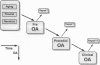
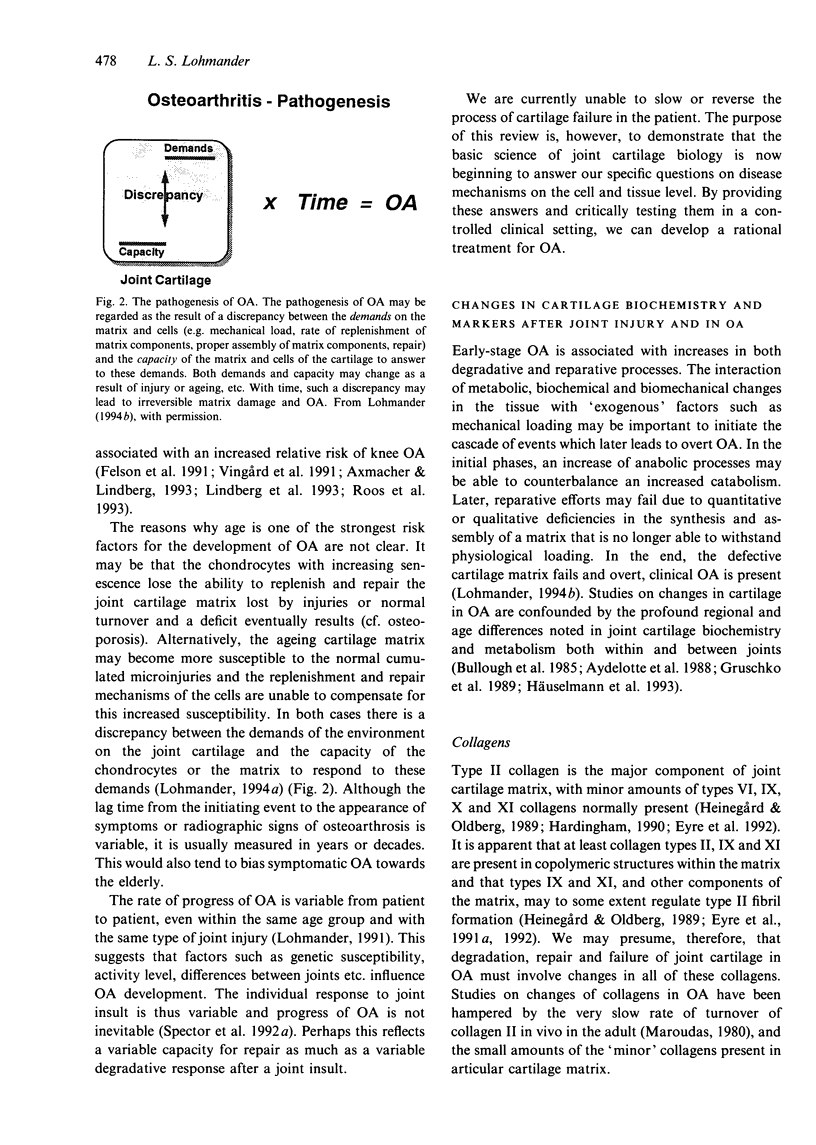
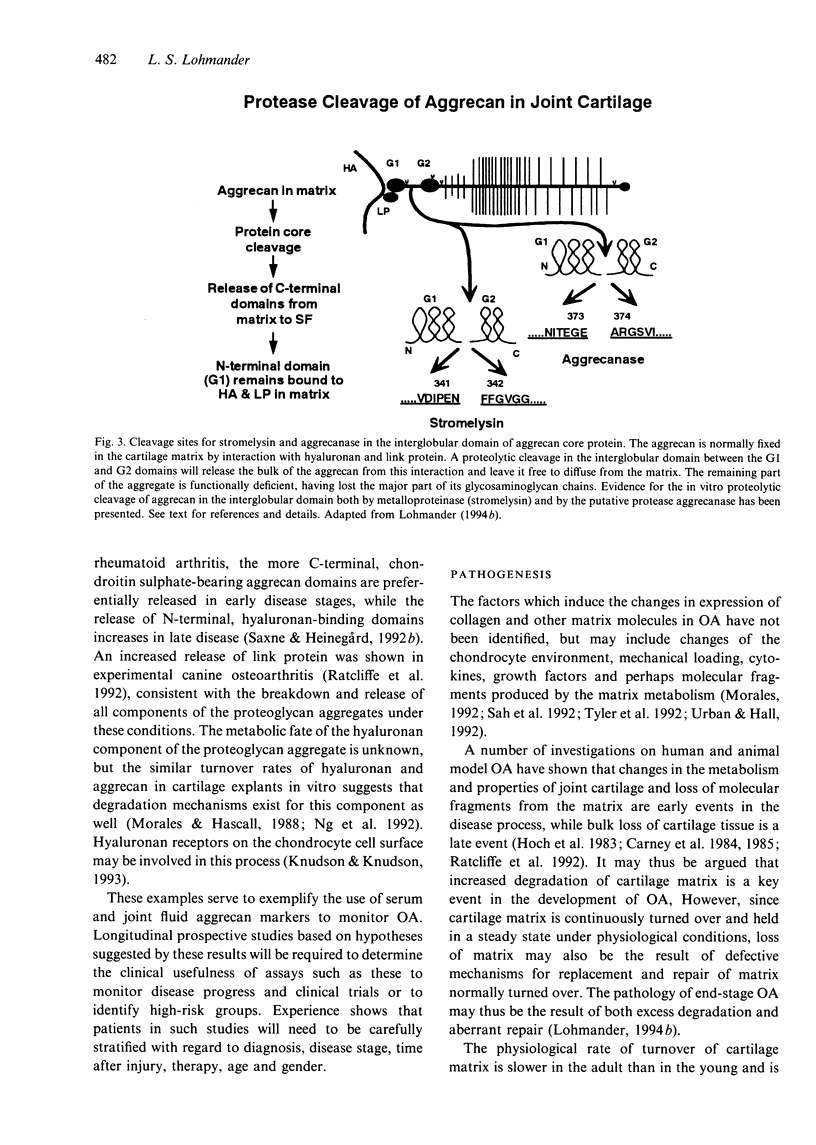
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Brandt K. D. Hypertrophic repair of canine articular cartilage in osteoarthritis after anterior cruciate ligament transection. J Rheumatol. 1991 Mar;18(3):428–435. [PubMed] [Google Scholar]
- Adams M. E. Cartilage hypertrophy following canine anterior cruciate ligament transection differs among different areas of the joint. J Rheumatol. 1989 Jun;16(6):818–824. [PubMed] [Google Scholar]
- Aigner T., Bertling W., Stöss H., Weseloh G., von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993 Mar;91(3):829–837. doi: 10.1172/JCI116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner T., Reichenberger E., Bertling W., Kirsch T., Stöss H., von der Mark K. Type X collagen expression in osteoarthritic and rheumatoid articular cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(4):205–211. doi: 10.1007/BF02899263. [DOI] [PubMed] [Google Scholar]
- Aigner T., Stöss H., Weseloh G., Zeiler G., von der Mark K. Activation of collagen type II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(6):337–345. doi: 10.1007/BF02899701. [DOI] [PubMed] [Google Scholar]
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman R. D., Dean D. D., Muniz O. E., Howell D. S. Prophylactic treatment of canine osteoarthritis with glycosaminoglycan polysulfuric acid ester. Arthritis Rheum. 1989 Jun;32(6):759–766. doi: 10.1002/anr.1780320614. [DOI] [PubMed] [Google Scholar]
- Altman R. D., Fries J. F., Bloch D. A., Carstens J., Cooke T. D., Genant H., Gofton P., Groth H., McShane D. J., Murphy W. A. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987 Nov;30(11):1214–1225. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Axmacher B., Lindberg H. Coxarthrosis in farmers. Clin Orthop Relat Res. 1993 Feb;(287):82–86. [PubMed] [Google Scholar]
- Aydelotte M. B., Greenhill R. R., Kuettner K. E. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tissue Res. 1988;18(3):223–234. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- Badley E. M., Tennant A. Changing profile of joint disorders with age: findings from a postal survey of the population of Calderdale, West Yorkshire, United Kingdom. Ann Rheum Dis. 1992 Mar;51(3):366–371. doi: 10.1136/ard.51.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsson S. Simultaneous preparation and quantitation of proteoglycans by precipitation with alcian blue. Anal Biochem. 1993 May 1;210(2):282–291. doi: 10.1006/abio.1993.1197. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Fife R. S., Braunstein E. M., Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum. 1991 Nov;34(11):1381–1386. doi: 10.1002/art.1780341106. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Myers S. L., Burr D., Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991 Dec;34(12):1560–1570. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- Brandt K. D. Should osteoarthritis be treated with nonsteroidal anti-inflammatory drugs? Rheum Dis Clin North Am. 1993 Aug;19(3):697–712. [PubMed] [Google Scholar]
- Brandt K. D., Thonar E. J. Lack of association between serum keratan sulfate concentrations and cartilage changes of osteoarthritis after transection of the anterior cruciate ligament in the dog. Arthritis Rheum. 1989 May;32(5):647–651. doi: 10.1002/anr.1780320521. [DOI] [PubMed] [Google Scholar]
- Brierley V. H., Ayad S., Grant M. E. Types II, VI and IX collagens in normal and osteoarthrotic human articular cartilage. Biochem Soc Trans. 1991 Nov;19(4):379S–379S. doi: 10.1042/bst019379s. [DOI] [PubMed] [Google Scholar]
- Brower A. C. Use of the radiograph to measure the course of rheumatoid arthritis. The gold standard versus fool's gold. Arthritis Rheum. 1990 Mar;33(3):316–324. doi: 10.1002/art.1780330303. [DOI] [PubMed] [Google Scholar]
- Bullough P. G., Yawitz P. S., Tafra L., Boskey A. L. Topographical variations in the morphology and biochemistry of adult canine tibial plateau articular cartilage. J Orthop Res. 1985;3(1):1–16. doi: 10.1002/jor.1100030101. [DOI] [PubMed] [Google Scholar]
- Bulstra S. K., Buurman W. A., Walenkamp G. H., Van der Linden A. J. Metabolic characteristics of in vitro cultured human chondrocytes in relation to the histopathologic grade of osteoarthritis. Clin Orthop Relat Res. 1989 May;(242):294–302. [PubMed] [Google Scholar]
- Burkhardt D., Ghosh P. Laboratory evaluation of antiarthritic drugs as potential chondroprotective agents. Semin Arthritis Rheum. 1987 Nov;17(2 Suppl 1):3–34. [PubMed] [Google Scholar]
