Abstract
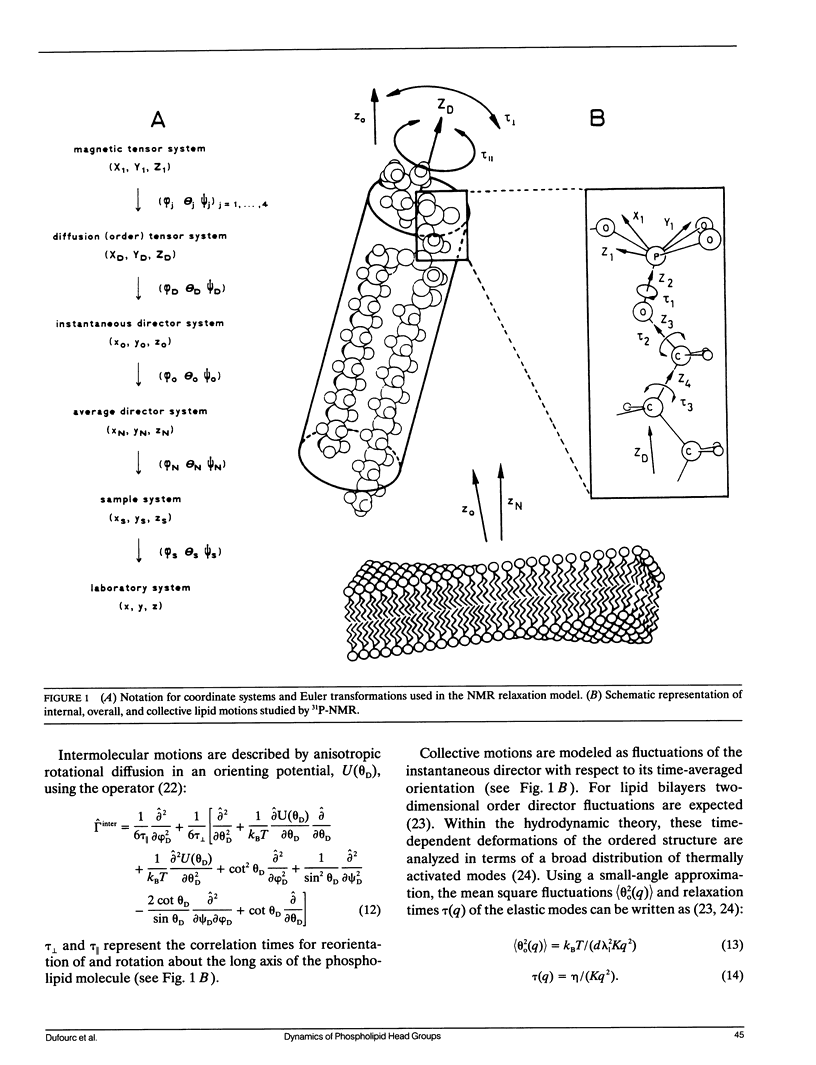
