Abstract
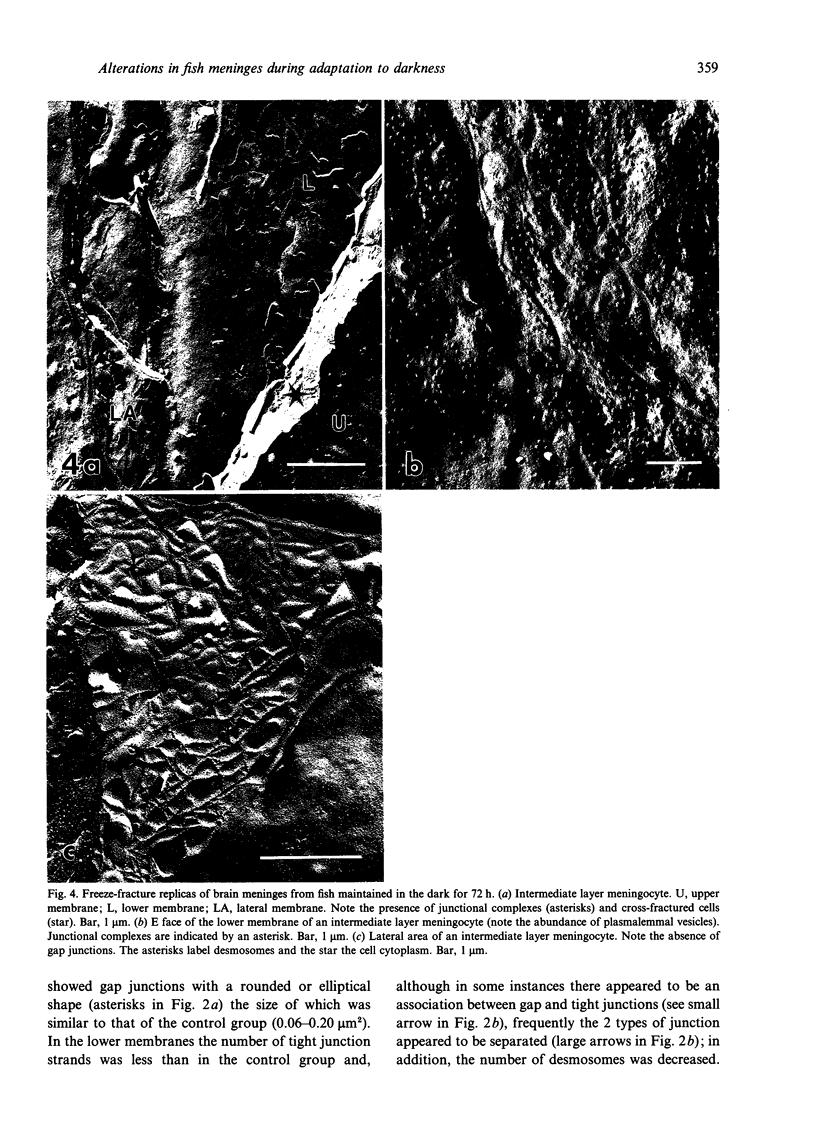
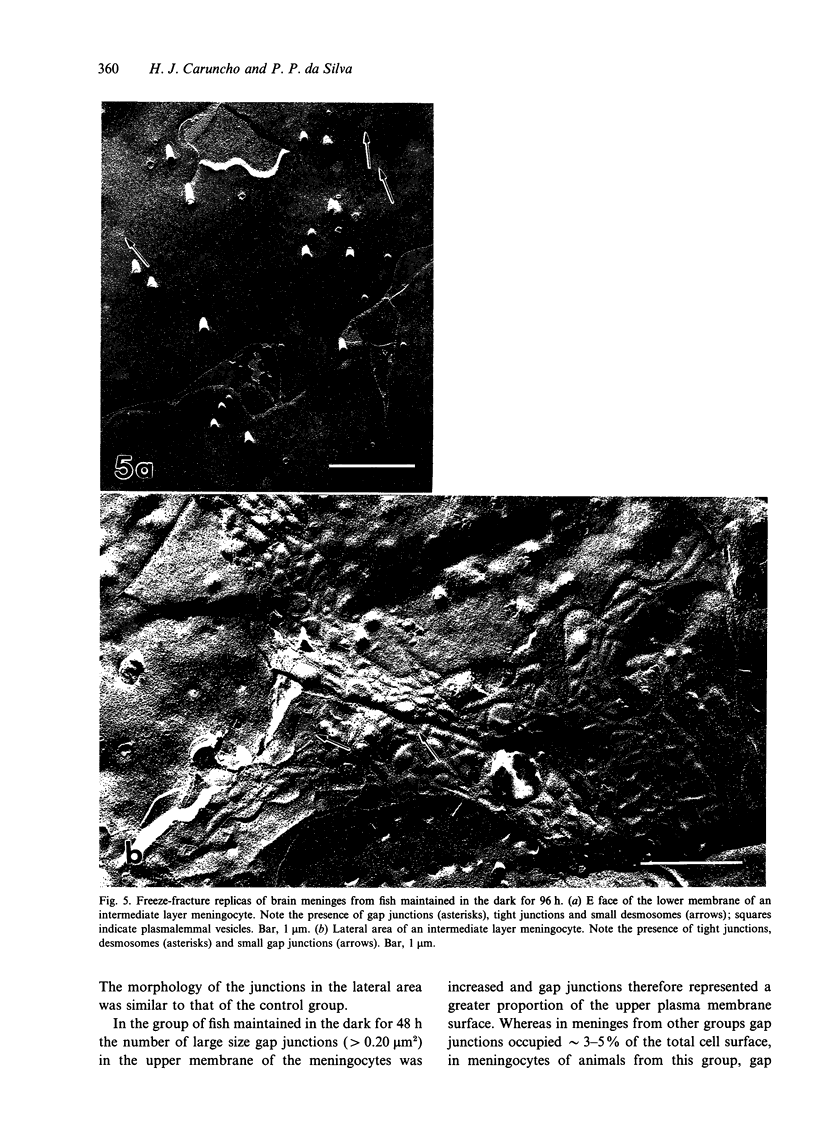
The morphological changes in the intermediate endomeningeal layer of the goldfish brain during light and dark adaptation were studied by freeze-fracture electron microscopy. During the different stages of adaptation no significant changes were found in the density of intramembrane particles and nuclear pores in these cells. The density of plasmalemmal vesicles in the meningocyte surface increased in the groups maintained in the dark for 48 and 72 h (maximum) and then decreased in the group maintained for 96 h in the dark to a basal level. There were also morphological changes in the junctional complexes. At the upper cell membranes (in contact with the outer layer) of meningocytes in the group maintained in the dark for 48 h, we found an increase in the surface occupied by gap junctions. In addition, gap junctions were absent in the lateral membranes of meningocytes from animals maintained in the dark for 72 h. The morphology of gap junctions in the group maintained in the dark for 96 h was similar to that of the control group. These results suggest that the cells of the teleost intermediate endomeningeal layer undergo important changes in activity during adaptative experiments.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benavente R., Dabauvalle M. C., Scheer U., Chaly N. Functional role of newly formed pore complexes in postmitotic nuclear reorganization. Chromosoma. 1989 Oct;98(4):233–241. doi: 10.1007/BF00327308. [DOI] [PubMed] [Google Scholar]
- Benowitz L. I., Shashoua V. E. Localization of a brain protein metabolically linked with behavioral plasticity in the goldfish. Brain Res. 1977 Nov 11;136(2):227–242. doi: 10.1016/0006-8993(77)90800-9. [DOI] [PubMed] [Google Scholar]
- Caruncho H. J., Pinto da Silva P., Anadon R. The morphology of teleost meningocytes as revealed by freeze-fracture. J Submicrosc Cytol Pathol. 1993 Jul;25(3):397–406. [PubMed] [Google Scholar]
- Caruncho H. J., Rodriguez-Moldes I., Anadón R. A freeze-fracture study of the soma membranes of monoaminergic neurons in the hypothalamic nucleus recessi posterioris of the rainbow trout (Salmo gairdneri Rich.). J Hirnforsch. 1990;31(5):575–584. [PubMed] [Google Scholar]