- Campion G. V., McCrae F., Schnitzer T. J., Lenz M. E., Dieppe P. A., Thonar E. J. Levels of keratan sulfate in the serum and synovial fluid of patients with osteoarthritis of the knee. Arthritis Rheum. 1991 Oct;34(10):1254–1259. doi: 10.1002/art.1780341008. [DOI] [PubMed] [Google Scholar]
- Carney S. L., Billingham M. E., Caterson B., Ratcliffe A., Bayliss M. T., Hardingham T. E., Muir H. Changes in proteoglycan turnover in experimental canine osteoarthritic cartilage. Matrix. 1992 Apr;12(2):137–147. doi: 10.1016/s0934-8832(11)80055-7. [DOI] [PubMed] [Google Scholar]
- Carney S. L., Billingham M. E., Muir H., Sandy J. D. Demonstration of increased proteoglycan turnover in cartilage explants from dogs with experimental osteoarthritis. J Orthop Res. 1984;2(3):201–206. doi: 10.1002/jor.1100020301. [DOI] [PubMed] [Google Scholar]
- Carney S. L., Billingham M. E., Muir H., Sandy J. D. Structure of newly synthesised (35S)-proteoglycans and (35S)-proteoglycan turnover products of cartilage explant cultures from dogs with experimental osteoarthritis. J Orthop Res. 1985;3(2):140–147. doi: 10.1002/jor.1100030203. [DOI] [PubMed] [Google Scholar]
- Carroll G. J., Bell M. C., Laing B. A., McCappin S., Blumer C., Leslie A. Reduction of the concentration and total amount of keratan sulphate in synovial fluid from patients with osteoarthritis during treatment with piroxicam. Ann Rheum Dis. 1992 Jul;51(7):850–854. doi: 10.1136/ard.51.7.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll G. Measurement of sulphated glycosaminoglycans and proteoglycan fragments in arthritic synovial fluid. Ann Rheum Dis. 1989 Jan;48(1):17–24. doi: 10.1136/ard.48.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case J. P., Lafyatis R., Remmers E. F., Kumkumian G. K., Wilder R. L. Transin/stromelysin expression in rheumatoid synovium. A transformation-associated metalloproteinase secreted by phenotypically invasive synoviocytes. Am J Pathol. 1989 Dec;135(6):1055–1064. [PMC free article] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R., Couchman J. R. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985 Feb;44(2):386–393. [PubMed] [Google Scholar]
- Chan W. P., Lang P., Stevens M. P., Sack K., Majumdar S., Stoller D. W., Basch C., Genant H. K. Osteoarthritis of the knee: comparison of radiography, CT, and MR imaging to assess extent and severity. AJR Am J Roentgenol. 1991 Oct;157(4):799–806. doi: 10.2214/ajr.157.4.1892040. [DOI] [PubMed] [Google Scholar]
- Clark I. M., Powell L. K., Ramsey S., Hazleman B. L., Cawston T. E. The measurement of collagenase, tissue inhibitor of metalloproteinases (TIMP), and collagenase-TIMP complex in synovial fluids from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1993 Mar;36(3):372–379. doi: 10.1002/art.1780360313. [DOI] [PubMed] [Google Scholar]
- Cruz T. F., Malcolm A. J., Adams M. E. The effect of maturation and anterior cruciate ligament transection on the level of keratan sulfate in the serum of dogs. J Rheumatol. 1989 Oct;16(10):1345–1350. [PubMed] [Google Scholar]
- Dahlberg L., Ryd L., Heinegård D., Lohmander L. S. Proteoglycan fragments in joint fluid. Influence of arthrosis and inflammation. Acta Orthop Scand. 1992 Aug;63(4):417–423. doi: 10.3109/17453679209154758. [DOI] [PubMed] [Google Scholar]
- Danielsson L., Lindberg H., Nilsson B. Prevalence of coxarthrosis. Clin Orthop Relat Res. 1984 Dec;(191):110–115. [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989 Aug;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. D., Muniz O. E., Rodriquez I., Carreno M. R., Morales S., Agundez A., Madan M. E., Altman R. D., Annefeld M., Howell D. S. Amelioration of lapine osteoarthritis by treatment with glycosaminoglycan-peptide association complex (Rumalon). Arthritis Rheum. 1991 Mar;34(3):304–313. doi: 10.1002/art.1780340308. [DOI] [PubMed] [Google Scholar]
- Docherty A. J., Murphy G. The tissue metalloproteinase family and the inhibitor TIMP: a study using cDNAs and recombinant proteins. Ann Rheum Dis. 1990 Jun;49 (Suppl 1):469–479. [PubMed] [Google Scholar]
- Dodge G. R., Poole A. R. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989 Feb;83(2):647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M., Watt I., Dieppe P. Influence of primary generalised osteoarthritis on development of secondary osteoarthritis. Lancet. 1983 Jul 2;2(8340):8–11. doi: 10.1016/s0140-6736(83)90003-x. [DOI] [PubMed] [Google Scholar]
- Dougados M., Gueguen A., Nguyen M., Thiesce A., Listrat V., Jacob L., Nakache J. P., Gabriel K. R., Lequesne M., Amor B. Longitudinal radiologic evaluation of osteoarthritis of the knee. J Rheumatol. 1992 Mar;19(3):378–384. [PubMed] [Google Scholar]
