Abstract
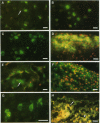
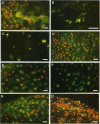
Connective tissues synthesise and secrete a family of matrix metalloproteinases (MMPs; collagenases, gelatinases and stromelysins) capable of degrading all the components of connective tissue matrices at physiological pH. We document the patterns of synthesis and distribution of MMPs and the tissue inhibitor of metalloproteinases-1 (TIMP-1) within the developing rabbit mandibular condyle using immunofluorescence microscopy. MMPs and TIMP-1 were detected both as bright intracellular accumulations within Golgi vesicles and also as diffuse matrix-bound extracellular deposits. Cells in the articular zone, proliferative zone, condylar cartilage and bone of the mandibular ramus were shown to produce all 3 classes of MMPs and TIMP-1 with the exception of stromelysin, which was not synthesised by cells of the bone of spongiosum. Temporal synthesis of MMPs and TIMP-1 within these regions varied during the period 18 d postcoitum to 14 d postnatum. Our results document unique patterns of MMP and TIMP-1 synthesis during embryonic and early postnatal development of condylar cartilage and support the concept that cells synthesise and secrete MMPs and TIMP-1 before undergoing proliferation and hypertrophy. A comparison of these results with data in the rabbit growth plate show many similarities, but some differences exist that probably reflect differences in the modes of growth of the 2 cartilages.
Full text
PDF


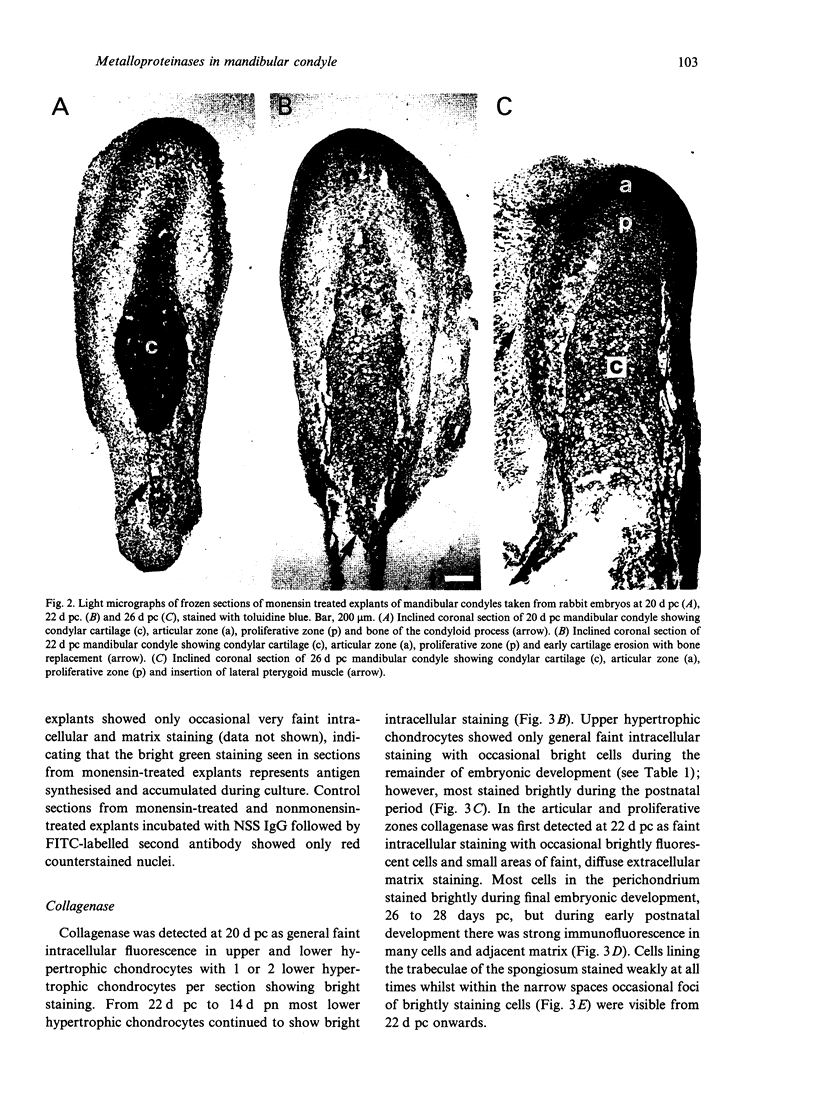
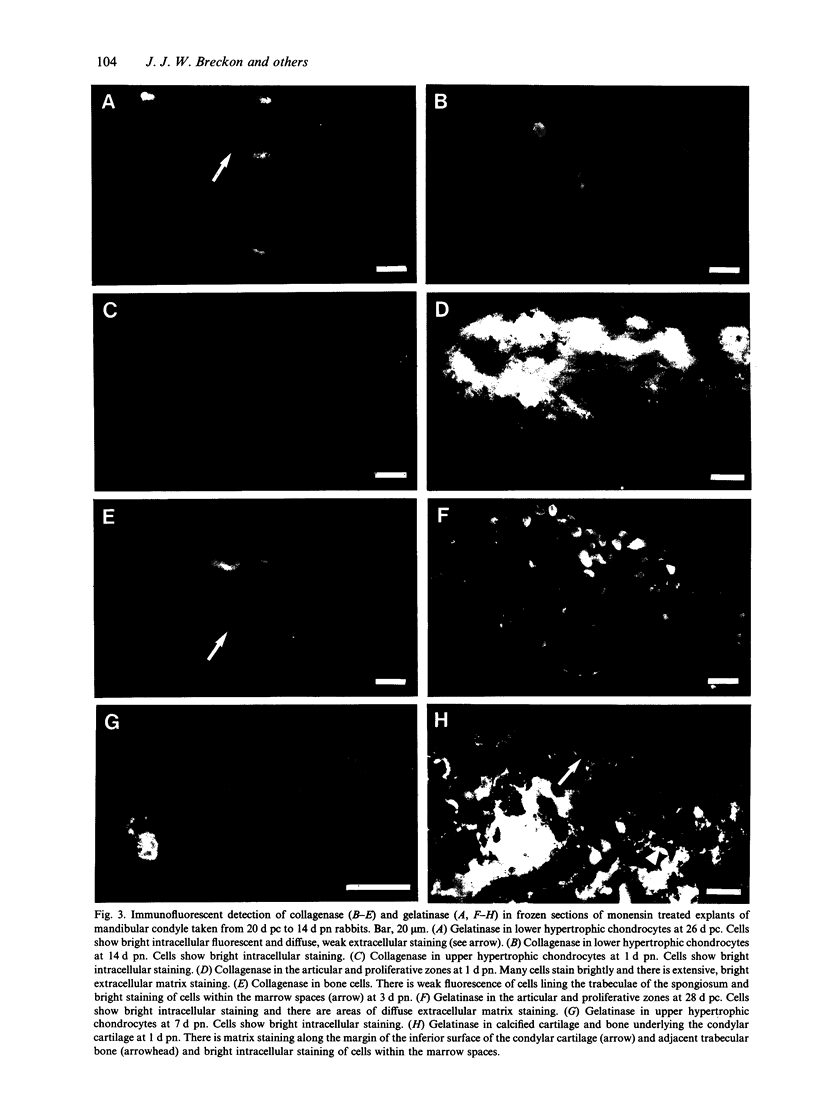

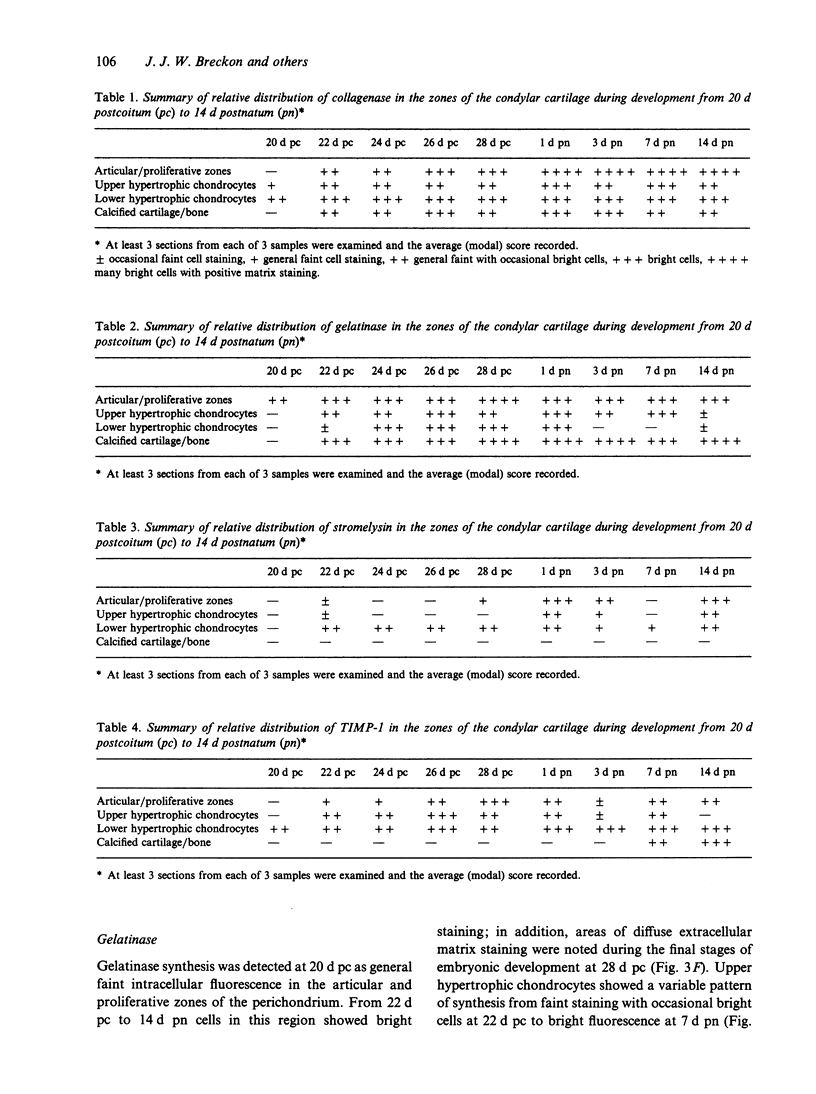




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson I., Berman I., Pita J. C. Proteoglycans from rabbit articular and growth plate cartilage. Ultracentrifugation, gel chromatography, and electron microscopy. J Biol Chem. 1983 Jul 25;258(14):8915–8921. [PubMed] [Google Scholar]
- BHASKAR S. N. Growth pattern of the rat mandible from 13 days insemination age to 30 days after birth. Am J Anat. 1953 Jan;92(1):1–53. doi: 10.1002/aja.1000920102. [DOI] [PubMed] [Google Scholar]
- Blackwood H. J. Growth of the mandibular condyle of the rat studied with tritiated thymidine. Arch Oral Biol. 1966 May;11(5):493–500. doi: 10.1016/0003-9969(66)90155-5. [DOI] [PubMed] [Google Scholar]
