Abstract
The cyclin-dependent kinase (CDK) inhibitor p21Waf1/Cip1/Sdi1 was identified initially as a gene induced in senescent cells and itself has been shown to cause permanent growth arrest/senescence. Reactive oxygen species (ROS), a byproduct of oxidative processes, can also induce an irreversible growth arrest similar to senescence. Here we show that p21 increased intracellular levels of ROS both in normal fibroblasts and in p53-negative cancer cells. N-acetyl-l-cysteine, an ROS inhibitor, rescued p21-induced senescence, showing that ROS elevation is necessary for induction of the permanent growth arrest phenotype. p16Ink4a, a CDK4- and CDK6-specific inhibitor, failed to increase ROS levels, and cell cycle arrest induced by p16 was reversible following its down-regulation, demonstrating the specificity of this p21 effect. A p21 mutant that lacked the ability to bind proliferating cell nuclear antigen (PCNA) retained the ability to induce both ROS and permanent growth arrest. All of these findings establish that p21 mediates senescence by a mechanism involving ROS accumulation which does not require either its PCNA binding or the CDK inhibitory functions shared with p16.
Keywords: CDK inhibitors/p21/PIG3/ROS/senescence
Introduction
Replicative senescence is a terminal differentiation state in which metabolically active cells are permanently and irreversibly arrested, with distinctive morphological changes (Hayflick and Moorehead, 1961) and phenotypic markers (Dimri et al., 1995). This phenotype was first observed in normal human fibroblasts in culture, which have a finite replicative lifespan and then become permanently growth arrested (Hayflick and Moorehead, 1961). Senescence can also be triggered in normal cells by oncogenes such as Ras (Serrano et al., 1997) or Raf (Zhu et al., 1998) or by tumor suppressors such as p53 (Sugrue et al., 1997). Thus, it has been suggested that senescence acts as a tumor suppressor mechanism by controlling the emergence of immortal cells (Sager, 1991; Smith and Pereira-Smith, 1996; Serrano et al., 1997).
Reactive oxygen species (ROS), byproducts of normal cellular oxidative processes (Orr and Sohal, 1994), have been shown to be involved in senescence (Q.Chen et al., 1995). Senescent cells are known to have higher levels of ROS than normal cells (Hagen et al., 1997). Moreover, oxidative stress caused by sublethal doses of H2O2 (Chen et al., 1998) or hyperoxia (von Zglinicki et al., 1995) can force human fibroblasts to arrest in a senescent-like fashion (Chen and Ames, 1994; Caldini et al., 1998; Dumont et al., 2000). Overexpression of antioxidant genes auch as superoxide dismutase or catalase causes extension of lifespan in Drosophila (Orr and Sohal, 1994; Parkes et al., 1998). This can also be observed in cell cultures maintained in low oxygen environments (Kinnula and Hassinen, 1981). All of these findings point to a strong relationship between oxidative damage, senescence and aging (for a review see Martin et al., 1996).
Up-regulation of the p53 tumor suppressor gene has been implicated in the induction of permanent growth arrest/senescence or apoptosis depending on many factors, including cellular context (Kastan et al., 1991; Lowe et al., 1993; Polyak et al., 1996; Sugrue et al., 1997; Sionov and Haupt, 1999). p53 overexpression has also been shown to cause the accumulation of ROS, presumably mediated by p53 transcriptional influence on pro-oxidant genes (Polyak et al., 1997). p21Waf1/Cip1/Sdi1, a p53-inducible protein (el-Deiry et al., 1993), is a necessary mediator of p53-induced cell cycle arrest (el-Deiry et al., 1993; Waldman et al., 1995, 1996), as indicated by the fact that p53 cannot induce arrest after DNA damage in p21-null mice (Brugarolas et al., 1995; Deng et al., 1995). p21 is a member of a family of cell cycle inhibitors that includes p27 and p57 (Sherr and Roberts, 1995), and it is capable of inhibiting cyclin-dependent kinases (CDKs) (Harper et al., 1993), key regulators of the cell cycle. It also acts to block DNA replication by binding to proliferating cell nuclear antigen (PCNA) (Waga et al., 1994). p21 expression has been observed in cultured human fibroblasts after prolonged passage, during which such cells undergo senescence (Noda et al., 1994). Moreover, p21 has been shown to be capable of inducing permanent growth arrest/senescence in a p53-independent manner (Fang et al., 1999; Wang et al., 1999). This phenotype was identified by use of a tetracycline (tet)-regulatable p21 system in which senescence could be distinguished from cell cycle inhibition associated with p21 expression by the ability to down-regulate p21 expression (Fang et al., 1999). In view of the implication of ROS in the induction of the permanent growth arrest/senescence phenotype, we investigated the possible role of ROS in p21-induced senescence.
Results
p21 increases ROS levels in EJ cells
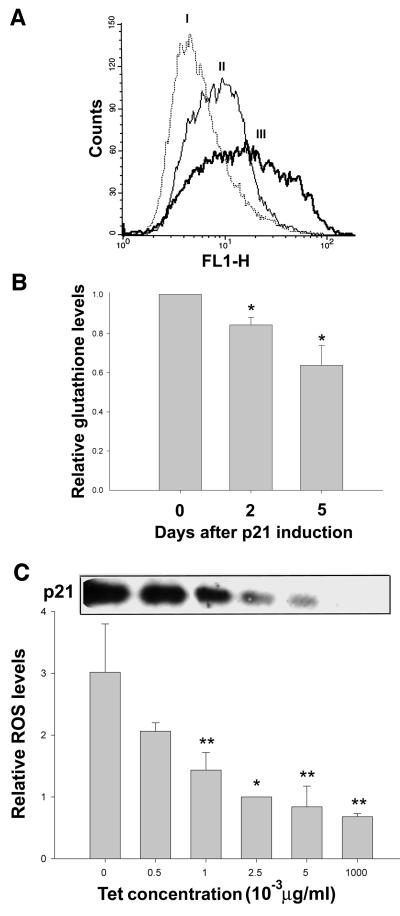
Expression of p21 in EJ tumor cells has been shown to cause growth arrest (Fang et al., 1999), which becomes irreversible after four of more days of expression. This permanent growth arrest is accompanied by the appearance of the senescence-specific marker SA-β-gal (Dimri et al., 1995) as well as morphological changes similar to those seen in senescence. In view of the known relationship between ROS accumulation and senescence (Q.Chen et al., 1995, 1998), we investigated whether ROS generation might contribute to this phenotype induced by prolonged p21 expression. To do so, we measured intracellular levels of ROS in EJ cells with tet-regulatable p21 expression (EJp21) by staining the cells with 2′,7′-dichlorofluorescin diacetate (DCF), a fluorescent marker of cellular oxidant production (Royall and Ischiropoulos, 1993). Figure 1A shows that ROS accumulation occurred by 2 days of tet removal and increased further by 5 days, when growth arrest had become irreversible. We further confirmed this result by measuring the levels of intracellular glutathione, one of the principal ROS buffers and a marker of oxidative stress (Dolphin et al., 1989). As shown Figure 1B, levels of glutathione decreased steadily after p21 induction, consistent with the effects of increased ROS levels. Moreover, when p21 protein levels were titrated using different concentrations of tet, ROS levels varied proportionally (Figure 1C), indicating that the accumulation of ROS was directly dependent on the levels of p21. Since EJ cells are known to lack functional p53 (Sugrue et al., 1997), these results establish that ROS accumulates as a result of p21 induction, independently of p53.