- Engström-Laurent A., Hellström S. The role of liver and kidneys in the removal of circulating hyaluronan. An experimental study in the rat. Connect Tissue Res. 1990;24(3-4):219–224. doi: 10.3109/03008209009152150. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Dickson I. R., Van Ness K. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988 Jun 1;252(2):495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., McDevitt C. A., Billingham M. E., Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980 Jun 15;188(3):823–837. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Weis M. A., Moskowitz R. W. Cartilage expression of a type II collagen mutation in an inherited form of osteoarthritis associated with a mild chondrodysplasia. J Clin Invest. 1991 Jan;87(1):357–361. doi: 10.1172/JCI114994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Wu J. J., Woods P. E., Weis M. A. The cartilage collagens and joint degeneration. Br J Rheumatol. 1991;30 (Suppl 1):10–15. [PubMed] [Google Scholar]
- Felson D. T., Anderson J. J., Naimark A., Walker A. M., Meenan R. F. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988 Jul 1;109(1):18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- Felson D. T. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. doi: 10.1093/oxfordjournals.epirev.a036019. [DOI] [PubMed] [Google Scholar]
- Felson D. T., Hannan M. T., Naimark A., Berkeley J., Gordon G., Wilson P. W., Anderson J. Occupational physical demands, knee bending, and knee osteoarthritis: results from the Framingham Study. J Rheumatol. 1991 Oct;18(10):1587–1592. [PubMed] [Google Scholar]
- Felson D. T., Naimark A., Anderson J., Kazis L., Castelli W., Meenan R. F. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987 Aug;30(8):914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- Felson D. T. The course of osteoarthritis and factors that affect it. Rheum Dis Clin North Am. 1993 Aug;19(3):607–615. [PubMed] [Google Scholar]
- Felson D. T., Zhang Y., Anthony J. M., Naimark A., Anderson J. J. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992 Apr 1;116(7):535–539. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- Fife R. S., Brandt K. D., Braunstein E. M., Myers S. L., Katz B. P., Ehlich J., Shelbourne K. D., Kalasinski L. A. The presence of cartilage matrix glycoprotein in serum as determined by immunolocation analysis is not a sensitive indicator of "early" osteoarthritis of the knee. J Lab Clin Med. 1991 Apr;117(4):332–338. [PubMed] [Google Scholar]
- Fife R. S. Identification of cartilage matrix glycoprotein in synovial fluid in human osteoarthritis. Arthritis Rheum. 1988 Apr;31(4):553–556. doi: 10.1002/art.1780310414. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M., Littman B. H. Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991 Sep;34(9):1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- Flannery C. R., Lark M. W., Sandy J. D. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992 Jan 15;267(2):1008–1014. [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Last K., Hardingham T. E., Murphy G., Hamilton J. A. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992 Sep 25;267(27):19470–19474. [PubMed] [Google Scholar]
- Fosang A. J., Tyler J. A., Hardingham T. E. Effect of interleukin-1 and insulin like growth factor-1 on the release of proteoglycan components and hyaluronan from pig articular cartilage in explant culture. Matrix. 1991 Feb;11(1):17–24. doi: 10.1016/s0934-8832(11)80223-4. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Kimpton W. G., Laurent T. C., Cahill R. N., Vakakis N. Uptake and degradation of hyaluronan in lymphatic tissue. Biochem J. 1988 Nov 15;256(1):153–158. doi: 10.1042/bj2560153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadher S. J., Eyre D. R., Wotton S. F., Schmid T. M., Woolley D. E. Degradation of cartilage collagens type II, IX, X and XI by enzymes derived from human articular chondrocytes. Matrix. 1990 Jul;10(3):154–163. doi: 10.1016/s0934-8832(11)80164-2. [DOI] [PubMed] [Google Scholar]
- Grant W. T., Sussman M. D., Balian G. A disulfide-bonded short chain collagen synthesized by degenerative and calcifying zones of bovine growth plate cartilage. J Biol Chem. 1985 Mar 25;260(6):3798–3803. [PubMed] [Google Scholar]
- Gravallese E. M., Darling J. M., Ladd A. L., Katz J. N., Glimcher L. H. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 1991 Sep;34(9):1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- Grushko G., Schneiderman R., Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res. 1989;19(2-4):149–176. doi: 10.3109/03008208909043895. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Bayliss M. T., Rayan V., Noble D. P. Effects of growth factors and cytokines on proteoglycan turnover in articular cartilage. Br J Rheumatol. 1992;31 (Suppl 1):1–6. [PubMed] [Google Scholar]
