Abstract
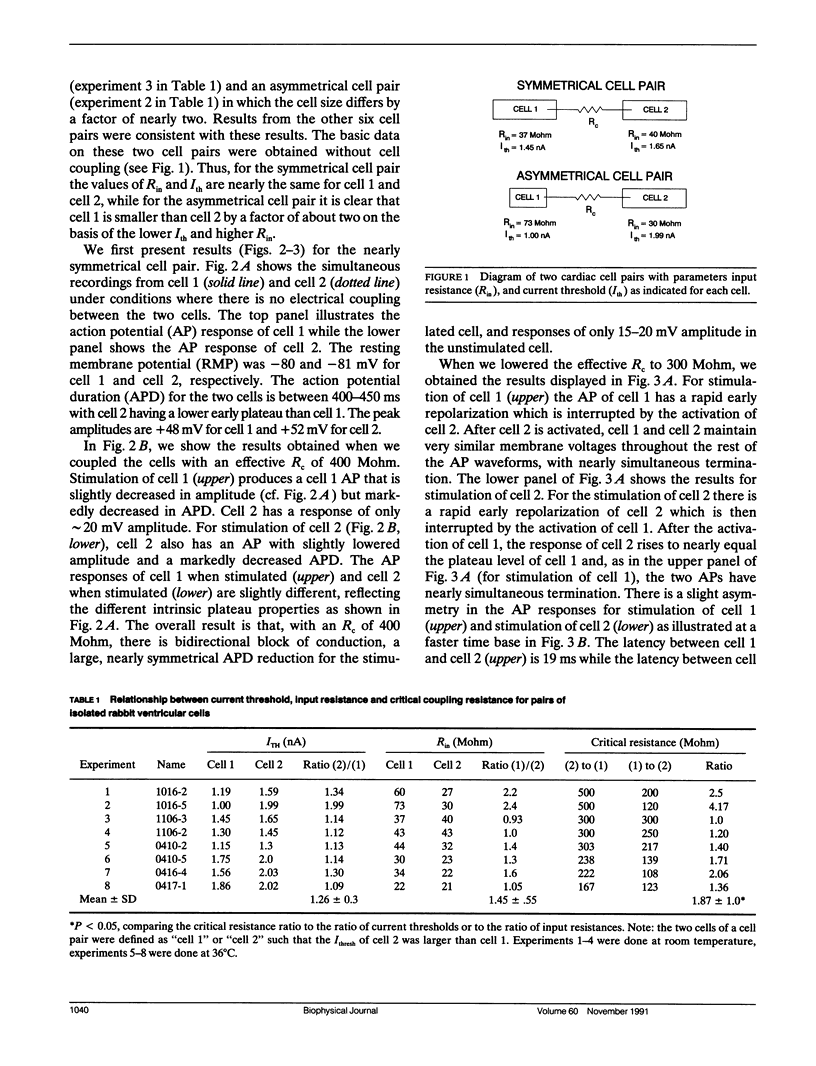
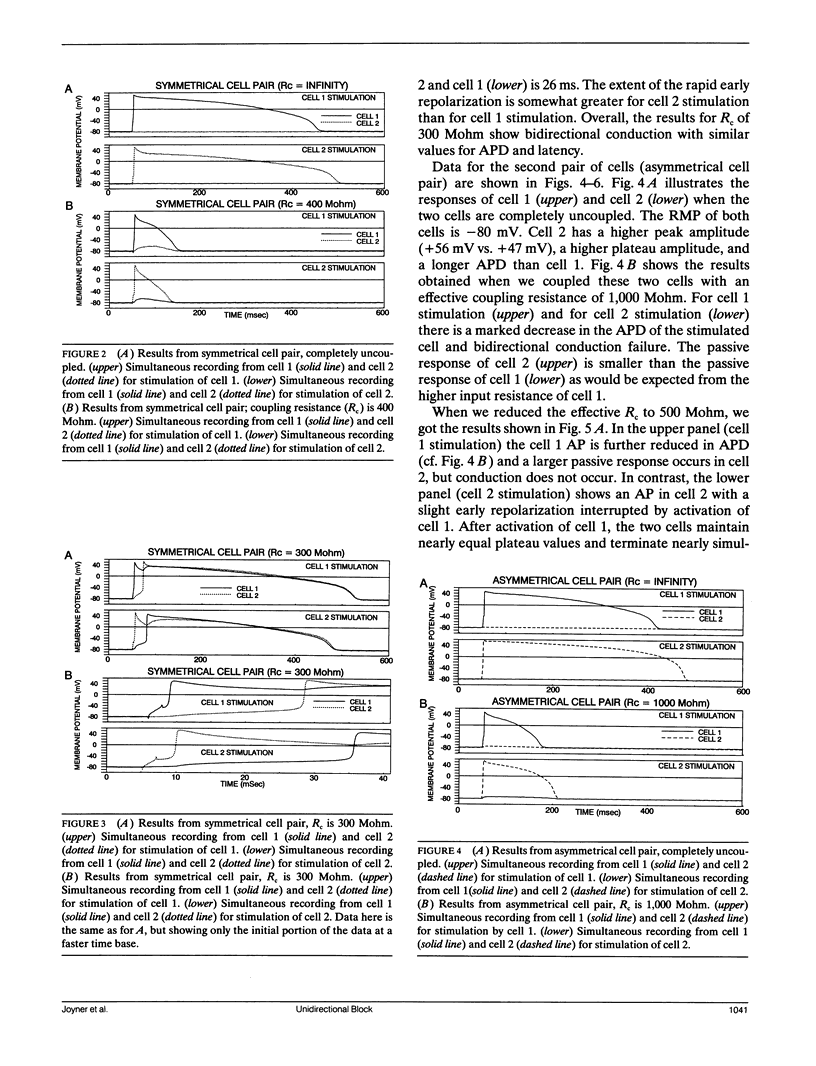
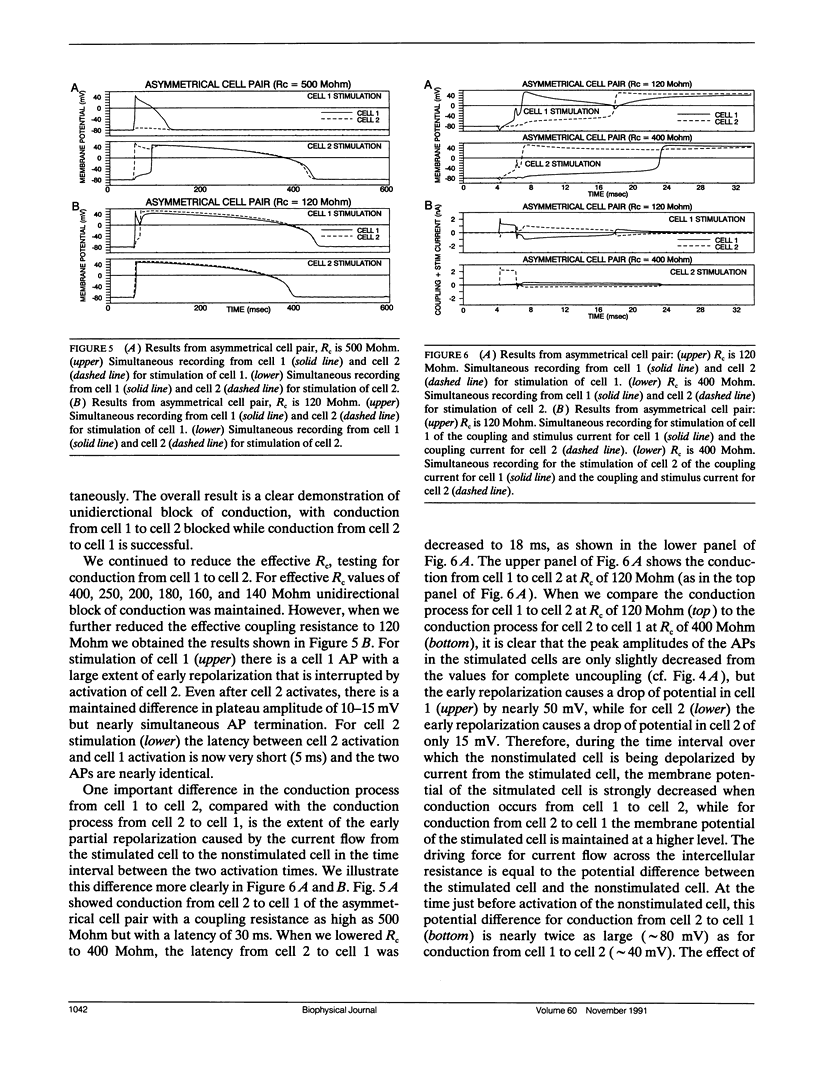
We have used pairs of electrically coupled cardiac cells to investigate the dependence of successful conduction of an action potential on three components of the conduction process: (a) the amount of depolarization required to be produced in the nonstimulated cell (the "sink" for current flow) to initiate an action potential in the nonstimulated cell, (b) the intercellular resistance as the path for intercellular current flow, and (c) the ability of the stimulated cell to maintain a high membrane potential to serve as the "source" of current during the conduction process. We present data from eight pairs of simultaneously recorded rabbit ventricular cells, with the two cells of each pair physically separated from each other. We used an electronic circuit to pass currents into and out of each cell such that these currents produced the effects of any desired level of intercellular resistance. The cells of equal size (as assessed by their current threshold and their input resistance for small depolarizations) show bidirectional failure of conduction at very high values of intercellular resistance which then converts to successful bidirectional conduction at lower values of intercellular resistance. For cell pairs with asymmetrical cell sizes, there is a large range of values of intercellular resistance over which unidirectional block occurs with conduction successful from the larger cell to the smaller cell but with conduction block from the smaller cell to the larger cell. We then further show that one important component which limits the conduction process is the large early repolarization which occurs in the stimulated cell during the process of conduction, a process that we term "source loading."
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allessie M. A., Bonke F. I., Schopman F. J. The mechanism of supraventricular tachycardia induced by a single premature beat in the isolated left atrium of the rabbit. I. Circus movement as a consequence of unidirectional block of the premature impulse. Recent Adv Stud Cardiac Struct Metab. 1975;5:303–308. [PubMed] [Google Scholar]