Fig. 1. Changes in ROS levels in EJp21 cells induced by p21 expression. (A) ROS levels as measured by FACS analysis after staining with the fluorescent probe DCF. The curves correspond to (I) cells cultured in the presence of tet (control) and 2 (II) or 5 (III) days after tet removal. The shift to the right of the curve due to increased fluorescence indicates an increase in the intracellular levels of ROS. (B) Intracellular glutathione levels were measured as described in Materials and methods at 2 or 5 days of p21 induction, compared with control cells. Results represent mean values of two experiments, and error bars show the standard deviation. *P <0.007. (C) Immunoblot analysis of p21 expression levels in lysates of EJp21 cells exposed to varying tet concentrations for 3 days. ROS levels in cultures were measured for the same tet concentrations at 3 days. Relative ROS levels were normalized to those at 2.5 × 10–3 µg/ml. Results represent mean values of three experiments for each concentration, and error bars show the standard deviation. *P <0.007; **P <0.05.
p21 increases ROS levels in normal human fibroblasts
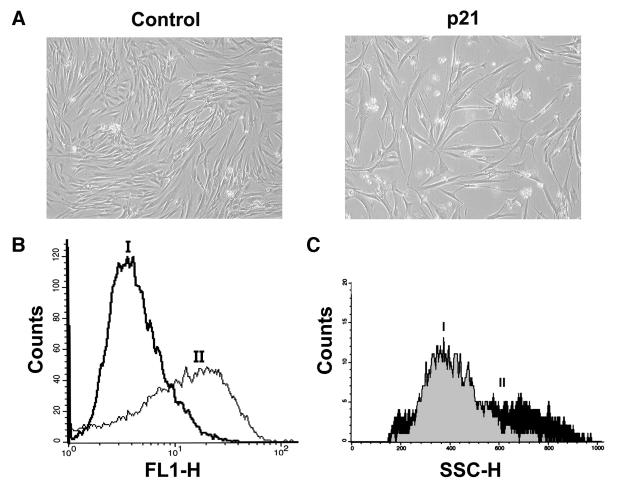
To assess whether ROS production in response to p21 also occurred in normal cells, we expressed p21 in human normal diploid fibroblasts (501T) by retroviral infection. As shown in Figure 2A and B, p21 caused both growth arrest and increased ROS levels, similar to the response observed in EJp21 cells. Cell size also increased in response to exogenous p21 (Figure 2C), as has been reported in naturally senescent fibroblasts (Angello et al., 1989). The levels of p21 expression induced by retrovirus gene transfer were comparable with those induced in response to UV irradiation, a p53-dependent inducer of p21 (data not shown). Thus, ROS increase and the permanent growth arrest phenotype were also induced by p21 expression in normal human cells.
Fig. 2. Increased ROS levels in 501T human fibroblasts in response to p21. (A) 501T cells infected with a retrovirus containing p21 and selected with puromycin for 1 week (p21), showing morphology changes and growth inhibition compared with the vector-infected cells (Control). Cells were photographed using a Nikon Eclipse TE200 microscope (100×). (B) ROS levels in cells infected with (I) a retrovirus control vector or (II) containing p21, 1 week after puromycin selection. (C) Cell size of 501T cells as measured by FACS analysis after infection with vector (I) or p21 retrovirus (II).
Blocking of ROS rescues p21-induced senescence in EJ cells
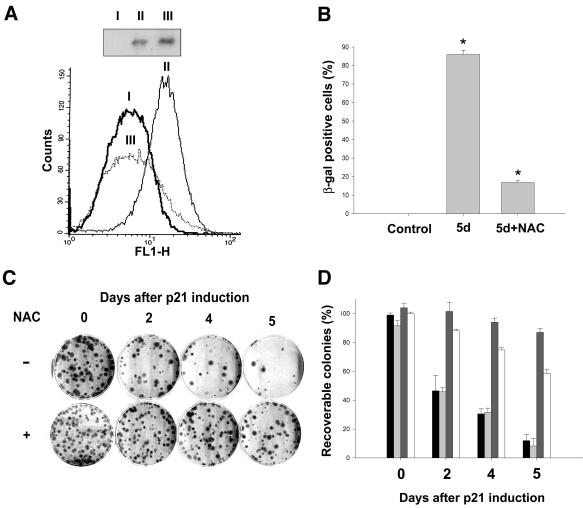
To determine whether accumulation of ROS played a major role in p21-induced permanent growth arrest/senescence, we attempted to block ROS accumulation in EJp21 cells with N-acetyl-l-cysteine (NAC), a reduced glutathione (GSH) provider and a direct scavenger of ROS (Staal et al., 1990). As shown in Figure 3A, cells incubated with 10 mM NAC showed no reduction in the level of induced p21 protein and maintained ROS levels comparable to those of uninduced cells. The number of cells that scored positive for SA-β-gal after 5 days was also substantially reduced in the presence of NAC, as compared with untreated p21-induced cultures (Figure 3B).