- Blumenthal N. C., Posner A. S., Silverman L. D., Rosenberg L. C. Effect of proteoglycans on in vitro hydroxyapatite formation. Calcif Tissue Int. 1979 Mar 13;27(1):75–82. doi: 10.1007/BF02441164. [DOI] [PubMed] [Google Scholar]
- Brown C. C., Hembry R. M., Reynolds J. J. Immunolocalization of metalloproteinases and their inhibitor in the rabbit growth plate. J Bone Joint Surg Am. 1989 Apr;71(4):580–593. [PubMed] [Google Scholar]
- Buckwalter J. A. Proteoglycan structure in calcifying cartilage. Clin Orthop Relat Res. 1983 Jan-Feb;(172):207–232. [PubMed] [Google Scholar]
- Cawston T. E., Murphy G., Mercer E., Galloway W. A., Hazleman B. L., Reynolds J. J. The interaction of purified rabbit bone collagenase with purified rabbit bone metalloproteinase inhibitor. Biochem J. 1983 May 1;211(2):313–318. doi: 10.1042/bj2110313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Reilly J. J., Jr, Kobzik L. Role of plasminogen activator in degradation of extracellular matrix protein by live human alveolar macrophages. Am Rev Respir Dis. 1988 Feb;137(2):412–419. doi: 10.1164/ajrccm/137.2.412. [DOI] [PubMed] [Google Scholar]
- De Bernard B., Stagni N., Colautti I., Vittur F., Bonucci E. Glycosaminoglycans and endochondral calcification. Clin Orthop Relat Res. 1977 Jul-Aug;(126):285–291. [PubMed] [Google Scholar]
- Dean D. D., Muniz O. E., Berman I., Pita J. C., Carreno M. R., Woessner J. F., Jr, Howell D. S. Localization of collagenase in the growth plate of rachitic rats. J Clin Invest. 1985 Aug;76(2):716–722. doi: 10.1172/JCI112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. D., Muniz O. E., Woessner J. F., Jr, Howell D. S. Production of collagenase and tissue inhibitor of metalloproteinases (TIMP) by rat growth plates in culture. Matrix. 1990 Oct;10(5):320–330. doi: 10.1016/s0934-8832(11)80188-5. [DOI] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Duterloo H. S., Jansen H. W. Chondrogenesis and osteogenesis in the mandibular condylar blastema. Rep Congr Eur Orthod Soc. 1969:109–118. [PubMed] [Google Scholar]
- Ehrlich M. G., Tebor G. B., Armstrong A. L., Mankin H. J. Comparative study of neutral proteoglycanase activity by growth plate zone. J Orthop Res. 1985;3(3):269–276. doi: 10.1002/jor.1100030303. [DOI] [PubMed] [Google Scholar]