- Hardingham T., Bayliss M. Proteoglycans of articular cartilage: changes in aging and in joint disease. Semin Arthritis Rheum. 1990 Dec;20(3 Suppl 1):12–33. doi: 10.1016/0049-0172(90)90044-g. [DOI] [PubMed] [Google Scholar]
- Hart D. J., Spector T. D., Brown P., Wilson P., Doyle D. V., Silman A. J. Clinical signs of early osteoarthritis: reproducibility and relation to x ray changes in 541 women in the general population. Ann Rheum Dis. 1991 Jul;50(7):467–470. doi: 10.1136/ard.50.7.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Inerot S., Olsson S. E., Saxne T. Cartilage proteoglycans in degenerative joint disease. J Rheumatol. 1987 May;14(Spec No):110–112. [PubMed] [Google Scholar]
- Heinegård D., Inerot S., Wieslander J., Lindblad G. A method for the quantification of cartilage proteoglycan structures liberated to the synovial fluid during developing degenerative joint disease. Scand J Clin Lab Invest. 1985 Sep;45(5):421–427. doi: 10.1080/00365518509155238. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Hoch D. H., Grodzinsky A. J., Koob T. J., Albert M. L., Eyre D. R. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- Homandberg G. A., Meyers R., Xie D. L. Fibronectin fragments cause chondrolysis of bovine articular cartilage slices in culture. J Biol Chem. 1992 Feb 25;267(6):3597–3604. [PubMed] [Google Scholar]
- Ike R. W. The role of arthroscopy in the differential diagnosis of osteoarthritis of the knee. Rheum Dis Clin North Am. 1993 Aug;19(3):673–696. [PubMed] [Google Scholar]
- Ilic M. Z., Handley C. J., Robinson H. C., Mok M. T. Mechanism of catabolism of aggrecan by articular cartilage. Arch Biochem Biophys. 1992 Apr;294(1):115–122. doi: 10.1016/0003-9861(92)90144-l. [DOI] [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Olsson S. E., Telhag H., Audell L. Proteoglycan alterations during developing experimental osteoarthritis in a novel hip joint model. J Orthop Res. 1991 Sep;9(5):658–673. doi: 10.1002/jor.1100090506. [DOI] [PubMed] [Google Scholar]
- KELLGREN J. H., LAWRENCE J. S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenstein P. L., Malemud C. J., Pathria M. N., Carter J. R., Sheon R. P., Moskowitz R. W. Early-onset primary osteoarthritis and mild chondrodysplasia. Radiographic and pathologic studies with an analysis of cartilage proteoglycans. Arthritis Rheum. 1990 May;33(5):674–684. doi: 10.1002/art.1780330510. [DOI] [PubMed] [Google Scholar]
- Kaye J. J. Radiologic assessment of osteoarthritis. New techniques. Rheum Dis Clin North Am. 1993 Aug;19(3):659–672. [PubMed] [Google Scholar]
- Knudson C. B., Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993 Oct;7(13):1233–1241. [PubMed] [Google Scholar]
- Kramer J. S., Yelin E. H., Epstein W. V. Social and economic impacts of four musculoskeletal conditions. A study using national community-based data. Arthritis Rheum. 1983 Jul;26(7):901–907. doi: 10.1002/art.1780260712. [DOI] [PubMed] [Google Scholar]
- Lawrence R. C., Hochberg M. C., Kelsey J. L., McDuffie F. C., Medsger T. A., Jr, Felts W. R., Shulman L. E. Estimates of the prevalence of selected arthritic and musculoskeletal diseases in the United States. J Rheumatol. 1989 Apr;16(4):427–441. [PubMed] [Google Scholar]
- Lefebvre V., Peeters-Joris C., Vaes G. Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta. 1990 May 22;1052(3):366–378. doi: 10.1016/0167-4889(90)90145-4. [DOI] [PubMed] [Google Scholar]
- Leipold H. R., Goldberg R. L., Lust G. Canine serum keratan sulfate and hyaluronate concentrations. Relationship to age and osteoarthritis. Arthritis Rheum. 1989 Mar;32(3):312–321. doi: 10.1002/anr.1780320313. [DOI] [PubMed] [Google Scholar]
- Lequesne M. Indices of severity and disease activity for osteoarthritis. Semin Arthritis Rheum. 1991 Jun;20(6 Suppl 2):48–54. doi: 10.1016/0049-0172(91)90027-w. [DOI] [PubMed] [Google Scholar]
- Lindberg H., Roos H., Gärdsell P. Prevalence of coxarthrosis in former soccer players. 286 players compared with matched controls. Acta Orthop Scand. 1993 Apr;64(2):165–167. doi: 10.3109/17453679308994561. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Dahlberg L., Ryd L., Heinegård D. Increased levels of proteoglycan fragments in knee joint fluid after injury. Arthritis Rheum. 1989 Nov;32(11):1434–1442. doi: 10.1002/anr.1780321113. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Hoerrner L. A., Dahlberg L., Roos H., Björnsson S., Lark M. W. Stromelysin, tissue inhibitor of metalloproteinases and proteoglycan fragments in human knee joint fluid after injury. J Rheumatol. 1993 Aug;20(8):1362–1368. [PubMed] [Google Scholar]