Fig. 3. Effects of NAC on the growth arrest/senescence phenotype of EJp21 cells. (A) ROS levels of EJp21 cells (I) in the presence of tetracycline, (II) after 5 days of p21 induction and (III) after 5 days of p21 induction with the addition of 10 mM NAC. Immunoblot analysis shows p21 expression levels in these cells. (B) The percentage of SA-β-gal-positive cells in EJp21 cells induced to express p21 by tet removal for 5 days in the presence or absence of 10 mM NAC. Control cells were kept in tet and no NAC for the duration of the experiment. Results represent mean values of two independent measures, and error bars show the standard deviation. *P <0.0001. (C) Representative plates from a colony formation assay, in which ∼100 cells were plated initially. Cells were maintained in the absence of tet for 0 (Control), 2, 4 or 5 days, and then tet was re-added to the medium. Cells were cultured for 14 more days followed by 10% formalin fixation and Giemsa staining. (D) Percentage of recoverable colonies for each condition described above, normalized to the number of colonies in the control plate. Bars correspond, from left to right, to control plates (black) and plates treated with 10 mM N-acetyl-l-alanine (light gray), 10 mM NAC (dark gray) or 2 mM GSH (white). Results represent mean values of at least two experiments, and error bars show the standard deviation.
Previous studies have shown that with increased time of p21 overexpression, there is a progressive decrease in the capacity of cells to form colonies after p21 repression by tet (Fang et al., 1999). To explore the importance of ROS in the irreversible growth arrest/senescence phenotype induced by p21, we examined the effect of exposure to 10 mM NAC on colony formation following p21 repression after p21 overexpression for different time periods. As shown in Figure 3C and D, colony formation of cells treated with NAC during the period of p21 induction was almost comparable to that of uninduced cells, even after 5 days of p21 expression. Figure 3D also shows the results of exposure to 10 mM N-acetyl-l-alanine, a compound with chemical structure similar to NAC but with no anti-ROS activity (Qiu et al., 1996), and 2 mM GSH, a widely used ROS inhibitor. Whereas N-acetyl-l-alanine failed to protect the cells from the permanent growth arrest phenotype induced by p21, exposure to GSH had an effect comparable with that of NAC. All of these findings indicate that ROS inhibitors were effective in preventing the onset of a permanent growth arrest in response to p21 expression, implicating ROS accumulation in this response.
p16 induces reversible growth arrest without elevation of ROS levels
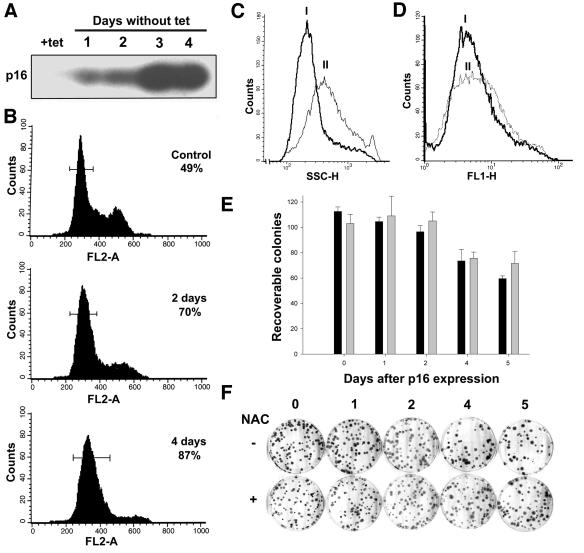
p16Ink4a is a CDK4 and CDK6 inhibitor that arrests cells in the G1 phase of the cell cycle (Serrano et al., 1993) and has been reported to be increased in senescent cells (Stein et al., 1999). Morever, it has been shown that p16 can induce growth arrest when overexpressed in cancer cells (Uhrbom et al., 1997). To determine whether p21-mediated ROS induction was specific or also resulted from overexpression of other CDK inhibitors, we measured ROS levels in EJ cells, which express no wild-type endogenous p16, containing tet-regulatable p16 (EJp16). As shown in Figure 4A and B, increased p16 levels were accompanied by cell cycle arrest in G1, together with increased cell size (Figure 4C). It is of note that ROS levels did not increase during p16 induction of growth arrest (Figure 4D). The growth inhibition associated with p16 was reversible following p16 repression (Figure 4E and F). After 5 days of p16 expression, >60% of cells were able to form colonies following tet repression of p16. Under these conditions, NAC did not change the number of rescued colonies significantly. These results indicate that cell cycle inhibition alone cannot account for ROS generation in response to p21 and strengthen the conclusion that ROS contributes importantly to the permanent growth arrest/senescence phenotype observed after p21 expression.
Fig. 4. Effects of p16 induction on the growth arrest/senescent phenotype of EJp16 cells. (A) EJp16 cell lysates obtained at varying time periods following tet removal were subjected to immunoblot analysis with anti-p16 antibody. (B) FACS analysis of EJp16 cells after propidium iodide staining at different times following tet removal. Percentages indicate the number of cells arrested in the G1 phase of the cell cycle, delimited by the bars in the graphics. (C) Cell size of EJp16 cells (I) cultured in tet or (II) after 6 days of tet removal, as measured by FACS analysis. (D) ROS levels as measured by FACS analysis after staining with the fluorescent probe DCF. The curves correspond to EJp16 cells (I) cultured in the presence of tet or (II) for 6 days following tet removal. (E) Percentage of recoverable colonies following p16 expression for different times. EJp16 cells were plated at a density of 100 cells per plate and were maintained in the absence of tet for 0 (control) up to 5 days, followed by tet addition. Cells were cultured for 14 more days followed by 10% formalin fixation and Giemsa staining. Black bars correspond to results with cultures in the absence of NAC, and gray bars correspond to results with cultures in 10 mM NAC, normalized to the number of colonies in the control plate. Results represent mean values of three experiments, and error bars show the standard deviation. (F) Representative plates from a colony formation assay.