- Clough G., Michel C. C. The role of vesicles in the transport of ferritin through frog endothelium. J Physiol. 1981 Jun;315:127–142. doi: 10.1113/jphysiol.1981.sp013737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen J. The endothelial vesicle system in cryofixed frog mesenteric capillaries analysed by ultrathin serial sectioning. J Electron Microsc Tech. 1991 Nov;19(3):291–304. doi: 10.1002/jemt.1060190305. [DOI] [PubMed] [Google Scholar]
- Frøkjaer-Jensen J., Wagner R. C., Andrews S. B., Hagman P., Reese T. S. Three-dimensional organization of the plasmalemmal vesicular system in directly frozen capillaries of the rete mirabile in the swim bladder of the eel. Cell Tissue Res. 1988 Oct;254(1):17–24. doi: 10.1007/BF00220012. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L. M., Perrelet A. Postsynaptic membrane domains in the molecular layer of the cerebellum: a correlation between presynaptic inputs and postsynaptic plasma membrane organization. Brain Res. 1984 Nov 12;321(2):255–266. doi: 10.1016/0006-8993(84)90178-1. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S. Nuclear transport. Curr Opin Cell Biol. 1989 Jun;1(3):441–446. doi: 10.1016/0955-0674(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Heltianu C., Dobrila L., Antohe F., Simionescu M. Evidence for thyroxine transport by the lung and heart capillary endothelium. Microvasc Res. 1989 Mar;37(2):188–203. doi: 10.1016/0026-2862(89)90037-x. [DOI] [PubMed] [Google Scholar]
- Hoffmann W. Goldfish ependymins: cerebrospinal fluid proteins of meningeal origin. Prog Brain Res. 1992;91:13–17. doi: 10.1016/s0079-6123(08)62310-9. [DOI] [PubMed] [Google Scholar]
- Milici A. J., Watrous N. E., Stukenbrok H., Palade G. E. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987 Dec;105(6 Pt 1):2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose Y., Kohno K., Ito R. Ultrastructural study on the meninx of the goldfish brain. J Comp Neurol. 1988 Apr 15;270(3):327–336. doi: 10.1002/cne.902700303. [DOI] [PubMed] [Google Scholar]
- Palade G. E., Bruns R. R. Structural modulations of plasmalemmal vesicles. J Cell Biol. 1968 Jun;37(3):633–649. doi: 10.1083/jcb.37.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piront M. L., Schmidt R. Inhibition of long-term memory formation by anti-ependymin antisera after active shock-avoidance learning in goldfish. Brain Res. 1988 Feb 23;442(1):53–62. doi: 10.1016/0006-8993(88)91431-x. [DOI] [PubMed] [Google Scholar]