- Flenniken A. M., Williams B. R. Developmental expression of the endogenous TIMP gene and a TIMP-lacZ fusion gene in transgenic mice. Genes Dev. 1990 Jul;4(7):1094–1106. doi: 10.1101/gad.4.7.1094. [DOI] [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilovic J., Hembry R. M., Reynolds J. J., Murphy G. Tissue inhibitor of metalloproteinases (TIMP) regulates extracellular type I collagen degradation by chondrocytes and endothelial cells. J Cell Sci. 1987 Mar;87(Pt 2):357–362. doi: 10.1242/jcs.87.2.357. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Grant W. T., Sussman M. D., Balian G. A disulfide-bonded short chain collagen synthesized by degenerative and calcifying zones of bovine growth plate cartilage. J Biol Chem. 1985 Mar 25;260(6):3798–3803. [PubMed] [Google Scholar]
- Heath J. K., Atkinson S. J., Meikle M. C., Reynolds J. J. Mouse osteoblasts synthesize collagenase in response to bone resorbing agents. Biochim Biophys Acta. 1984 Nov 6;802(1):151–154. doi: 10.1016/0304-4165(84)90046-1. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Cawston T. E., Dingle J. T., Reynolds J. J. Characterization of a specific antiserum for mammalian collagenase from several species: immunolocalization of collagenase in rabbit chondrocytes and uterus. J Cell Sci. 1986 Mar;81:105–123. doi: 10.1242/jcs.81.1.105. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Reynolds J. J. Immunolocalization of tissue inhibitor of metalloproteinases (TIMP) in human cells. Characterization and use of a specific antiserum. J Cell Sci. 1985 Feb;73:105–119. doi: 10.1242/jcs.73.1.105. [DOI] [PubMed] [Google Scholar]
- Luder H. U., Leblond C. P., von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988 Jul;182(3):197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- Marchi F., Luder H. U., Leblond C. P. Changes in cells's secretory organelles and extracellular matrix during endochondral ossification in the mandibular condyle of the growing rat. Am J Anat. 1991 Jan;190(1):41–73. doi: 10.1002/aja.1001900106. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Meikle M. C., Bord S., Hembry R. M., Compston J., Croucher P. I., Reynolds J. J. Human osteoblasts in culture synthesize collagenase and other matrix metalloproteinases in response to osteotropic hormones and cytokines. J Cell Sci. 1992 Dec;103(Pt 4):1093–1099. doi: 10.1242/jcs.103.4.1093. [DOI] [PubMed] [Google Scholar]
- Mercier P., Ehrlich M. G., Armstrong A., Mankin H. J. Elaboration of neutral proteoglycanase by growth-plate tissue cultures. J Bone Joint Surg Am. 1987 Jan;69(1):76–82. [PubMed] [Google Scholar]