p21’s ability to induce ROS and permanent growth arrest is independent of its PCNA-binding function
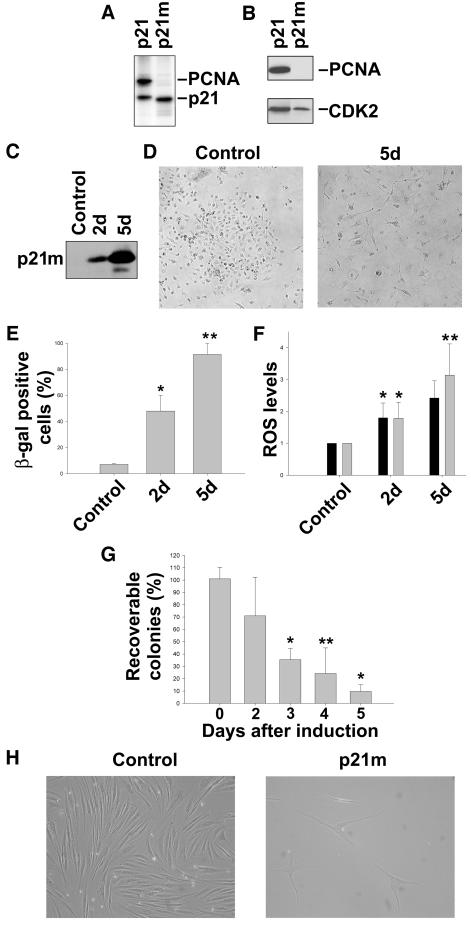
An important difference between the p21 and p16 families of CDK inhibitors is the ability of the first group to bind to PCNA. To elucidate whether its PCNA-binding function was responsible for the ability of p21 to induce ROS and permanent growth arrest, we established a tet-regulatable EJ cell line expressing a mutant p21 (p21m) that lacks the ability to bind to PCNA (EJp21m). p21m has two mutations, M147A and F150A, which were constructed based on crystal structural analysis that indicates that M147 and F150 are both involved in critical contacts with PCNA (Gibbs et al., 1997). To verify that p21m was deficient in binding to PCNA, transient transfection experiments were carried out followed by hemagglutinin (HA) immunoprecipitation of extracts containing metabolically 35S-labeled proteins. As shown in Figure 5A, HA-tagged wild-type p21 stoichiometrically co-immunoprecipitated a 35 kDa species (lane 1), while p21m was completely deficient in this interaction (lane 2). Immunoblot analysis of the same immunoprecipitates revealed that the 35 kDa species was PCNA (Figure 5B, top panel). Previous studies have revealed the association between endogenous p21, PCNA, CDK and cyclins, using antibodies that recognize CDK or cyclins (Xiong et al., 1992). In the present transient transfection study, HA-p21–PCNA was the predominant complex observed due to the presence of high levels of both proteins. However, consistent with the findings of Xiong et al. (1992), the results of immunoprecipitation/immunoblot experiments revealed the presence of CDK2 in both the HA-p21 and HA-p21m immunoprecipitates (Figure 5B, lower panel).
Fig. 5. The mutant form of p21 that does not bind to PCNA induces permanent growth arrest and ROS in EJ and normal human fibroblasts. (A) Autoradiography of a 12.5% SDS–PAGE that separates αHA-immunoprecipitates derived from 35S-metabolically labeled extracts of 293T cells transfected with HA-p21 or HA-p21m constructs. Polypeptides corresponding in sizes to p21 and PCNA, respectively, are indicated. (B) Immunoblot analysis of PCNA and CDK2 expression levels in the same metabolically labeled extracts. (C) Immunoblot analysis of p21 expression levels in lysates of EJp21m cells after 2 or 5 days of tet removal. (D) EJp21m cells cultured in the presence of tet (Control) or after 5 days of tet removal (5d). Cells were photographed using a Nikon Eclipse TE200 microscope (100×). (E) Percentage of SA-β-gal-positive cells in EJp21m cells induced to express mutant p21 by tet removal for 2 (2d) or 5 (5d) days, compared with cells cultured in the presence of tet (Control). Results represent mean values of three independent measures, and error bars show the standard deviation. *P <0.005; **P <0.00001. (F) ROS levels in EJp21m cells after 2 (2d) or 5 (5d) days of tet removal, compared with levels in EJp21 cells in the same conditions. Control cells were cultured in the presence of tet. *P <0.05; **P <0.001. (G) Percentage of recoverable colonies following mutant p21 expression for different times. EJp21m cells were plated at a density of 100 cells per plate and were maintained in the absence of tet for 0 (control) up to 5 days, followed by tet addition. Cells were cultured for 14 more days followed by 10% formalin fixation and Giemsa staining. Results represent mean values of three experiments normalized to the number of colonies in the control plate, and error bars show the standard deviation. *P <0.00005; **P <0.0005. (H) 501T cells infected with a retrovirus containing mutant p21 after 1 week selection with puromycin (p21m), showing morphology changes and growth inhibition compared with the vector-infected cells (Control). Cells were photographed using a Nikon Eclipse TE200 microscope (100×).
Induction of p21m (Figure 5C) caused the cells to arrest in G1 (data not shown) in a senescent-like fashion, with expression of SA-β-gal (Figure 5D and E). Moreover, ROS levels increased up to 3-fold by 5 days (Figure 5F), and growth arrest also became irreversible after 4–5 days (Figure 5G). To assess the effects of p21m in normal cells, we infected 501T fibroblasts with a retrovirus containing p21m. As shown in Figure 5H, infected cells became growth arrested and adopted a senescent-like morphology with increased ROS levels similar to those seen in 501T cells infected with a retrovirus containing wild-type p21. These results together show that the ability of p21 to induce permanent growth arrest and ROS in both normal and cancer cells is independent of its PCNA-binding properties.
Further analysis of p21-induced ROS
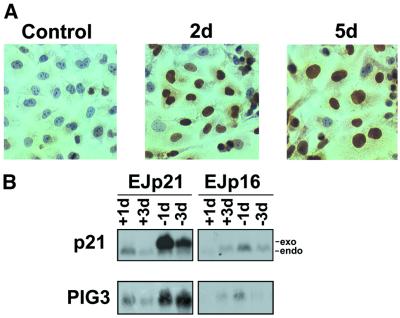
Recent studies have indicated that p21 can be phosphorylated and translocated to the cytoplasm, where it may exhibit new functions (Zhou et al., 2001). To assess p21 subcellular localization, we performed p21 immunostaining following p21 up-regulation in EJp21 cells. As shown in Figure 6A, induced p21was localized predominantly to the nucleus after both 2 and 5 days. These results indicate that if cytoplasmatic localization plays a role in ROS induction, it must be due to a small fraction of the protein.