- Schmidt J. T., Edwards D. L., Stuermer C. The re-establishment of synaptic transmission by regenerating optic axons in goldfish: time course and effects of blocking activity by intraocular injection of tetrodotoxin. Brain Res. 1983 Jun 13;269(1):15–27. doi: 10.1016/0006-8993(83)90958-7. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Rother S., Schlingensiepen K. H., Brysch W. Neuronal plasticity depending on a glycoprotein synthesized in goldfish leptomeninx. Prog Brain Res. 1992;91:7–12. doi: 10.1016/s0079-6123(08)62309-2. [DOI] [PubMed] [Google Scholar]
- Severs N. J. Caveolae: static inpocketings of the plasma membrane, dynamic vesicles or plain artifact? J Cell Sci. 1988 Jul;90(Pt 3):341–348. doi: 10.1242/jcs.90.3.341. [DOI] [PubMed] [Google Scholar]
- Shashoua V. E. Brain metabolism and the acquisition of new behaviors. III. Evidence for secretion of two proteins into the brain extracellular fluid after training. Brain Res. 1979 Apr 27;166(2):349–358. doi: 10.1016/0006-8993(79)90220-8. [DOI] [PubMed] [Google Scholar]
- Shashoua V. E. Extracellular fluid proteins of goldfish brain: studies of concentration and labeling patterns. Neurochem Res. 1981 Oct;6(10):1129–1147. doi: 10.1007/BF00964418. [DOI] [PubMed] [Google Scholar]
- Shashoua V. E. The role of brain extracellular proteins in neuroplasticity and learning. Cell Mol Neurobiol. 1985 Jun;5(1-2):183–207. doi: 10.1007/BF00711092. [DOI] [PubMed] [Google Scholar]
- Shashoua V. E. The role of ependymin in the development of long lasting synaptic changes. J Physiol (Paris) 1988;83(3):232–239. [PubMed] [Google Scholar]
- Wagner R. C., Andrews S. B. Cryofixation of vascular endothelium. J Electron Microsc Tech. 1991 Nov;19(3):276–290. doi: 10.1002/jemt.1060190304. [DOI] [PubMed] [Google Scholar]
- Weiler R., Kohler K., Kirsch M., Wagner H. J. Glutamate and dopamine modulate synaptic plasticity in horizontal cell dendrites of fish retina. Neurosci Lett. 1988 May 3;87(3):205–209. doi: 10.1016/0304-3940(88)90449-1. [DOI] [PubMed] [Google Scholar]
- Weiler R., Schultz K. Ionotropic non-N-methyl-D-aspartate agonists induce retraction of dendritic spinules from retinal horizontal cells. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6533–6537. doi: 10.1073/pnas.90.14.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]