- Barold S. S., Fracp M. B., Coumel P. Mechanisms of atrioventricular junctional tachycardia. Role of reentry and concealed accessory bypass tracts. Am J Cardiol. 1977 Jan;39(1):97–106. doi: 10.1016/s0002-9149(77)80018-0. [DOI] [PubMed] [Google Scholar]
- Bernstein R. C., Frame L. H. Ventricular reentry around a fixed barrier. Resetting with advancement in an in vitro model. Circulation. 1990 Jan;81(1):267–280. doi: 10.1161/01.cir.81.1.267. [DOI] [PubMed] [Google Scholar]
- Bonke F. I., Allessie M. A., Bouman L. N. Reentry in the atrium. Bull Schweiz Akad Med Wiss. 1975 Aug;31(1-3):33–44. [PubMed] [Google Scholar]
- Chen P. S., Wolf P. D., Dixon E. G., Danieley N. D., Frazier D. W., Smith W. M., Ideker R. E. Mechanism of ventricular vulnerability to single premature stimuli in open-chest dogs. Circ Res. 1988 Jun;62(6):1191–1209. doi: 10.1161/01.res.62.6.1191. [DOI] [PubMed] [Google Scholar]
- Cranefield P. F., Klein H. O., Hoffman B. F. Conduction of the cardiac impulse. 1. Delay, block, and one-way block in depressed Purkinje fibers. Circ Res. 1971 Feb;28(2):199–219. doi: 10.1161/01.res.28.2.199. [DOI] [PubMed] [Google Scholar]
- De la Fuente D., Sasyniuk B., Moe G. K. Conduction through a narrow isthmus in isolated canine atrial tissue. A model of the W-P-W syndrome. Circulation. 1971 Nov;44(5):803–809. doi: 10.1161/01.cir.44.5.803. [DOI] [PubMed] [Google Scholar]
- Durrer D., Lie K. I., Janse M. J., Schuilenburg R. M. Mechanisms of tachyarrhythmias, past and present. Eur J Cardiol. 1978 Sep;8(2):281–297. [PubMed] [Google Scholar]
- Frazier D. W., Wolf P. D., Wharton J. M., Tang A. S., Smith W. M., Ideker R. E. Stimulus-induced critical point. Mechanism for electrical initiation of reentry in normal canine myocardium. J Clin Invest. 1989 Mar;83(3):1039–1052. doi: 10.1172/JCI113945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. F., Jr, Heger J. J., Prystowsky E. N., Zipes D. P. Cellular electrophysiologic abnormalities of diseased human ventricular myocardium. Am J Cardiol. 1983 Jan 1;51(1):137–144. doi: 10.1016/s0002-9149(83)80024-1. [DOI] [PubMed] [Google Scholar]
- Hamamoto H., Kinoshita E., Tomoda H., Goto Y. The evidence of transmural unidirectional block by experimental current induced ventricular tachycardia in the canine heart. Jpn Circ J. 1987 Feb;51(2):181–187. doi: 10.1253/jcj.51.181. [DOI] [PubMed] [Google Scholar]
