Abstract
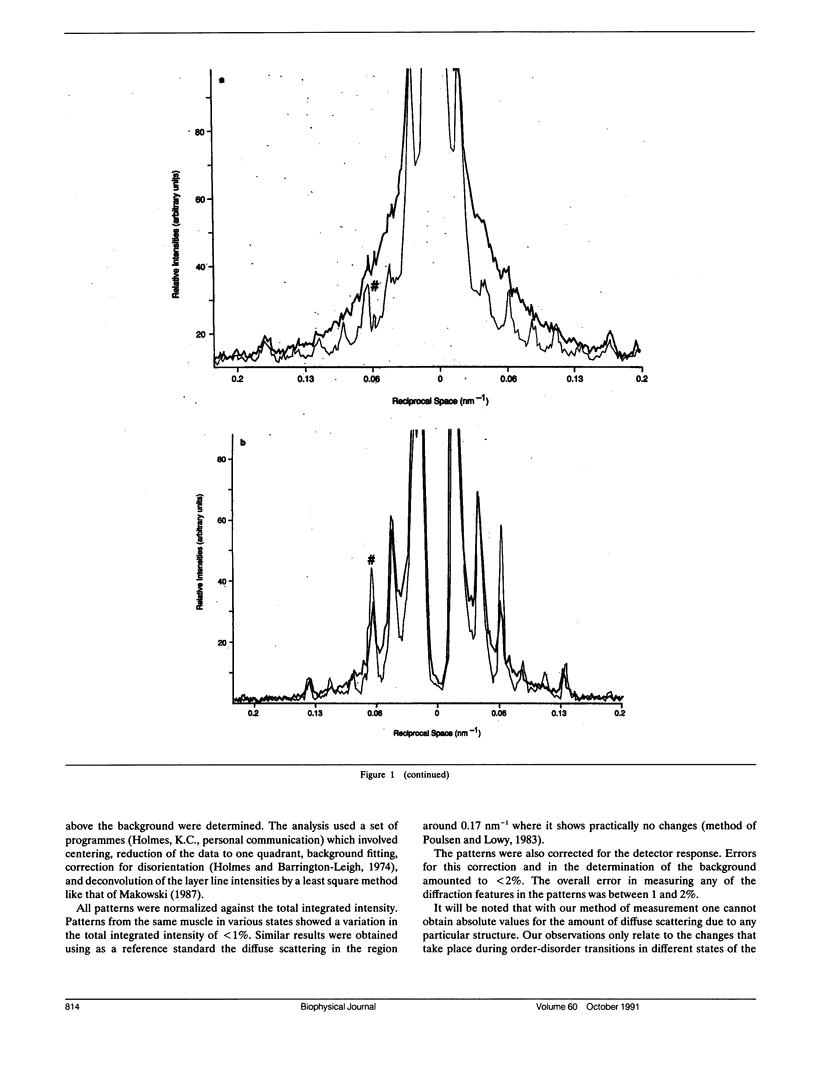
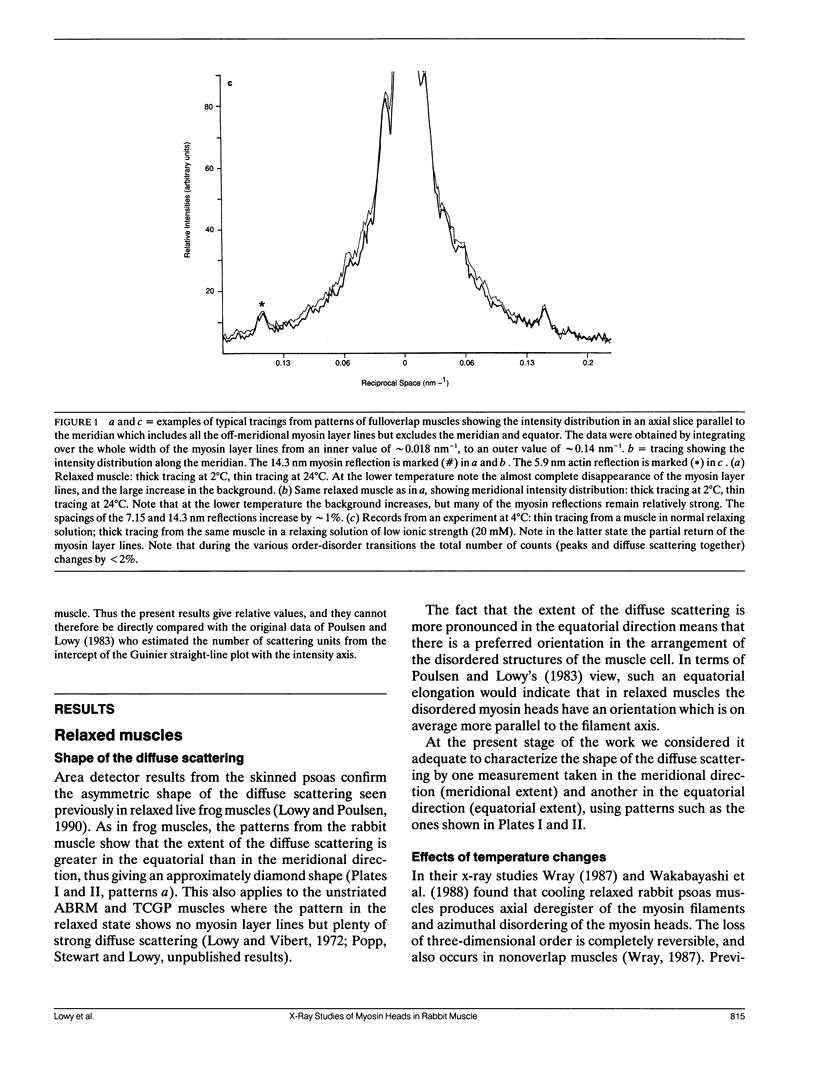
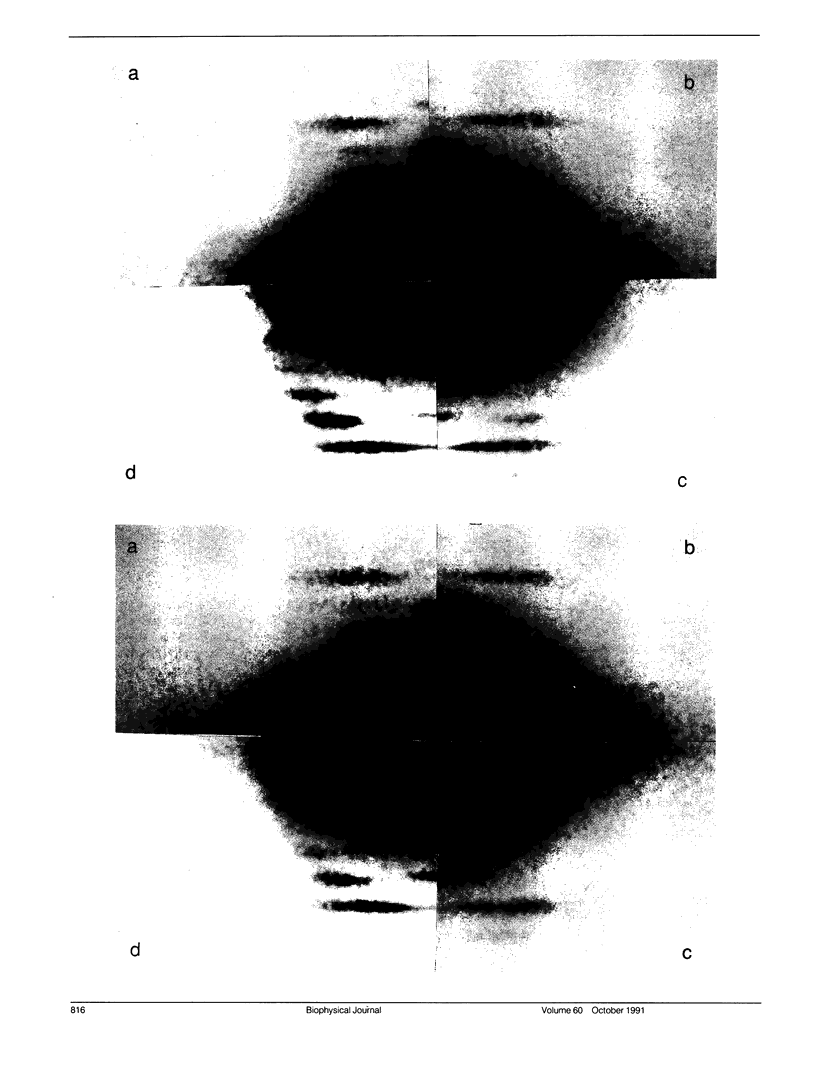
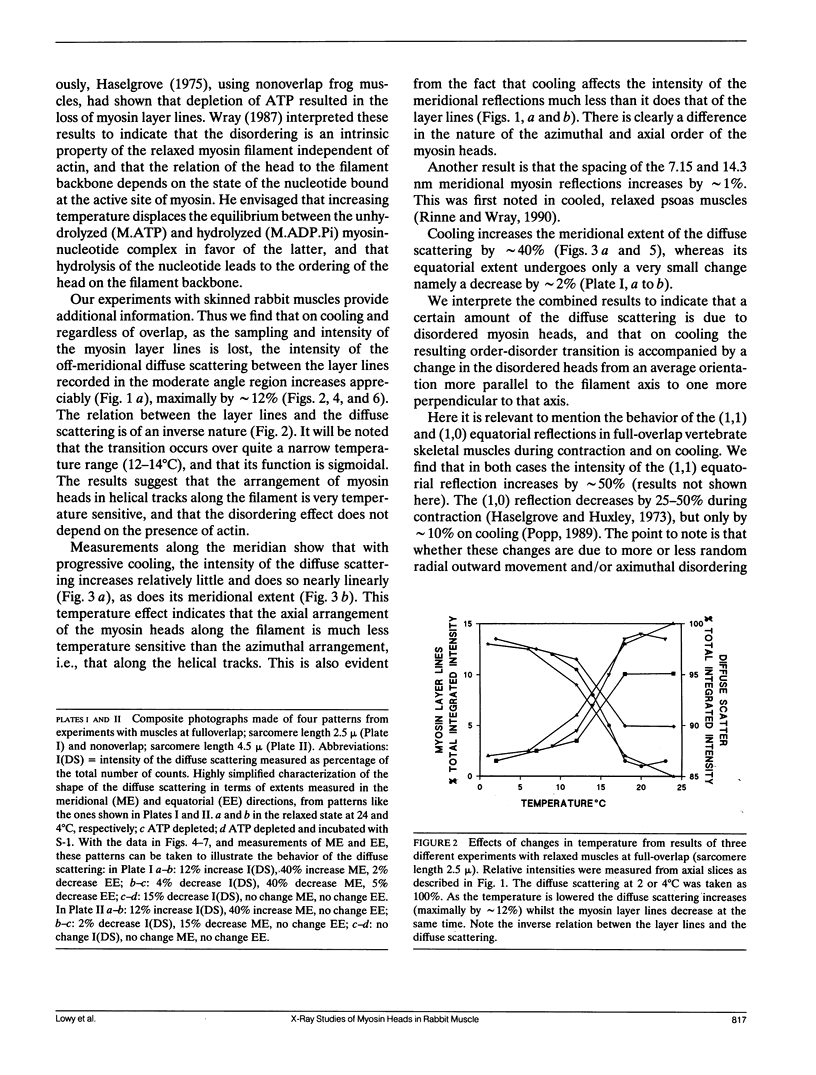
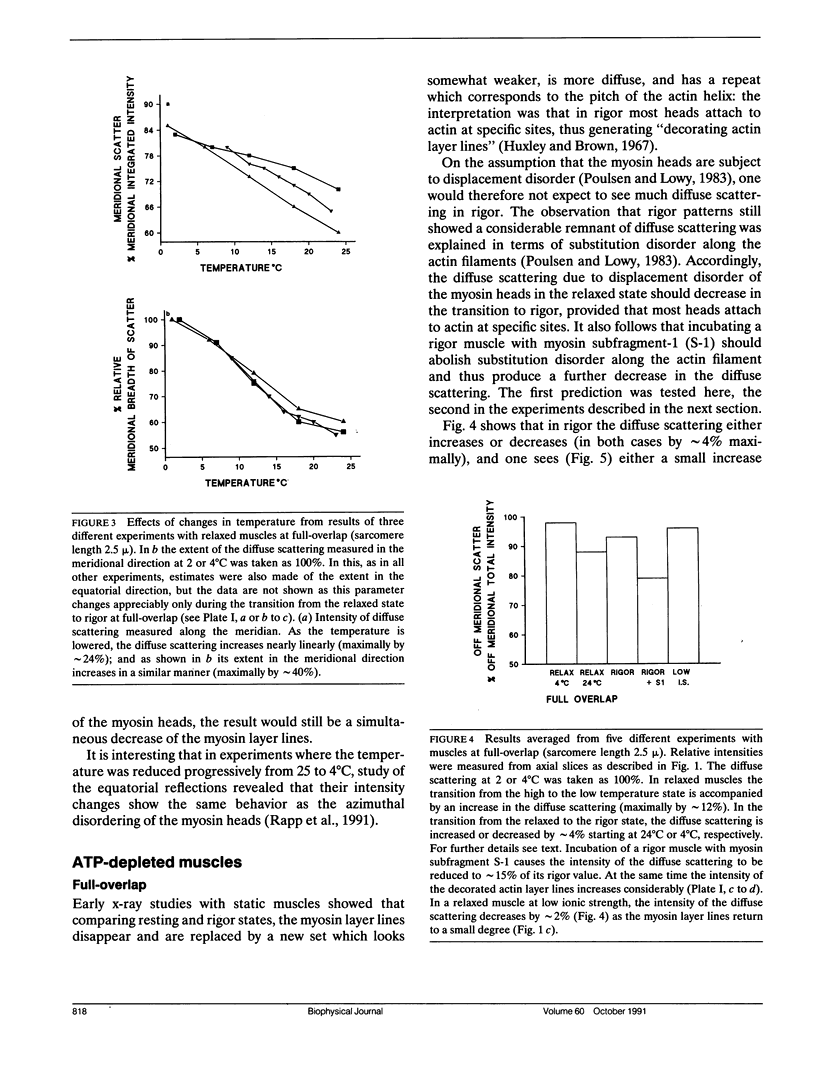
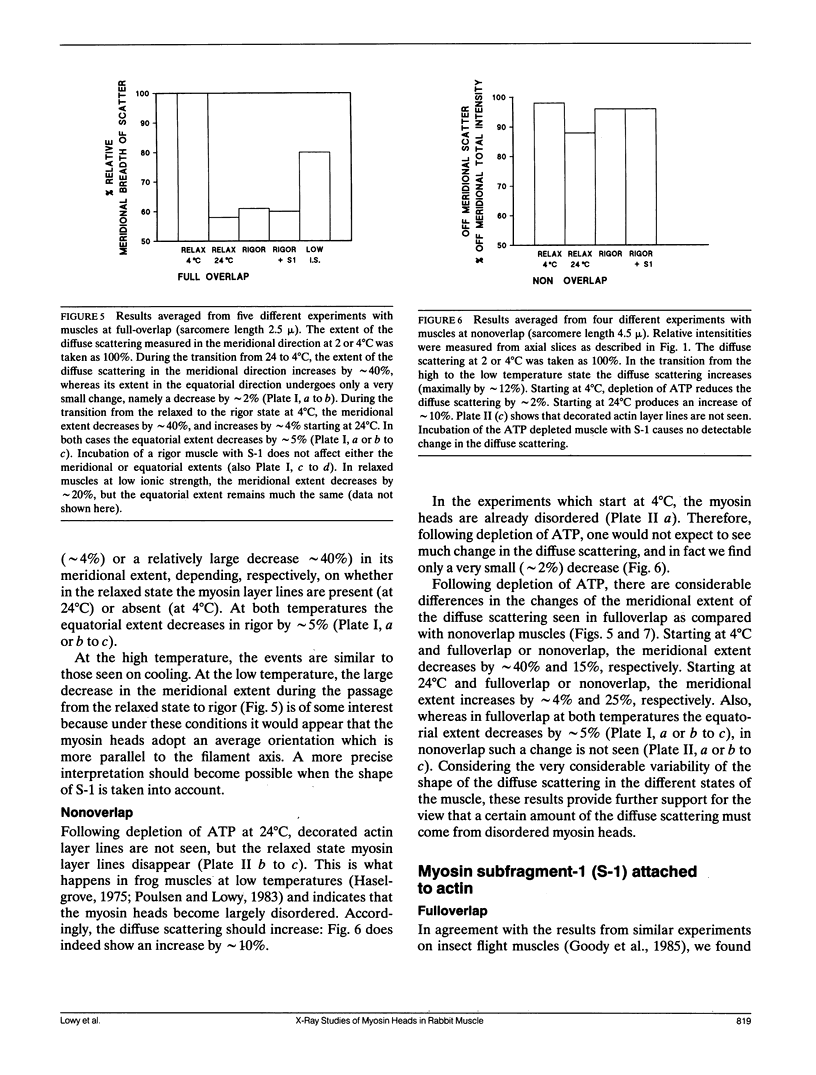
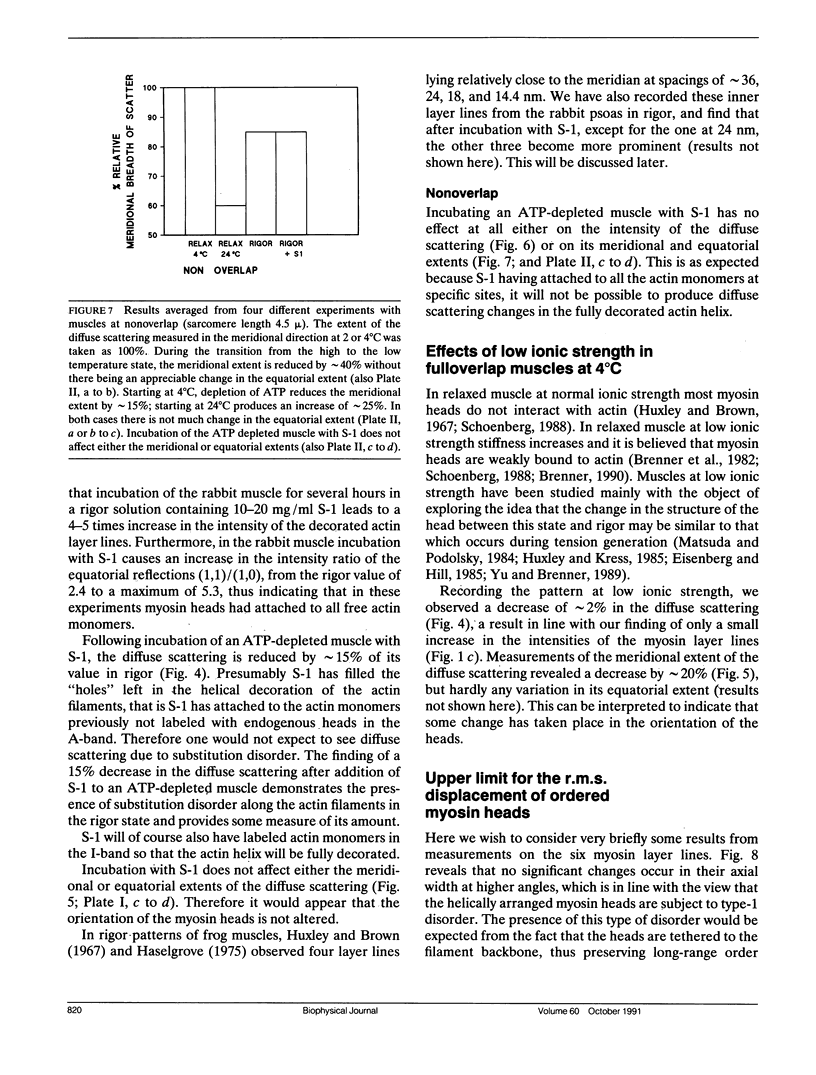
Using x-rays from a laboratory source and an area detector, myosin layer lines and the diffuse scattering between them in the moderate angle region have been recorded. At full overlap, incubation of rigor muscles with S-1 greatly reduces the diffuse scattering. Also, three of the four actin-based layer lines lying close to the meridian (Huxley, H. E., and W. Brown, 1967. J. Mol. Biol. 30:384-434; Haselgrove, J. C. 1975. J. Mol. Biol. 92:113-143) increase, suggesting fuller labeling of the actin filaments. These results are consistent with the idea (Poulsen, F. R., and J. Lowy, 1983. Nature [Lond.]. 303:146-152) that some of the diffuse scattering in rigor muscles is due to a random mixture of actin monomers with and without attached myosin heads (substitution disorder). In relaxed muscles, regardless of overlap, lowering the temperature from 24 to 4 degrees C practically abolishes the myosin layer lines (a result first obtained by Wray, J.S. 1987. J. Muscle Res. Cell Motil. 