- Lohmander L. S., Hoerrner L. A., Lark M. W. Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum. 1993 Feb;36(2):181–189. doi: 10.1002/art.1780360207. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Lark M. W., Dahlberg L., Walakovits L. A., Roos H. Cartilage matrix metabolism in osteoarthritis: markers in synovial fluid, serum, and urine. Clin Biochem. 1992 Jun;25(3):167–174. doi: 10.1016/0009-9120(92)90250-v. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S. Markers of cartilage metabolism in arthrosis. A review. Acta Orthop Scand. 1991 Dec;62(6):623–632. doi: 10.3109/17453679108994513. [DOI] [PubMed] [Google Scholar]
- Lohmander S. Turnover of proteoglycans in guinea pig costal cartilage. Arch Biochem Biophys. 1977 Apr 15;180(1):93–101. doi: 10.1016/0003-9861(77)90012-1. [DOI] [PubMed] [Google Scholar]
- Loulakis P., Shrikhande A., Davis G., Maniglia C. A. N-terminal sequence of proteoglycan fragments isolated from medium of interleukin-1-treated articular-cartilage cultures. Putative site(s) of enzymic cleavage. Biochem J. 1992 Jun 1;284(Pt 2):589–593. doi: 10.1042/bj2840589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysholm J., Hamberg P., Gillquist J. The correlation between osteoarthrosis as seen on radiographs and on arthroscopy. Arthroscopy. 1987;3(3):161–165. doi: 10.1016/s0749-8063(87)80058-0. [DOI] [PubMed] [Google Scholar]
- Malemud C. J. The role of growth factors in cartilage metabolism. Rheum Dis Clin North Am. 1993 Aug;19(3):569–580. [PubMed] [Google Scholar]
- Manicourt D. H., Lenz M. E., Thonar E. J. Levels of serum keratan sulfate rise rapidly and remain elevated following anterior cruciate ligament transection in the dog. J Rheumatol. 1991 Dec;18(12):1872–1876. [PubMed] [Google Scholar]
- Manicourt D. H., Pita J. C. Progressive depletion of hyaluronic acid in early experimental osteoarthritis in dogs. Arthritis Rheum. 1988 Apr;31(4):538–544. doi: 10.1002/art.1780310411. [DOI] [PubMed] [Google Scholar]
- Manicourt D. H., Pita J. C., Thonar E. J., Howell D. S. Proteoglycans nondissociatively extracted from different zones of canine normal articular cartilage: variations in the sedimentation profile of aggregates with degree of physiological stress. Connect Tissue Res. 1991;26(4):231–246. doi: 10.3109/03008209109152441. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Maroudas A. Glycosaminoglycan turn-over in articular cartilage. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):293–313. doi: 10.1098/rstb.1975.0054. [DOI] [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- McCachren S. S., Haynes B. F., Niedel J. E. Localization of collagenase mRNA in rheumatoid arthritis synovium by in situ hybridization histochemistry. J Clin Immunol. 1990 Jan;10(1):19–27. doi: 10.1007/BF00917494. [DOI] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J Bone Joint Surg Br. 1976 Feb;58(1):94–101. doi: 10.1302/0301-620X.58B1.131804. [DOI] [PubMed] [Google Scholar]
- McDevitt C. A., Pahl J. A., Ayad S., Miller R. R., Uratsuji M., Andrish J. T. Experimental osteoarthritic articular cartilage is enriched in guanidine soluble type VI collagen. Biochem Biophys Res Commun. 1988 Nov 30;157(1):250–255. doi: 10.1016/s0006-291x(88)80040-8. [DOI] [PubMed] [Google Scholar]
- Mehraban F., Finegan C. K., Moskowitz R. W. Serum keratan sulfate. Quantitative and qualitative comparisons in inflammatory versus noninflammatory arthritides. Arthritis Rheum. 1991 Apr;34(4):383–392. doi: 10.1002/art.1780340403. [DOI] [PubMed] [Google Scholar]
- Middleton J. F., Tyler J. A. Upregulation of insulin-like growth factor I gene expression in the lesions of osteoarthritic human articular cartilage. Ann Rheum Dis. 1992 Apr;51(4):440–447. doi: 10.1136/ard.51.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales T. I., Hascall V. C. Correlated metabolism of proteoglycans and hyaluronic acid in bovine cartilage organ cultures. J Biol Chem. 1988 Mar 15;263(8):3632–3638. [PubMed] [Google Scholar]
- Moskowitz R. W., Howell D. S., Goldberg V. M., Muniz O., Pita J. C. Cartilage proteoglycan alterations in an experimentally induced model of rabbit osteoarthritis. Arthritis Rheum. 1979 Feb;22(2):155–163. doi: 10.1002/art.1780220208. [DOI] [PubMed] [Google Scholar]
- Moskowitz R. W., Reese J. H., Young R. G., Fein-Krantz D., Malemud C. J., Caplan A. I. The effects of Rumalon, a glycosaminoglycan peptide complex, in a partial meniscectomy model of osteoarthritis in rabbits. J Rheumatol. 1991 Feb;18(2):205–209. [PubMed] [Google Scholar]
- Nakata K., Ono K., Miyazaki J., Olsen B. R., Muragaki Y., Adachi E., Yamamura K., Kimura T. Osteoarthritis associated with mild chondrodysplasia in transgenic mice expressing alpha 1(IX) collagen chains with a central deletion. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2870–2874. doi: 10.1073/pnas.90.7.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlich A. G., Wiest I., von der Mark K. Immunohistochemical analysis of interstitial collagens in cartilage of different stages of osteoarthrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(4):249–255. doi: 10.1007/BF02899269. [DOI] [PubMed] [Google Scholar]