- Hellam D. C., Studt J. W. Linear analysis of membrane conductance and capacitance in cardiac Purkinje fibres. J Physiol. 1974 Dec;243(3):661–694. doi: 10.1113/jphysiol.1974.sp010771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse M. J., Capucci A., Coronel R., Fabius M. A. Variability of recovery of excitability in the normal canine and the ischaemic porcine heart. Eur Heart J. 1985 Nov;6 (Suppl 500):41–52. doi: 10.1093/eurheartj/6.suppl_d.41. [DOI] [PubMed] [Google Scholar]
- Joyner R. W. Mechanisms of unidirectional block in cardiac tissues. Biophys J. 1981 Jul;35(1):113–125. doi: 10.1016/S0006-3495(81)84778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner R. W., Overholt E. D., Ramza B., Veenstra R. D. Propagation through electrically coupled cells: two inhomogeneously coupled cardiac tissue layers. Am J Physiol. 1984 Oct;247(4 Pt 2):H596–H609. doi: 10.1152/ajpheart.1984.247.4.H596. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Veenstra R., Rawling D., Chorro A. Propagation through electrically coupled cells. Effects of a resistive barrier. Biophys J. 1984 May;45(5):1017–1025. doi: 10.1016/S0006-3495(84)84247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama A., Eguchi K., Shibayama R. Circus movement tachycardia induced by a single premature stimulus on the ventricular sheet--evaluation of the leading circle hypothesis in the canine ventricular muscle. Jpn Circ J. 1986 Jan;50(1):65–73. doi: 10.1253/jcj.50.65. [DOI] [PubMed] [Google Scholar]
- Kienzle M. G., Tan R. C., Ramza B. M., Young M. L., Joyner R. W. Alterations in endocardial activation of the canine papillary muscle early and late after myocardial infarction. Circulation. 1987 Oct;76(4):860–874. doi: 10.1161/01.cir.76.4.860. [DOI] [PubMed] [Google Scholar]
- Kim S. S., Lal R., Ruffy R. Paroxysmal nonreentrant supraventricular tachycardia due to simultaneous fast and slow pathway conduction in dual atrioventricular node pathways. J Am Coll Cardiol. 1987 Aug;10(2):456–461. doi: 10.1016/s0735-1097(87)80032-3. [DOI] [PubMed] [Google Scholar]
- Lazzara R., Scherlag B. J. Generation of arrhythmias in myocardial ischemia and infarction. Am J Cardiol. 1988 Jan 15;61(2):20A–26A. doi: 10.1016/0002-9149(88)90737-0. [DOI] [PubMed] [Google Scholar]