Fig. 6. Further analysis of p21-induced ROS. (A) Immunostaining with p21 antibody of EJp21 cells in the presence of tet for 2 days (Control) and after 2 (2d) or 5 (5d) days of p21 induction by tet removal. Cells were photographed using a Nikon Microphot-FXA microscope (400×). (B) PIG3 is induced by p21 or mutant p21 but not p16. Northern blot analysis of p21 and PIG3 RNA levels in EJp21 cells in the presence of tetracycline for 1 or 3 days (+1d and +3d) and after 1 or 3 days of induction (–1d and –3d), compared with levels in EJp16 in the same conditions. Exo, induced p21; endo, endogenous p21.
It has also been reported that p53 increases the levels of intracellular ROS and that this effect is necessary for p53-induced responses, such as apoptosis (Polyak et al., 1997). p53-mediated induction of ROS has been proposed to be dependent on its transactivation of genes capable of generating ROS. PIG3, a gene homolog of oxidoreductase genes from several species, is one of the p53-responsive genes that has been suggested to be important in p53 induction of increased ROS levels (Polyak et al., 1997). PIG3 is induced with delayed kinetics as compared with other p53 target genes and its levels are increased during p53-mediated growth arrest (Flatt et al., 2000). To investigate further the mechanisms of ROS induction by p21, we measured the levels of PIG3 mRNA in EJp21 cells after tet removal. PIG3 mRNA levels increased significantly in EJp21 after 5 days of induction (Figure 6B). In contrast, p16 expression did not increase PIG3 RNA, consistent with the inability of p16 to induce ROS.
Discussion
Our present studies demonstrate that up-regulation of the CDK inhibitor p21 caused increased ROS levels in both normal and tumor cells, and this increase was proportional to the level of p21. ROS accumulation was not a general consequence of cell cycle arrest, since another CDK inhibitor, p16, failed to increase ROS levels. That ROS accumulation in response to p21 was causative of the permanent cell cycle arrest induced by p21 derives from two lines of evidence. NAC, an inhibitor of ROS (Staal et al., 1990), was able to block ROS accumulation in response to tet-regulatable p21, although it had no effect on the cell cycle inhibition or morphology changes accompanying p21 up-regulation. In striking contrast, NAC inhibited SA-β-gal staining of p21-expressing cells and protected the cells from the permanent growth arrest phenotype induced by p21 expression. Prolonged expression of p16, like p21, can induce senescence-like arrest in cancer cells (Uhrbom et al., 1997). We similarly observed growth arrest in both normal and tumor cells induced by p16. However, growth arrest following p16 down-regulation was found to be reversible, consistent with previous studies that reported reversibility of p16-mediated arrest (Uhrbom et al., 1997; Rossi et al., 1998). It is of note that this reversible growth arrest phenotype correlated with the absence of ROS accumulation in response to p16. All of these findings argue strongly that ROS accumulation is responsible for the permanent growth arrest/senescence phenotype induced by prolonged p21 expression.
It has been shown that naturally senescent fibroblasts exhibit increased ROS (Q.Chen et al., 1995), p16 (Alcorta et al., 1996) and p21 levels (Noda et al., 1994), although p21 up-regulation has been reported to be transient in such cells (Alcorta et al., 1996; Hara et al., 1996). Our findings that p21 increases ROS accumulation, which acts as mediator of the permanent growth arrest phenotype in the absence of continued p21 or p16 expression, provide a possible explanation for these observations. While we show that ROS is necessary for the permanent growth arrest/senescence phenotype induced by p21, our studies do not exclude the importance of the initial growth arrest associated with p21’s known cell cycle inhibitory functions. In fact, we have observed that in response to oxidative stress (H2O2), wild-type p21 fibroblasts undergo growth arrest but p21–/– fibroblasts fail to arrest and undergo apoptosis at increased H2O2 levels (S.Macip, S.Lee and S.Aaronson, unpublished data). This suggests that both p21 growth inhibition and ROS accumulation in response to p21 contribute to the permanent growth arrest phenotype.
It has been established that p21 inhibits cell cycle progression in both G1 and G2 through inhibition of several CDKs (Harper et al., 1993), including CDK2, CDK3, CDK4, CDK6 and CDC2, as well as PCNA, an essential DNA replication factor (Waga et al., 1994). These functions, which reside in different domains, are each sufficient to cause growth arrest (J.Chen et al., 1995; Luo et al., 1995). In contrast, p16 is a member of a family of CDK inhibitors, together with p15, p18 and p19 (Sherr and Roberts, 1995), which specifically inhibit CDK4 and CDK6 and have no effect on PCNA. A p21 mutant, which lacks PCNA-binding ability (Luo et al., 1995; Ando et al., 2001), has been reported to retain the ability to induce growth arrest specifically in G1, implying that PCNA binding is required for maintenance of the p21 G2 arrest function (Ando et al., 2001). We showed that this mutant retained the ability to induce both ROS accumulation and permanent growth arrest, demonstrating that p21 binding to PCNA and its G2 arrest function are not required for these responses.
PIG3, a p53 target gene involved in the ROS pathway, has been proposed to be important for induction of ROS and apoptosis after p53 expression (Polyak et al., 1997). p21 has been implicated in transcription control through interaction with the co-activator p300 (Perkins et al., 1997; Snowden et al., 2000). It has also been proposed that p21 can function as a direct modulator of transcription independently of its CDK inhibition properties (Dotto, 2000), based on the negative role of p21 on differentiation (Di Cunto et al., 1998). We observed that p21 induced up-regulation of PIG3 at both the RNA and protein levels, but that PIG3 levels were not increased in response to p16 expression. An approach to identify additional candidate genes which may influence ROS accumulation in response to p21 currently is being pursued. This approach may help to characterize those genes whose expression alterations are necessary and sufficient for p21-dependent induction of ROS and permanent growth arrest.
Permanent growth arrest/senescence has been proposed to be a tumor suppression mechanism to control the emergence of immortal cells (Sager, 1991; Smith and Pereira-Smith, 1996; Serrano et al., 1997). For instance, p53 (Sugrue et al., 1997) and Rb (Xu et al., 1997) can trigger senescence when reintroduced into immortal tumor cells, which have lost the functions of such genes in the course of tumor evolution. Depending on various factors, induction of p53 after genotoxic stress can result in either senescence or apoptosis, presumably to avoid replication of damaged DNA (Sionov and Haupt, 1999). Our results implicate p21, a known p53 effector, and ROS in the p53 senescent response. The role of ROS in p21-induced senescence underscores the importance of oxidative balance in tumor prevention and aging.