- Ng C. K., Handley C. J., Preston B. N., Robinson H. C. The extracellular processing and catabolism of hyaluronan in cultured adult articular cartilage explants. Arch Biochem Biophys. 1992 Oct;298(1):70–79. doi: 10.1016/0003-9861(92)90095-e. [DOI] [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes F. R., Stabler C. L. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989 Jul-Aug;17(4):505–513. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Immobilization of the knee prevents osteoarthritis after anterior cruciate ligament transection. Arthritis Rheum. 1982 Oct;25(10):1201–1208. doi: 10.1002/art.1780251009. [DOI] [PubMed] [Google Scholar]
- Palmoski M., Brandt K. Hyaluronate-binding by proteoglycans. Comparison of mildly and severely osteoarthritic regions of human femoral cartilage. Clin Chim Acta. 1976 Jul 1;70(1):87–95. doi: 10.1016/0009-8981(76)90008-5. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., DiBattista J. A., Roughley P., McCollum R., Martel-Pelletier J. Cytokines and inflammation in cartilage degradation. Rheum Dis Clin North Am. 1993 Aug;19(3):545–568. [PubMed] [Google Scholar]
- Pelletier J. P., Faure M. P., DiBattista J. A., Wilhelm S., Visco D., Martel-Pelletier J. Coordinate synthesis of stromelysin, interleukin-1, and oncogene proteins in experimental osteoarthritis. An immunohistochemical study. Am J Pathol. 1993 Jan;142(1):95–105. [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Mehraban F., Malemud C. J. Immunological analysis of proteoglycan structural changes in the early stage of experimental osteoarthritic canine cartilage lesions. J Orthop Res. 1992 Jul;10(4):511–523. doi: 10.1002/jor.1100100406. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Mineau F., Faure M. P., Martel-Pelletier J. Imbalance between the mechanisms of activation and inhibition of metalloproteases in the early lesions of experimental osteoarthritis. Arthritis Rheum. 1990 Oct;33(10):1466–1476. doi: 10.1002/art.1780331003. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Billingham M. E., Saed-Nejad F., Muir H., Hardingham T. E. Increased release of matrix components from articular cartilage in experimental canine osteoarthritis. J Orthop Res. 1992 May;10(3):350–358. doi: 10.1002/jor.1100100307. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Doherty M., Maini R. N., Hardingham T. E. Increased concentrations of proteoglycan components in the synovial fluids of patients with acute but not chronic joint disease. Ann Rheum Dis. 1988 Oct;47(10):826–832. doi: 10.1136/ard.47.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A., Shurety W., Caterson B. The quantitation of a native chondroitin sulfate epitope in synovial fluid lavages and articular cartilage from canine experimental osteoarthritis and disuse atrophy. Arthritis Rheum. 1993 Apr;36(4):543–551. doi: 10.1002/art.1780360416. [DOI] [PubMed] [Google Scholar]
- Rizkalla G., Reiner A., Bogoch E., Poole A. R. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest. 1992 Dec;90(6):2268–2277. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S., Weightman B., Urban J., Chappell D. Mechanical and biochemical properties of human articular cartilage in osteoarthritic femoral heads and in autopsy specimens. J Bone Joint Surg Br. 1986 Mar;68(2):278–288. doi: 10.1302/0301-620X.68B2.3958016. [DOI] [PubMed] [Google Scholar]
- Rogachefsky R. A., Dean D. D., Howell D. S., Altman R. D. Treatment of canine osteoarthritis with insulin-like growth factor-1 (IGF-1) and sodium pentosan polysulfate. Osteoarthritis Cartilage. 1993 Apr;1(2):105–114. doi: 10.1016/s1063-4584(05)80025-1. [DOI] [PubMed] [Google Scholar]
- Ronzière M. C., Ricard-Blum S., Tiollier J., Hartmann D. J., Garrone R., Herbage D. Comparative analysis of collagens solubilized from human foetal, and normal and osteoarthritic adult articular cartilage, with emphasis on type VI collagen. Biochim Biophys Acta. 1990 Apr 19;1038(2):222–230. doi: 10.1016/0167-4838(90)90209-x. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Adams M. E., Billingham M. E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984 Apr;27(4):388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Flannery C. R., Neame P. J., Lohmander L. S. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992 May;89(5):1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Saxne T., Castro F., Rydholm U., Svantesson H. Cartilage derived proteoglycans in body fluids of children. Inverse correlation with age. J Rheumatol. 1989 Oct;16(10):1341–1344. [PubMed] [Google Scholar]