- Lesh M. D., Pring M., Spear J. F. Cellular uncoupling can unmask dispersion of action potential duration in ventricular myocardium. A computer modeling study. Circ Res. 1989 Nov;65(5):1426–1440. doi: 10.1161/01.res.65.5.1426. [DOI] [PubMed] [Google Scholar]
- Lin F. C., Yeh S. J., Wu D. Determinants of simultaneous fast and slow pathway conduction in patients with dual atrioventricular nodal pathways. Am Heart J. 1985 May;109(5 Pt 1):963–970. doi: 10.1016/0002-8703(85)90236-4. [DOI] [PubMed] [Google Scholar]
- MOE G. K., MENDEZ C., HAN J. ABERRANT A-V IMPULSE PROPAGATION IN THE DOG HEART: A STUDY OF FUNCTIONAL BUNDLE BRANCH BLOCK. Circ Res. 1965 Mar;16:261–286. doi: 10.1161/01.res.16.3.261. [DOI] [PubMed] [Google Scholar]
- MOE G. K., PRESTON J. B., BURLINGTON H. Physiologic evidence for a dual A-V transmission system. Circ Res. 1956 Jul;4(4):357–375. doi: 10.1161/01.res.4.4.357. [DOI] [PubMed] [Google Scholar]
- Mahmud R., Denker S. T., Lehmann M. H., Addas A., Akhtar M. Unidirectional retrograde atrioventricular nodal block in man: determinants of reversibility by vagal antagonism. Am Heart J. 1985 Sep;110(3):568–574. doi: 10.1016/0002-8703(85)90076-6. [DOI] [PubMed] [Google Scholar]
- Mazgalev T., Dreifus L. S., Bianchi J., Michelson E. L. The mechanism of AV junctional reentry: role of the atrionodal junction. Anat Rec. 1981 Sep;201(1):179–188. doi: 10.1002/ar.1092010119. [DOI] [PubMed] [Google Scholar]
- Okumura K., Henthorn R. W., Epstein A. E., Plumb V. J., Waldo A. L. Further observations on transient entrainment: importance of pacing site and properties of the components of the reentry circuit. Circulation. 1985 Dec;72(6):1293–1307. doi: 10.1161/01.cir.72.6.1293. [DOI] [PubMed] [Google Scholar]
- Overholt E. D., Joyner R. W., Veenstra R. D., Rawling D., Wiedmann R. Unidirectional block between Purkinje and ventricular layers of papillary muscles. Am J Physiol. 1984 Oct;247(4 Pt 2):H584–H595. doi: 10.1152/ajpheart.1984.247.4.H584. [DOI] [PubMed] [Google Scholar]
