changes in rodent muscle fibre types during post-natal growth, undernutrition and exercise. J Physiol. 1979 Nov;296:453–469. doi: 10.1113/jphysiol.1979.sp013016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P. D., Parsons D., Oakley C. R. Differentiation of fiber types in skeletal muscle from the sequential inactivation of myofibrillar actomyosin ATPase during acid preincubation. Histochemistry. 1983;77(4):543–555. doi: 10.1007/BF00495808. [DOI] [PubMed] [Google Scholar]
- Holmäng A., Svedberg J., Jennische E., Björntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats. Am J Physiol. 1990 Oct;259(4 Pt 1):E555–E560. doi: 10.1152/ajpendo.1990.259.4.E555. [DOI] [PubMed] [Google Scholar]
- Ianuzzo D., Patel P., Chen V., O'Brien P., Williams C. Thyroidal trophic influence on skeletal muscle myosin. Nature. 1977 Nov 3;270(5632):74–76. doi: 10.1038/270074a0. [DOI] [PubMed] [Google Scholar]
- Ishihara A., Inoue N., Katsuta S. The relationship of voluntary running to fibre type composition, fibre area and capillary supply in rat soleus and plantaris muscles. Eur J Appl Physiol Occup Physiol. 1991;62(3):211–215. doi: 10.1007/BF00643744. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Kelly A., Lyons G., Gambki B., Rubinstein N. Influences of testosterone on contractile proteins of the guinea pig temporalis muscle. Adv Exp Med Biol. 1985;182:155–168. doi: 10.1007/978-1-4684-4907-5_13. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Lowrie M. B., More A. F., Vrbová G. The effect of load on the phenotype of the developing rat soleus muscle. Pflugers Arch. 1989 Nov;415(2):204–208. doi: 10.1007/BF00370593. [DOI] [PubMed] [Google Scholar]
- Nwoye L., Mommaerts W. F., Simpson D. R., Seraydarian K., Marusich M. Evidence for a direct action of thyroid hormone in specifying muscle properties. Am J Physiol. 1982 Mar;242(3):R401–R408. doi: 10.1152/ajpregu.1982.242.3.R401. [DOI] [PubMed] [Google Scholar]
- Ripoll E., Sillau A. H., Banchero N. Changes in the capillarity of skeletal muscle in the growing rat. Pflugers Arch. 1979 Jun 12;380(2):153–158. doi: 10.1007/BF00582151. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 1978 Feb;62(2):473–485. doi: 10.1016/0012-1606(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Sillau A. H., Banchero N. Effect of maturation on capillary density, fiber size and composition in rat skeletal muscle. Proc Soc Exp Biol Med. 1977 Mar;154(3):461–466. doi: 10.3181/00379727-154-39694. [DOI] [PubMed] [Google Scholar]
- Smith D., Green H., Thomson J., Sharratt M. Capillary and size interrelationships in developing rat diaphragm, EDL, and soleus muscle fiber types. Am J Physiol. 1989 Jan;256(1 Pt 1):C50–C58. doi: 10.1152/ajpcell.1989.256.1.C50. [DOI] [PubMed] [Google Scholar]
- Smith D., Green H., Thomson J., Sharratt M. Oxidative potential in developing rat diaphragm, EDL, and soleus muscle fibers. Am J Physiol. 1988 May;254(5 Pt 1):C661–C668. doi: 10.1152/ajpcell.1988.254.5.C661. [DOI] [PubMed] [Google Scholar]
- Sugie H., Verity M. A. Postnatal histochemical fiber type differentiation in normal and hypothyroid rat soleus muscle. Muscle Nerve. 1985 Oct;8(8):654–660. doi: 10.1002/mus.880080805. [DOI] [PubMed] [Google Scholar]
- Tamaki N. Effect of growth on muscle capillarity and fiber type composition in rat diaphragm. Eur J Appl Physiol Occup Physiol. 1985;54(1):24–29. doi: 10.1007/BF00426293. [DOI] [PubMed] [Google Scholar]
- Tomanek R. J. A histochemical study of postnatal differentiation of skeletal muscle with reference to functional overload. Dev Biol. 1975 Feb;42(2):305–314. doi: 10.1016/0012-1606(75)90337-1. [DOI] [PubMed] [Google Scholar]
- Vaughan H. S., Aziz-Ullah, Goldspink G., Nowell N. W. Sex and stock differences in the histochemical myofibrillar adenosine triphosphatase reaction in the soleus muscle of the mouse. J Histochem Cytochem. 1974 Mar;22(3):155–159. doi: 10.1177/22.3.155. [DOI] [PubMed] [Google Scholar]
- Vrbová G., Navarrete R., Lowrie M. Matching of muscle properties and motoneurone firing patterns during early stages of development. J Exp Biol. 1985 Mar;115:113–123. doi: 10.1242/jeb.115.1.113. [DOI] [PubMed] [Google Scholar]
- Watt P. W., Goldspink G., Ward P. S. Changes in fiber type composition in growing muscle as a result of dynamic exercise and static overload. Muscle Nerve. 1984 Jan;7(1):50–53. doi: 10.1002/mus.880070108. [DOI] [PubMed] [Google Scholar]
- Wernig A., Irintchev A., Weisshaupt P. Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. J Physiol. 1990 Sep;428:639–652. doi: 10.1113/jphysiol.1990.sp018232. [DOI] [PMC free article] [PubMed] [Google Scholar]