- Saxne T., Glennås A., Kvien T. K., Melby K., Heinegård D. Release of cartilage macromolecules into the synovial fluid in patients with acute and prolonged phases of reactive arthritis. Arthritis Rheum. 1993 Jan;36(1):20–25. doi: 10.1002/art.1780360105. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992 Sep;31(9):583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D. Synovial fluid analysis of two groups of proteoglycan epitopes distinguishes early and late cartilage lesions. Arthritis Rheum. 1992 Apr;35(4):385–390. doi: 10.1002/art.1780350404. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D., Wollheim F. A. Therapeutic effects on cartilage metabolism in arthritis as measured by release of proteoglycan structures into the synovial fluid. Ann Rheum Dis. 1986 Jun;45(6):491–497. doi: 10.1136/ard.45.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol. 1985 Feb;100(2):598–605. doi: 10.1083/jcb.100.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibel M. J., Duncan A., Robins S. P. Urinary hydroxy-pyridinium crosslinks provide indices of cartilage and bone involvement in arthritic diseases. J Rheumatol. 1989 Jul;16(7):964–970. [PubMed] [Google Scholar]
- Seibel M. J., Macaulay W., Jelsma R., Saed-Nejad F., Ratcliffe A. Antigenic properties of keratan sulfate: influence of antigen structure, monoclonal antibodies, and antibody valency. Arch Biochem Biophys. 1992 Aug 1;296(2):410–418. doi: 10.1016/0003-9861(92)90591-j. [DOI] [PubMed] [Google Scholar]
- Shinmei M., Ito K., Matsuyama S., Yoshihara Y., Matsuzawa K. Joint fluid carboxy-terminal type II procollagen peptide as a marker of cartilage collagen biosynthesis. Osteoarthritis Cartilage. 1993 Apr;1(2):121–128. doi: 10.1016/s1063-4584(05)80027-5. [DOI] [PubMed] [Google Scholar]
- Shinmei M., Miyauchi S., Machida A., Miyazaki K. Quantitation of chondroitin 4-sulfate and chondroitin 6-sulfate in pathologic joint fluid. Arthritis Rheum. 1992 Nov;35(11):1304–1308. doi: 10.1002/art.1780351110. [DOI] [PubMed] [Google Scholar]
- Shuckett R., Malemud C. J. Distinct cartilage proteoglycan chromatographic elution patterns in advanced human hip osteoarthritis: correlations with histologic analysis. J Rheumatol. 1990 Mar;17(3):357–363. [PubMed] [Google Scholar]
- Shuckett R., Malemud C. J. Proteoglycans synthesized by chondrocytes of human nonarthritic and osteoarthritic cartilage. Proc Soc Exp Biol Med. 1989 Mar;190(3):275–281. doi: 10.3181/00379727-190-42860. [DOI] [PubMed] [Google Scholar]
- Smedsrød B., Melkko J., Risteli L., Risteli J. Circulating C-terminal propeptide of type I procollagen is cleared mainly via the mannose receptor in liver endothelial cells. Biochem J. 1990 Oct 15;271(2):345–350. doi: 10.1042/bj2710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville K., Faulkner G., Langman M. Non-steroidal anti-inflammatory drugs and bleeding peptic ulcer. Lancet. 1986 Mar 1;1(8479):462–464. doi: 10.1016/s0140-6736(86)92927-2. [DOI] [PubMed] [Google Scholar]
- Spector T. D., Dacre J. E., Harris P. A., Huskisson E. C. Radiological progression of osteoarthritis: an 11 year follow up study of the knee. Ann Rheum Dis. 1992 Oct;51(10):1107–1110. doi: 10.1136/ard.51.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. D., Woodward L., Hall G. M., Hammond A., Williams A., Butler M. G., James I. T., Hart D. J., Thompson P. W., Scott D. L. Keratan sulphate in rheumatoid arthritis, osteoarthritis, and inflammatory diseases. Ann Rheum Dis. 1992 Oct;51(10):1134–1137. doi: 10.1136/ard.51.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. P., Schils J. P., Bergfeld J. A., Andrish J. T., Weiker G. G., Anderson T. E., Piraino D. W., Richmond B. J., Medendorp S. V. Prospective study of osseous, articular, and meniscal lesions in recent anterior cruciate ligament tears by magnetic resonance imaging and arthroscopy. Am J Sports Med. 1993 Jul-Aug;21(4):551–557. doi: 10.1177/036354659302100412. [DOI] [PubMed] [Google Scholar]
- Steven M. M. Prevalence of chronic arthritis in four geographical areas of the Scottish Highlands. Ann Rheum Dis. 1992 Feb;51(2):186–194. doi: 10.1136/ard.51.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet M. B., Coelho A., Schnitzler C. M., Schnitzer T. J., Lenz M. E., Jakim I., Kuettner K. E., Thonar E. J. Serum keratan sulfate levels in osteoarthritis patients. Arthritis Rheum. 1988 May;31(5):648–652. doi: 10.1002/art.1780310510. [DOI] [PubMed] [Google Scholar]
- Sweet M. B., Thonar E. J., Immelman A. R., Solomon L. Biochemical changes in progressive osteoarthrosis. Ann Rheum Dis. 1977 Oct;36(5):387–398. doi: 10.1136/ard.36.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Jr, Oegema T. R., Jr Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J Bone Joint Surg Am. 1979 Apr;61(3):407–416. [PubMed] [Google Scholar]