- Pressler M. L. Cable analysis in quiescent and active sheep Purkinje fibres. J Physiol. 1984 Jul;352:739–757. doi: 10.1113/jphysiol.1984.sp015319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan W., Rudy Y. Unidirectional block and reentry of cardiac excitation: a model study. Circ Res. 1990 Feb;66(2):367–382. doi: 10.1161/01.res.66.2.367. [DOI] [PubMed] [Google Scholar]
- Reddy C. P., Lynch M. Abolition and modification of reentry within the His-Purkinje system by procainamide in man. Circulation. 1978 Dec;58(6):1010–1022. doi: 10.1161/01.cir.58.6.1010. [DOI] [PubMed] [Google Scholar]
- Salerno J. A., Tavazzi L., Chimienti M., Ray M., Bobba P. Paroxysmal atrioventricular tachycardia involving an anomalous pathway with antegrade unidirectional block. Eur J Cardiol. 1979 Apr;9(4):285–305. [PubMed] [Google Scholar]
- Smith J. M., Cohen R. J. Simple finite-element model accounts for wide range of cardiac dysrhythmias. Proc Natl Acad Sci U S A. 1984 Jan;81(1):233–237. doi: 10.1073/pnas.81.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R. C., Joyner R. W. Electrotonic influences on action potentials from isolated ventricular cells. Circ Res. 1990 Nov;67(5):1071–1081. doi: 10.1161/01.res.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Ursell P. C., Gardner P. I., Albala A., Fenoglio J. J., Jr, Wit A. L. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985 Mar;56(3):436–451. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- Wald R. W., Waxman M. B., Downar E. The effect of antiarrhythmic drugs on depressed conduction and unidirectional block in sheep Purkinje fibers. Circ Res. 1980 May;46(5):612–619. doi: 10.1161/01.res.46.5.612. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. Effects of calcium and sodium concentrations on atrioventricular conduction: experimental study in rabbit hearts with clinical implications on heart block and slow calcium channel blocking agent usage. Am Heart J. 1981 Nov;102(5):883–891. doi: 10.1016/0002-8703(81)90040-5. [DOI] [PubMed] [Google Scholar]
- Waxman M. B., Downar E., Wald R. W. Unidirectional block in Purkinje fibers. Can J Physiol Pharmacol. 1980 Aug;58(8):925–933. doi: 10.1139/y80-141. [DOI] [PubMed] [Google Scholar]
- Weingart R., Maurer P. Action potential transfer in cell pairs isolated from adult rat and guinea pig ventricles. Circ Res. 1988 Jul;63(1):72–80. doi: 10.1161/01.res.63.1.72. [DOI] [PubMed] [Google Scholar]