Materials and methods
Cell culture and treatments
EJ cells with a tet-regulated expression system (Fang et al., 1999) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (FBS), penicillin–streptomycin (50 units/ml), hygromycin (100 µg/ml) and geneticin (750 µg/ml), plus 1 µg/ml tet to repress expression of p21 (EJp21) or p16 (EJp16). Fresh medium with tet was added every 3 days. To induce p21 or p16 expression, cells were washed three times with phosphate-buffered saline (PBS) and seeded in medium in the absence of tet. NAC (Sigma), N-acetyl-l-alanine (Sigma) or GSH (Sigma) were added to the medium, when required, for the duration of the experiment. 293T cells were maintained in DMEM supplemented with 10% FBS and penicillin–streptomycin (50 U/ml).
Plasmid construction and DNA transfection
EJ-ETH clones that contain the transactivator have been described previously (Sugrue et al., 1997). The ptet puro expression vector was constructed by substitution of the neomycin marker with puromycin. ptet-p16 was constructed by subcloning p16Ink4a cDNA into the BamHI–AscI sites of the ptet puro vector. Transfection with this expression vector into EJ-ETH cells was performed using the standard calcium phosphate method. Transfectants were double selected in the presence of hygromycin (100 µg/ml) and puromycin (2 µg/ml), and individual clones of stable transfectants were selected for further analysis. Transfection with p21 or p21m constructs (generously provided by Y.Xiong) into 293T was performed using the standard calcium phosphate method.
DCF staining
Cells were incubated with 5 µg/ml of DCF (Molecular Probes) for 30 min, then washed with PBS, trypsinized and collected in 1 ml of PBS. Fluorescent stained cells were transferred to polystyrene tubes with cell-strainer caps (Falcon) and subjected to fluorescence-activated cell sorting (FACS; Beckton Dickinson FACScan) using Cell Quest 3.2 (Beckton Dickinson) software for acquisition and analysis.
Cell cycle analysis
Cells were stained with propidium iodide using the CycleTEST Plus DNA reagent kit (Beckton Dickinson), following the instructions provided. Fluorescent stained cells were transferred to polystyrene tubes with cell-strainer caps (Falcon) and subjected to FACS (Beckton Dickinson FACScan) using Cell Quest 3.2 (Beckton Dickinson) software for acquisition and analysis.
Colorimetric assay for glutathione
A colorimetric assay to determine intracellular glutathione concentrations (Oxford Biomedical Research, GT10) was used as described by the manufacturer. Briefly, cells were collected, washed and incubated with the provided reagents, and then the ODs of samples were measured with a spectophotometer set at 400 nm.
Immunobolot analysis
Cells cultured in the presence or absence of tet were washed twice with ice-cold PBS and lysed in EBC buffer [50 mM Tris pH 8, 120 mM NaCl, 0.5% NP-40, 100 mM sodium fluoride, 2 mM sodium vanadate, 2 mM phenylmethylsulfonyl fluoride (PMSF) and 10 µg/ml aprotinin]. Lysates were cleared by centrifugation at 14 000 r.p.m. for 20 min at 4°C. Protein concentrations were then determined using the BCA protein assay (Pierce). A 40 µg aliquot of total cell protein per sample was subjected to 14% SDS–PAGE and transferred to an Immobilon (Millipore) polyvinvylidene difluoride filter. p21 was detected using Ab-1 monoclonal antibody (Oncogene Science) and p16 was detected using C-20 polyclonal antibody (Santa Cruz Biotechnology). Antibodies for PCNA and CDK2 were obtained from Boehringer Mannheim and Upstate, respectively. An enhanced chemiluminescence (ECL) detection system (Amersham) was used.
Immunocytochemistry
Cells were seeded onto glass coverslips and maintained in medium with or without tet for 2–5 days. Coverslips were rinsed with PBS, fixed with 1% paraformaldehyde in PBS for 10 min at room temperature, washed twice with PBS, incubated in pre-cooled 2:1 ethanol/acetic acid for 5 min at –20°C, washed twice with PBS, incubated for 15 min in 3% hydrogen peroxide in PBS and then blocked in normal horse serum for 30 min at room temperature. Cells were stained for p21 using 2.5 µg/ml of Ab-1 monoclonal p21 antibody (Oncogene Science) overnight at 4°C. Following staining, cells were washed twice with PBS and then incubated for 30 min at room temperature in biotinylated horse anti-mouse secondary antibody (Vector Lab). Following two washes with PBS, cells were incubated in streptavidin–horseradish peroxidase (Zymed), washed three more times with PBS and once with 0.5% Triton X-100, and then incubated for 5 min in the dark with diaminobenzidine (DAB; Sigma).
Metabolic labeling, extract preparation and immunoprecipitation analysis
Transfected 293T cells were washed 40–48 h after transfection with 1× PBS and starved for 30 min with DMEM lacking l-methionine/l-cysteine (Life Technologies) supplemented with 10% dialyzed FBS. Medium was then changed to include 100 µCi/ml Easy Tag Express [35S] Protein Labelling Mix (NEN Life Science) and cells were incubated for 2 h. Following harvesting of the cells by centrifugation, pellets were resuspended in 0.2 ml/plate of buffer A (10 mM Tris–HCl pH 7.4, 10 mM NaCl, 0.5% NP-40, 1 mM PMSF, 2 µg/ml antipain, 2 µg/ml leupeptin) and sonicated (20 s ×6). Buffer B was then added (0.3 ml/plate). The mixture was rotated for 60 min at 4°C and supernatants following centrifugation (100 000 g, 4°C, for 60 min) were saved.
Extracts (100 µl) were then mixed with 10 µg of α-HA (12CA5) and 10 µg of protein A–agarose beads (Upstate Biotechnology) for 1 h at 4°C. The beads were washed three times with 0.5 ml of buffer C (buffers A and B mixed in equal volumes) and then twice with 1× PBS. Bound proteins were released by boiling the beads for 3 min in the presence of 20 µl of 4× concentrated Laemmli loading buffer and separated by 15% SDS–PAGE.