- Thonar E. J., Lenz M. E., Klintworth G. K., Caterson B., Pachman L. M., Glickman P., Katz R., Huff J., Kuettner K. E. Quantification of keratan sulfate in blood as a marker of cartilage catabolism. Arthritis Rheum. 1985 Dec;28(12):1367–1376. doi: 10.1002/art.1780281209. [DOI] [PubMed] [Google Scholar]
- Thonar E. J., Shinmei M., Lohmander L. S. Body fluid markers of cartilage changes in osteoarthritis. Rheum Dis Clin North Am. 1993 Aug;19(3):635–657. [PubMed] [Google Scholar]
- Thornton D. J., Morris H. G., Cockin G. H., Huckerby T. N., Nieduszynski I. A., Carlstedt I., Hardingham T. E., Ratcliffe A. Structural and immunological studies of keratan sulphates from mature bovine articular cartilage. Biochem J. 1989 May 15;260(1):277–282. doi: 10.1042/bj2600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzaicos C., Fraser J. R., Tsotsis E., Kimpton W. G. Inhibition of hyaluronan uptake in lymphatic tissue by chondroitin sulphate proteoglycan. Biochem J. 1989 Dec 15;264(3):823–828. doi: 10.1042/bj2640823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelhart D., Thonar E. J., Pietryla D. W., Williams J. M. Elevation in urinary levels of pyridinium cross-links of collagen following chymopapain-induced degradation of articular cartilage in the rabbit knee provides evidence of metabolic changes in bone. Osteoarthritis Cartilage. 1993 Jul;1(3):185–192. doi: 10.1016/s1063-4584(05)80090-1. [DOI] [PubMed] [Google Scholar]
- Vikkula M., Palotie A., Ritvaniemi P., Ott J., Ala-Kokko L., Sievers U., Aho K., Peltonen L. Early-onset osteoarthritis linked to the type II procollagen gene. Detailed clinical phenotype and further analyses of the gene. Arthritis Rheum. 1993 Mar;36(3):401–409. doi: 10.1002/art.1780360317. [DOI] [PubMed] [Google Scholar]
- Vingård E., Alfredsson L., Goldie I., Hogstedt C. Occupation and osteoarthrosis of the hip and knee: a register-based cohort study. Int J Epidemiol. 1991 Dec;20(4):1025–1031. doi: 10.1093/ije/20.4.1025. [DOI] [PubMed] [Google Scholar]
- Walakovits L. A., Moore V. L., Bhardwaj N., Gallick G. S., Lark M. W. Detection of stromelysin and collagenase in synovial fluid from patients with rheumatoid arthritis and posttraumatic knee injury. Arthritis Rheum. 1992 Jan;35(1):35–42. doi: 10.1002/art.1780350106. [DOI] [PubMed] [Google Scholar]
- Williams C. J., Jimenez S. A. Heredity, genes and osteoarthritis. Rheum Dis Clin North Am. 1993 Aug;19(3):523–543. [PubMed] [Google Scholar]
- Williams J. M., Downey C., Thonar E. J. Increase in levels of serum keratan sulfate following cartilage proteoglycan degradation in the rabbit knee joint. Arthritis Rheum. 1988 Apr;31(4):557–560. doi: 10.1002/art.1780310415. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Ongchi D. R., Thonar E. J. Repair of articular cartilage injury following intra-articular chymopapain-induced matrix proteoglycan loss. J Orthop Res. 1993 Sep;11(5):705–716. doi: 10.1002/jor.1100110513. [DOI] [PubMed] [Google Scholar]
- Witsch-Prehm P., Miehlke R., Kresse H. Presence of small proteoglycan fragments in normal and arthritic human cartilage. Arthritis Rheum. 1992 Sep;35(9):1042–1052. doi: 10.1002/art.1780350909. [DOI] [PubMed] [Google Scholar]
- Witter J., Roughley P. J., Webber C., Roberts N., Keystone E., Poole A. R. The immunologic detection and characterization of cartilage proteoglycan degradation products in synovial fluids of patients with arthritis. Arthritis Rheum. 1987 May;30(5):519–529. doi: 10.1002/art.1780300506. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Wu J. J., Lark M. W., Chun L. E., Eyre D. R. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991 Mar 25;266(9):5625–5628. [PubMed] [Google Scholar]
- Wurster N. B., Lust G. Synthesis of fibronectin in normal and osteoarthritic articular cartilage. Biochim Biophys Acta. 1984 Jul 16;800(1):52–58. doi: 10.1016/0304-4165(84)90093-x. [DOI] [PubMed] [Google Scholar]
- Xie D. L., Meyers R., Homandberg G. A. Fibronectin fragments in osteoarthritic synovial fluid. J Rheumatol. 1992 Sep;19(9):1448–1452. [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yelin E. Arthritis. The cumulative impact of a common chronic condition. Arthritis Rheum. 1992 May;35(5):489–497. [PubMed] [Google Scholar]
- Zafarullah M., Pelletier J. P., Cloutier J. M., Martel-Pelletier J. Elevated metalloproteinase and tissue inhibitor of metalloproteinase mRNA in human osteoarthritic synovia. J Rheumatol. 1993 Apr;20(4):693–697. [PubMed] [Google Scholar]
- von der Mark K., Kirsch T., Nerlich A., Kuss A., Weseloh G., Glückert K., Stöss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992 Jul;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]