8:62 (a). Abstr.), whilst the diffuse scattering between these layer lines increases appreciably. Similar changes occur in the passage from rest to peak tetanic tension in live frog muscle (Lowy, J., and F.R. Poulsen. 1990. Biophys. J. 57:977-985). Cooling the psoas demonstrates that the intensity relation between the layer lines and the diffuse scattering is of an inverse nature, and that the transition occurs over a narrow temperature range (12-14 degrees C) with a sigmoidal function. From these results it would appear that the helical arrangement of the myosin heads is very temperature sensitive, and that the disordering effect does not depend on the presence of actin. Measurements along the meridian reveal that the intensity of the diffuse scattering increases relatively little and does so in a nearly linear manner: evidently the axial order of the myosin heads is much less temperature sensitive. The combined data support the view (Poulsen, F. R., and J. Lowy. 1983. Nature [Lond.]. 303:146-152) that in relaxed muscles a significant part of the diffuse scattering originates from disordered myosin heads. The observation that the extent of the diffuse scattering is greater in the equatorial than in the meridional direction suggests that the disordered myosin heads have an orientation which is on average more parallel to the filament axis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT G. F. X-RAY DIFFRACTION STUDIES ON STRIATED AND SMOOTH MUSCLES. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:467–472. doi: 10.1098/rspb.1964.0057. [DOI] [PubMed] [Google Scholar]
- Egelman E. H., Francis N., DeRosier D. J. F-actin is a helix with a random variable twist. Nature. 1982 Jul 8;298(5870):131–135. doi: 10.1038/298131a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Goody R. S., Reedy M. C., Hofmann W., Holmes K. C., Reedy M. K. Binding of myosin subfragment 1 to glycerinated insect flight muscle in the rigor state. Biophys J. 1985 Feb;47(2 Pt 1):151–169. doi: 10.1016/s0006-3495(85)83889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Kress M., Bordas J., Koch M. H. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol. 1982 Jul 15;158(4):637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Knight P., Trinick J. Structure of the myosin projections on native thick filaments from vertebrate skeletal muscle. J Mol Biol. 1984 Aug 15;177(3):461–482. doi: 10.1016/0022-2836(84)90295-x. [DOI] [PubMed] [Google Scholar]
- LOWY J., HANSON J. Ultrastructure of invertebrate smooth muscles. Physiol Rev Suppl. 1962 Jul;5:34–47. [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R. Studies of the diffuse x-ray scattering from contracting frog skeletal muscles. Biophys J. 1990 May;57(5):977–985. doi: 10.1016/S0006-3495(90)82617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R. Time-resolved X-ray diffraction studies of the structural behaviour of myosin heads in a living contracting unstriated muscle. Nature. 1982 Sep 23;299(5881):308–312. doi: 10.1038/299308a0. [DOI] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R., Vibert P. J. Myosin filaments in vertebrate smooth muscle. Nature. 1970 Mar 14;225(5237):1053–1054. doi: 10.1038/2251053a0. [DOI] [PubMed] [Google Scholar]
- Lowy J., Poulsen F. R. X-ray study of myosin heads in contracting frog skeletal muscle. J Mol Biol. 1987 Apr 20;194(4):595–600. doi: 10.1016/0022-2836(87)90236-1. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Podolsky R. J. X-ray evidence for two structural states of the actomyosin cross-bridge in muscle fibers. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2364–2368. doi: 10.1073/pnas.81.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrón R., Craig R. Disorder induced in nonoverlap myosin cross-bridges by loss of adenosine triphosphate. Biophys J. 1989 Nov;56(5):927–933. doi: 10.1016/S0006-3495(89)82738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrón R., Huxley H. E. The effect of the ATP analogue AMPPNP on the structure of crossbridges in vertebrate skeletal muscles: X-ray diffraction and mechanical studies. J Muscle Res Cell Motil. 1984 Dec;5(6):613–655. doi: 10.1007/BF00713923. [DOI] [PubMed] [Google Scholar]
- Poole K. J., Rapp G., Maéda Y., Goody R. S. The time course of changes in the equatorial diffraction patterns from different muscle types on photolysis of caged-ATP. Adv Exp Med Biol. 1988;226:391–404. [PubMed] [Google Scholar]
