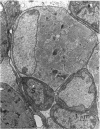
Abstract
Quantitative analyses of the satellite cell content in the biceps brachii muscle of mice genetically selected for high (QL) and low (QS) bodyweight showed that selection alters the total number of satellite cell nuclei rather than the relative proportions of nuclei in the myofibre and satellite cell populations. These findings are in accordance with those previously published for other tissues of these mice and support the hypothesis that regulatory mechanisms remain unaltered by selection pressure. Size at birth, however, is a reflection of nutritional status as well as genetic background, and comparisons between differently sized littermates within each of the lines showed a significant increase in satellite cell density in larger compared with smaller individuals. These differences between littermates were not accompanied by any alteration in myofibre nuclear density. It is therefore suggested that whilst both genetic and nutritional factors exert their effects on muscle growth through an influence on satellite cell division, both do so at different stages during the programme of satellite cell differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguas A. P. The use of osmium tetroxide-potassium ferrocyanide as an extracellular tracer in electron microscopy. Stain Technol. 1982 Mar;57(2):69–73. doi: 10.3109/10520298209066530. [DOI] [PubMed] [Google Scholar]
- Allen R. E., Merkel R. A., Young R. B. Cellular aspects of muscle growth: myogenic cell proliferation. J Anim Sci. 1979 Jul;49(1):115–127. doi: 10.2527/jas1979.491115x. [DOI] [PubMed] [Google Scholar]
- Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol. 1986 May;115(1):140–147. doi: 10.1016/0012-1606(86)90235-6. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 1986 May;115(1):129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat Rec. 1975 Jun;182(2):215–235. doi: 10.1002/ar.1091820207. [DOI] [PubMed] [Google Scholar]
- Campion D. R., Marks H. L., Reagan J. O., Barrett J. B. Composition and muscle cellularity of Japanese Quail after selection for high body weight under an optimal or suboptimal nutritional environment. Poult Sci. 1982 Feb;61(2):212–217. doi: 10.3382/ps.0610212. [DOI] [PubMed] [Google Scholar]
- Campion D. R., Marks H. L., Richardson L. R. An analysis of satellite cell content in the semimembranosus muscle of Japanese quail (Coturnix coturnix japonica) selected for rapid growth. Acta Anat (Basel) 1982;112(1):9–13. doi: 10.1159/000145491. [DOI] [PubMed] [Google Scholar]
- Campion D. R. The muscle satellite cell: a review. Int Rev Cytol. 1984;87:225–251. doi: 10.1016/s0074-7696(08)62444-4. [DOI] [PubMed] [Google Scholar]
- Cardasis C. A., Cooper G. W. An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: a satellite cell-muscle fiber growth unit. J Exp Zool. 1975 Mar;191(3):347–358. doi: 10.1002/jez.1401910305. [DOI] [PubMed] [Google Scholar]
- Cossu G., Ranaldi G., Senni M. I., Molinaro M., Vivarelli E. 'Early' mammalian myoblasts are resistant to phorbol ester-induced block of differentiation. Development. 1988 Jan;102(1):65–69. doi: 10.1242/dev.102.1.65. [DOI] [PubMed] [Google Scholar]
- ENESCO M., PUDDY D. INCREASE IN THE NUMBER OF NUCLEI AND WEIGHT IN SKELETAL MUSCLE OF RATS OF VARIOUS AGES. Am J Anat. 1964 Mar;114:235–244. doi: 10.1002/aja.1001140204. [DOI] [PubMed] [Google Scholar]
- Falconer D. S., Gauld I. K., Roberts R. C. Cell numbers and cell sizes in organs of mice selected for large and small body size. Genet Res. 1978 Jun;31(3):287–301. doi: 10.1017/s0016672300018061. [DOI] [PubMed] [Google Scholar]
- Falconer D. S. Replicated selection for body weight in mice. Genet Res. 1973 Dec;22(3):291–321. doi: 10.1017/s0016672300013094. [DOI] [PubMed] [Google Scholar]
- Fowler S. P., Campion D. R., Marks H. L., Reagan J. O. An analysis of skeletal muscle response to selection for rapid growth in Japanese quail (Coturnix coturnix Japonica). Growth. 1980 Sep;44(3):235–252. [PubMed] [Google Scholar]
- Gibson M. C., Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve. 1983 Oct;6(8):574–580. doi: 10.1002/mus.880060807. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAREN A. GENETIC AND ENVIRONMENTAL EFFECTS ON FOETAL AND PLACENTAL GROWTH IN MICE. J Reprod Fertil. 1965 Feb;9:79–98. doi: 10.1530/jrf.0.0090079. [DOI] [PubMed] [Google Scholar]
- Moss F. P., Leblond C. P. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971 Aug;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Moss F. P. The relationship between the dimensions of the fibres and the number of nuclei during restricted growth, degrowth and compensatory growth of skeletal muscle. Am J Anat. 1968 May;122(3):565–571. doi: 10.1002/aja.1001220309. [DOI] [PubMed] [Google Scholar]
- Ontell M., Dunn R. F. Neonatal muscle growth: a quantitative study. Am J Anat. 1978 Aug;152(4):539–555. doi: 10.1002/aja.1001520408. [DOI] [PubMed] [Google Scholar]
- Ontell M. Muscle satellite cells: a validated technique for light microscopic identification and a quantitative study of changes in their population following denervation. Anat Rec. 1974 Feb;178(2):211–227. doi: 10.1002/ar.1091780206. [DOI] [PubMed] [Google Scholar]
- Ontell M. Muscle satellite cells: a validated technique for light microscopic identification and a quantitative study of changes in their population following denervation. Anat Rec. 1974 Feb;178(2):211–227. doi: 10.1002/ar.1091780206. [DOI] [PubMed] [Google Scholar]
- Penney R. K., Prentis P. F., Marshall P. A., Goldspink G. Differentiation of muscle and the determination of ultimate tissue size. Cell Tissue Res. 1983;228(2):375–388. doi: 10.1007/BF00204886. [DOI] [PubMed] [Google Scholar]
- Purchas R. W., Romsos D. R., Allen R. E., Merkel R. A. Muscle growth and satellite cell proliferative activity in obese (OB/OB) mice. J Anim Sci. 1985 Mar;60(3):644–651. doi: 10.2527/jas1985.603644x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge G. J. Differences in body compositions, growth and food intakes between mice which have been selected for a small and large body size. Br J Nutr. 1981 Nov;46(3):441–450. doi: 10.1079/bjn19810052. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H., Hellhammer U. The number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec. 1977 Oct;189(2):169–175. doi: 10.1002/ar.1091890204. [DOI] [PubMed] [Google Scholar]
- Schultz E. A quantitative study of the satellite cell population in postnatal mouse lumbrical muscle. Anat Rec. 1974 Dec;180(4):589–595. doi: 10.1002/ar.1091800405. [DOI] [PubMed] [Google Scholar]