- Mignatti P., Robbins E., Rifkin D. B. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986 Nov 21;47(4):487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
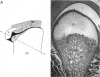
- Mills D. K., Daniel J. C., Scapino R. Histological features and in-vitro proteoglycan synthesis in the rabbit craniomandibular joint disc. Arch Oral Biol. 1988;33(3):195–202. doi: 10.1016/0003-9969(88)90045-3. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Galloway W. A., Barnes M. J., Bunning R. A., Mercer E., Reynolds J. J., Burgeson R. E. Metalloproteinases from rabbit bone culture medium degrade types IV and V collagens, laminin and fibronectin. Biochem J. 1981 Dec 1;199(3):807–811. doi: 10.1042/bj1990807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., McGarrity A. M., Reynolds J. J., Henderson B. Gelatinase (type IV collagenase) immunolocalization in cells and tissues: use of an antiserum to rabbit bone gelatinase that identifies high and low Mr forms. J Cell Sci. 1989 Mar;92(Pt 3):487–495. doi: 10.1242/jcs.92.3.487. [DOI] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Reynolds J. J. Characterization of a specific antiserum to rabbit stromelysin and demonstration of the synthesis of collagenase and stromelysin by stimulated rabbit articular chondrocytes. Coll Relat Res. 1986 Oct;6(4):351–363. doi: 10.1016/s0174-173x(86)80005-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Nagase H., Brinckerhoff C. E. Relationship of procollagenase activator, stromelysin and matrix metalloproteinase 3. Coll Relat Res. 1988 Jul;8(4):389–391. doi: 10.1016/s0174-173x(88)80009-8. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ward R., Hembry R. M., Reynolds J. J., Kühn K., Tryggvason K. Characterization of gelatinase from pig polymorphonuclear leucocytes. A metalloproteinase resembling tumour type IV collagenase. Biochem J. 1989 Mar 1;258(2):463–472. doi: 10.1042/bj2580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Brinckerhoff C. E., Vater C. A., Harris E. D., Jr Biosynthesis and secretion of procollagenase by rabbit synovial fibroblasts. Inhibition of procollagenase secretion by monensin and evidence for glycosylation of procollagenase. Biochem J. 1983 Aug 15;214(2):281–288. doi: 10.1042/bj2140281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S., Hogan B. L., Wills A. J., Heath J. K., Edwards D. R. Developmental expression of tissue inhibitor of metalloproteinase (TIMP) RNA. Development. 