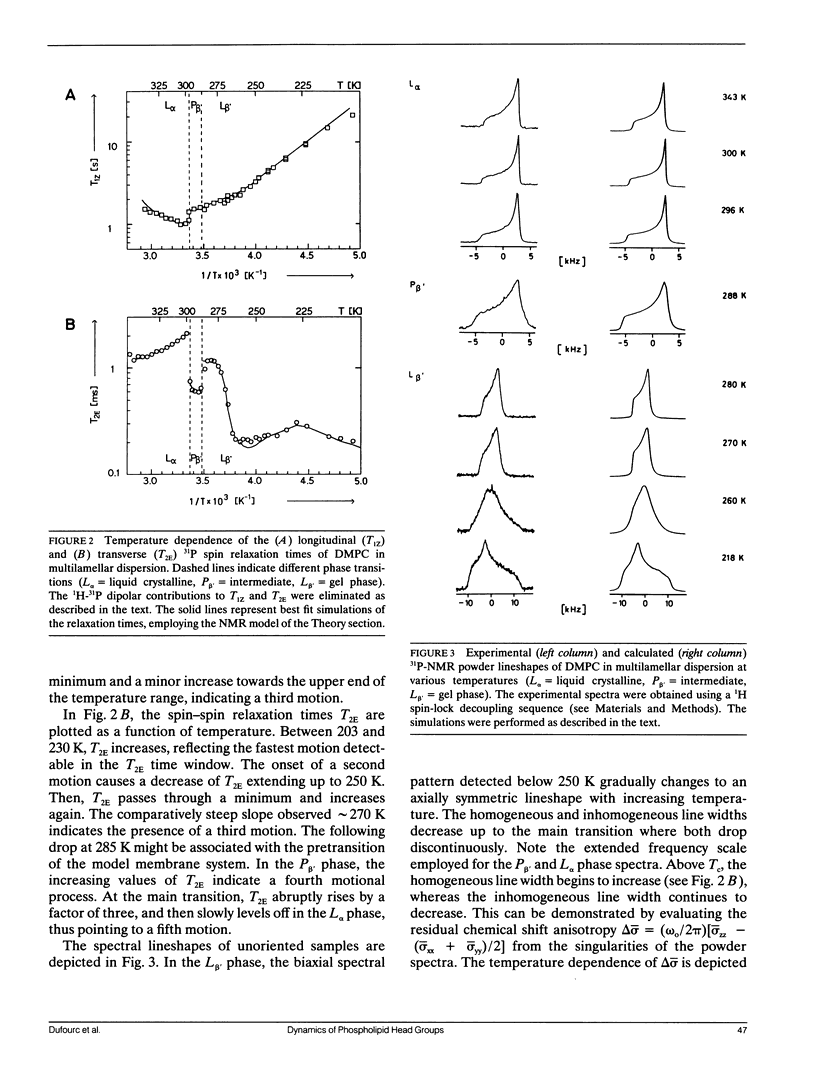
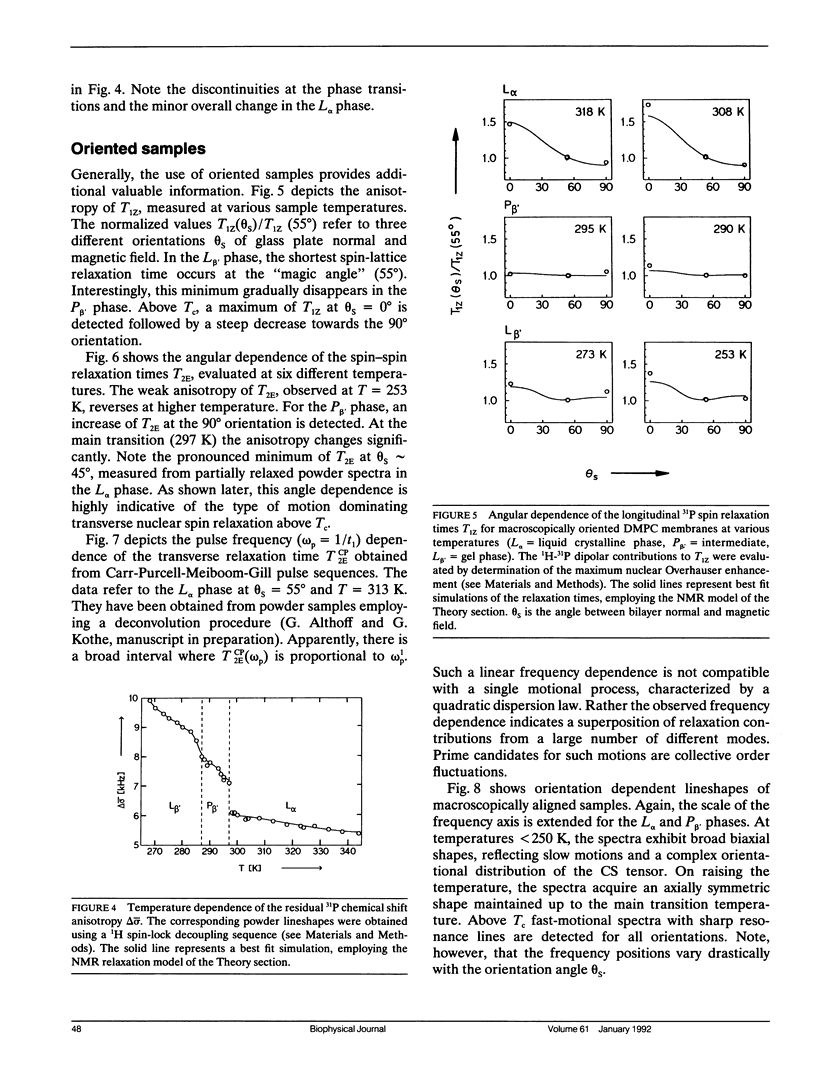