- Poulsen F. R., Lowy J., Cooke P. H., Bartels E. M., Elliott G. F., Hughes R. A. Diffuse X-ray scatter from myosin heads in oriented synthetic filaments. Biophys J. 1987 Jun;51(6):959–967. doi: 10.1016/S0006-3495(87)83423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen F. R., Lowy J. Small-angle X-ray scattering from myosin heads in relaxed and rigor frog skeletal muscles. Nature. 1983 May 12;303(5913):146–152. doi: 10.1038/303146a0. [DOI] [PubMed] [Google Scholar]
- Reedy M. K. Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in the rigor cross-bridge lattice. J Mol Biol. 1968 Jan 28;31(2):155–176. doi: 10.1016/0022-2836(68)90437-3. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. Characterization of the myosin adenosine triphosphate (M.ATP) crossbridge in rabbit and frog skeletal muscle fibers. Biophys J. 1988 Jul;54(1):135–148. doi: 10.1016/S0006-3495(88)82938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieszek A. The fine structure of the contractile apparatus of the anterior byssus retractor muscle of Mytilus edulis. J Ultrastruct Res. 1973 May;43(3):313–343. doi: 10.1016/s0022-5320(73)80041-3. [DOI] [PubMed] [Google Scholar]
- Squire J. M., Harford J. J. Actin filament organization and myosin head labelling patterns in vertebrate skeletal muscles in the rigor and weak binding states. J Muscle Res Cell Motil. 1988 Aug;9(4):344–358. doi: 10.1007/BF01773878. [DOI] [PubMed] [Google Scholar]
- Vibert P. J. Domain structure of the myosin head in correlation-averaged images of shadowed molecules. J Muscle Res Cell Motil. 1988 Apr;9(2):147–155. doi: 10.1007/BF01773736. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T., Akiba T., Hirose K., Tomioka A., Tokunaga M., Suzuki M., Toyoshima C., Sutoh K., Yamamoto K., Matsumoto T. Temperature-induced change of thick filament and location of the functional sites of myosin. Adv Exp Med Biol. 1988;226:39–48. [PubMed] [Google Scholar]
- Yu L. C., Brenner B. Structures of actomyosin crossbridges in relaxed and rigor muscle fibers. Biophys J. 1989 Mar;55(3):441–453. doi: 10.1016/S0006-3495(89)82838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]