1989 Mar;105(3):575–583. doi: 10.1242/dev.105.3.575. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Sodek J. Initial characterization of a neutral metalloproteinase, active on native 3/4-collagen fragments, synthesized by ROS 17/2.8 osteoblastic cells, periodontal fibroblasts, and identified in gingival crevicular fluid. J Dent Res. 1987 Jul;66(7):1271–1282. doi: 10.1177/00220345870660071201. [DOI] [PubMed] [Google Scholar]
- Reynolds J. J., Hembry R. M. Immunolocalization of metalloproteinases and TIMP in normal and pathological tissues. Matrix Suppl. 1992;1:375–382. [PubMed] [Google Scholar]
- Sahlberg C., Reponen P., Tryggvason K., Thesleff I. Association between the expression of murine 72 kDa type IV collagenase by odontoblasts and basement membrane degradation during mouse tooth development. Arch Oral Biol. 1992 Dec;37(12):1021–1030. doi: 10.1016/0003-9969(92)90034-6. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol. 1985 Feb;100(2):598–605. doi: 10.1083/jcb.100.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J. Identification and partial characterization of an inhibitor of collagenase from rabbit bone. Biochem J. 1977 Nov 1;167(2):353–360. doi: 10.1042/bj1670353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Thomson B. M., Atkinson S. J., McGarrity A. M., Hembry R. M., Reynolds J. J., Meikle M. C. Type I collagen degradation by mouse calvarial osteoblasts stimulated with 1,25-dihydroxyvitamin D-3: evidence for a plasminogen-plasmin-metalloproteinase activation cascade. Biochim Biophys Acta. 1989 Nov 20;1014(2):125–132. doi: 10.1016/0167-4889(89)90024-4. [DOI] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata S., Ito Y., Tanaka R., Shimizu S. Gelatinases of metastatic cell lines of murine colonic carcinoma as detected by substrate-gel electrophoresis. Biochem Biophys Res Commun. 1988 Feb 29;151(1):158–162. doi: 10.1016/0006-291x(88)90573-6. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Tanaka R., Ito Y., Shimizu S. Gelatinases of murine metastatic tumor cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):228–234. doi: 10.1016/s0006-291x(89)80202-5. [DOI] [PubMed] [Google Scholar]
- Yasui N., Hori H., Nagai Y. Production of collagenase inhibitor by the growth cartilage of embryonic chick bone: isolation and partial characterization. Coll Relat Res. 1981;1(1):59–72. doi: 10.1016/s0174-173x(80)80008-2. [DOI] [PubMed] [Google Scholar]