Northern blot analyisis
Total RNA was extracted from cells using TriZol (Gibco-BRL). RNA concentrations were measured spectophotometrically at 260 nm. A 20 µg aliquot of total RNA was electrophoresed at 70 mV for 2–3 h in a 1.6% agarose/formaldehyde gel, transferred to a positively charged nylon membrane and probed with a 32P-labeled PIG3 probe obtained from the verified IMAGE clone 859359 (supplied by ResGen). After washing once with 2× SSC/0.1% SDS at 50°C, twice with 0.5× SSC/0.1% SDS at 50°C, and twice with 0.1× SSC/0.1% SDS at room temperature, the blots were autoradiographed.
Senescence-associated β-galactosidase (SA-β-gal) staining
Cells were washed in PBS and fixed with 2% formaldehyde/0.2% glutaraldehyde in PBS for 5 min at room temperature. Plates were stained as described previously (Dimri et al., 1995).
Acknowledgments
Acknowledgements
We thank I.George, from the Mount Sinai Flow Cytometry Core Facility, and R.F.Qiao, S.Yao and J.Leung for technical assistance. Y.Xiong generously provided the PCNA binding mutant p21. S.M. received support from the Ministerio de Educacion y Cultura of Spain and is the recipient of a postdoctoral fellowship from the Forchheimer foundation. A.C. received support from a NIH predoctoral training grant in cancer biology. Z.-Q.P. is an Irma T.Hirschl Scholar. This work was supported in part by NIH grants CA80058 and CA85214 (to S.A.A.), CA78356 and CA82211 (to S.W.L.), Public Health Service Grant GM55059 (to Z.-Q.P.) and by the Peter Sharp Foundation (to S.A.A.).
References
- Alcorta D.A., Xiong,Y., Phelps,D., Hannon,G., Beach,D. and Barrett,J.C. (1996) Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl Acad. Sci. USA, 93, 13742–13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T., Kawabe,T., Ohara,H., Ducommun,B., Itoh,M. and Okamoto,T. (2001) Involvement of the interaction between p21 and proliferating cell nuclear antigen for the maintenance of G2/M arrest after DNA damage. J. Biol. Chem., 276, 42971–42977. [DOI] [PubMed] [Google Scholar]
- Angello J.C., Pendergrass,W.R., Norwood,T.H. and Prothero,J. (1989) Cell enlargement: one possible mechanism underlying cellular senescence. J. Cell Physiol., 140, 288–294. [DOI] [PubMed] [Google Scholar]
- Brugarolas J., Chandrasekaran,C., Gordon,J.I., Beach,D., Jacks,T. and Hannon,G.J. (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature, 377, 552–557. [DOI] [PubMed] [Google Scholar]
- Caldini R., Chevanne,M., Mocali,A., Tombaccini,D. and Paoletti,F. (1998) Premature induction of aging in sublethally H2O2-treated young MRC5 fibroblasts correlates with increased glutathione peroxidase levels and resistance to DNA breakage. Mech. Ageing Dev., 105, 137–150. [DOI] [PubMed] [Google Scholar]
- Chen J., Jackson,P.K., Kirschner,M.W. and Dutta,A. (1995) Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature, 374, 386–388. [DOI] [PubMed] [Google Scholar]
- Chen Q. and Ames,B.N. (1994) Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc. Natl Acad. Sci. USA, 91, 4130–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Fischer,A., Reagan,J.D., Yan,L.J. and Ames,B.N. (1995) Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl Acad. Sci. USA, 92, 4337–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.M., Bartholomew,J.C., Campisi,J., Acosta,M., Reagan,J.D. and Ames,B.N. (1998) Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem. J., 332, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Zhang,P., Harper,J.W., Elledge,S.J. and Leder,P. (1995) Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell, 82, 675–684. [DOI] [PubMed] [Google Scholar]
- Di Cunto F., Topley,G., Calautti,E., Hsiao,J., Ong,L., Seth,P.K. and Dotto,G.P. (1998) Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science, 280, 1069–1072. [DOI] [PubMed] [Google Scholar]
- Dimri G.P. et al. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA, 92, 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin D., Poulson,R. and Avramovic,O. (1989) Glutathione: Chemical, Bochemical and Medical Aspects, Volumes A&B. John Wiley and Sons.
- Dotto G.P. (2000) p21WAF1/Cip1: more than a break to the cell cycle? Biochim. Biophys. Acta, 1471, 43–56. [DOI] [PubMed] [Google Scholar]
- Dumont P., Burton,M., Chen,Q.M., Gonos,E.S., Frippiat,C., Mazarati,J., Eliaers,F., Remacle,J. and Toussaint,O. (2000) Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radical Biol. Med., 28, 361–373. [DOI] [PubMed] [Google Scholar]
- el-Deiry W.S. et al. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell, 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Fang L., Igarashi,M., Leung,J., Sugrue,M.M., Lee,S.W. and Aaronson,S.A. (1999) p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene, 18, 2789–2797. [DOI] [PubMed] [Google Scholar]
- Flatt P.M. et al. (2000) p53-dependent expression of PIG3 during proliferation, genotoxic stress and reversible growth arrest. Cancer Lett., 156, 63–72. [DOI] [PubMed] [Google Scholar]
- Gibbs E., Kelman,Z., Gulbis,J.M., O’Donnell,M., Kuriyan,J., Burgers,P.M. and Hurwitz,J. (1997) The influence of the proliferating cell nuclear antigen-interacting domain of p21CIP1 on DNA synthesis catalyzed by the human and Saccharomyces cerevisiae polymerase delta holoenzymes. J. Biol. Chem., 272, 2373–2381. [DOI] [PubMed] [Google Scholar]
- Hagen T.M., Yowe,D.L., Bartholomew,J.C., Wehr,C.M., Do,K.L., Park,J.Y. and Ames,B.N. (1997) Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc. Natl Acad. Sci. USA, 94, 3064–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara E., Smith,R., Parry,D., Tahara,H., Stone,S. and Peters,G. (1996) Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol., 16, 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J.W., Adami,G.R., Wei,N., Keyomarsi,K. and Elledge,S.J. (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell, 75, 805–816. [DOI] [PubMed] [Google Scholar]
- Hayflick L. and Moorehead,P. (1961) The serial cultivation of human diploid strains. Exp. Cell Res., 25, 585–621. [DOI] [PubMed] [Google Scholar]
- Kastan M.B., Onyekwere,O., Sidransky,D., Vogelstein,B. and Craig,R.W. (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res., 51, 6304–6311. [PubMed] [Google Scholar]
- Kinnula V.L. and Hassinen,I.E. (1981) Effects of hypoxia and fasting on the cytochrome concentration in intestinal epithelial villous cell mitochondria. Role of changes in the life-span of the cells. Acta Physiol. Scand., 112, 387–393. [DOI] [PubMed] [Google Scholar]
- Lowe S.W., Schmitt,E.M., Smith,S.W., Osborne,B.A. and Jacks,T. (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature, 362, 847–849. [DOI] [PubMed] [Google Scholar]
- Luo Y., Hurwitz,J. and Massague,J. (1995) Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature, 375, 159–161. [DOI] [PubMed] [Google Scholar]
- Martin G.M., Austad,S.N. and Johnson,T.E. (1996) Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nature Genet., 13, 25–34. [DOI] [PubMed] [Google Scholar]
- Noda A., Ning,Y., Venable,S.F., Pereira-Smith,O.M. and Smith,J.R. (1994) Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res., 211, 90–98. [DOI] [PubMed] [Google Scholar]
- Orr W.C. and Sohal,R.S. (1994) Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science, 263, 1128–1130. [DOI] [PubMed] [Google Scholar]
- Parkes T.L., Elia,A.J., Dickinson,D., Hilliker,A.J., Phillips,J.P. and Boulianne,G.L. (1998) Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nature Genet., 19, 171–174. [DOI] [PubMed] [Google Scholar]
- Perkins N.D., Felzien,L.K., Betts,J.C., Leung,K., Beach,D.H. and Nabel,G.J. (1997) Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science, 275, 523–527. [DOI] [PubMed] [Google Scholar]
- Polyak K., Waldman,T., He,T.C., Kinzler,K.W. and Vogelstein,B. (1996) Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev., 10, 1945–1952. [DOI] [PubMed] [Google Scholar]
- Polyak K., Xia,Y., Zweier,J.L., Kinzler,K.W. and Vogelstein,B. (1997) A model for p53-induced apoptosis. Nature, 389, 300–305. [DOI] [PubMed] [Google Scholar]
- Qiu X., Forman,H.J., Schonthal,A.H. and Cadenas,E. (1996) Induction of p21 mediated by reactive oxygen species formed during the metabolism of aziridinylbenzoquinones by HCT116 cells. J. Biol. Chem., 271, 31915–31921. [DOI] [PubMed] [Google Scholar]
- Rossi F.M., Guicherit,O.M., Spicher,A., Kringstein,A.M., Fatyol,K., Blakely,B.T. and Blau,H.M. (1998) Tetracycline-regulatable factors with distinct dimerization domains allow reversible growth inhibition by p16. Nature Genet., 20, 389–393. [DOI] [PubMed] [Google Scholar]
- Royall J.A. and Ischiropoulos,H. (1993) Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys., 302, 348–355. [DOI] [PubMed] [Google Scholar]
- Sager R. (1991) Senescence as a mode of tumor suppression. Environ. Health Perspect., 93, 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon,G.J. and Beach,D. (1993) A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature, 366, 704–707. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lin,A.W., McCurrach,M.E., Beach,D. and Lowe,S.W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell, 88, 593–602. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev., 9, 1149–1163. [DOI] [PubMed] [Google Scholar]
- Sionov R.V. and Haupt,Y. (1999) The cellular response to p53: the decision between life and death. Oncogene, 18, 6145–6157. [DOI] [PubMed] [Google Scholar]
- Smith J.R. and Pereira-Smith,O.M. (1996) Replicative senescence: implications for in vivo aging and tumor suppression. Science, 273, 63–67. [DOI] [PubMed] [Google Scholar]
- Snowden A.W., Anderson,L.A., Webster,G.A. and Perkins,N.D. (2000) A novel transcriptional repression domain mediates p21WAF1/CIP1 induction of p300 transactivation. Mol. Cell. Biol., 20, 2676–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F.J., Roederer,M. and Herzenberg,L.A. (1990) Intracellular thiols regulate activation of nuclear factor κB and transcription of human immunodeficiency virus. Proc. Natl Acad. Sci. USA, 87, 9943–9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G.H., Drullinger,L.F., Soulard,A. and Dulic,V. (1999) Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell. Biol., 19, 2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue M.M., Shin,D.Y., Lee,S.W. and Aaronson,S.A. (1997) Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl Acad. Sci. USA, 94, 9648–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrbom L., Nister,M. and Westermark,B. (1997) Induction of senescence in human malignant glioma cells by p16INK4A. Oncogene, 15, 505–514. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T., Saretzki,G., Docke,W. and Lotze,C. (1995) Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res., 220, 186–193. [DOI] [PubMed] [Google Scholar]
- Waga S., Hannon,G.J., Beach,D. and Stillman,B. (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature, 369, 574–578. [DOI] [PubMed] [Google Scholar]
- Waldman T., Kinzler,K.W. and Vogelstein,B. (1995) p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res., 55, 5187–5190. [PubMed] [Google Scholar]
- Waldman T., Lengauer,C., Kinzler,K.W. and Vogelstein,B. (1996) Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature, 381, 713–716. [DOI] [PubMed] [Google Scholar]
- Wang Y., Blandino,G. and Givol,D. (1999) Induced p21waf expression in H1299 cell line promotes cell senescence and protects against cytotoxic effect of radiation and doxorubicin. Oncogene, 18, 2643–2649. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang,H. and Beach,D. (1992) D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell, 71, 505–514. [DOI] [PubMed] [Google Scholar]
- Xu H.J. et al. (1997) Reexpression of the retinoblastoma protein in tumor cells induces senescence and telomerase inhibition. Oncogene, 15, 2589–2596. [DOI] [PubMed] [Google Scholar]
- Zhou B.P., Liao,Y., Xia,W., Spohn,B., Lee,M.H. and Hung,M.C. (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nature Cell Biol., 3, 245–252. [DOI] [PubMed] [Google Scholar]
- Zhu J., Woods,D., McMahon,M. and Bishop,J.M. (1998) Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev., 12, 2997–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]