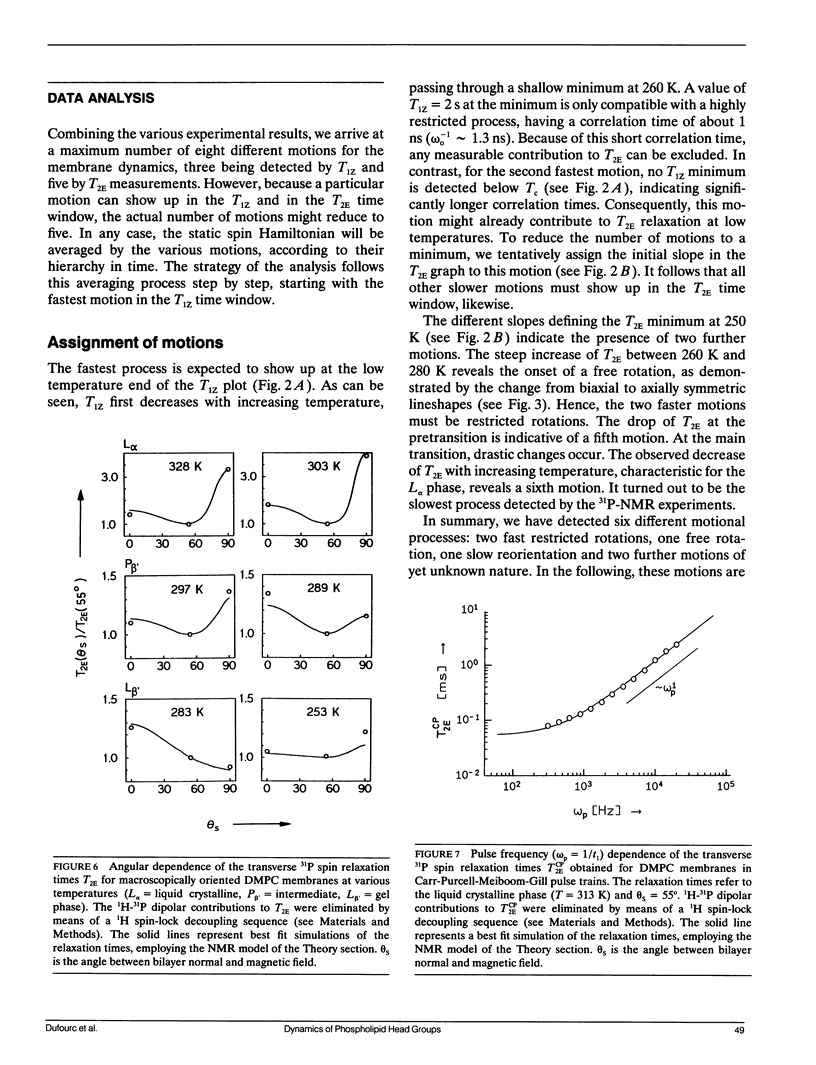
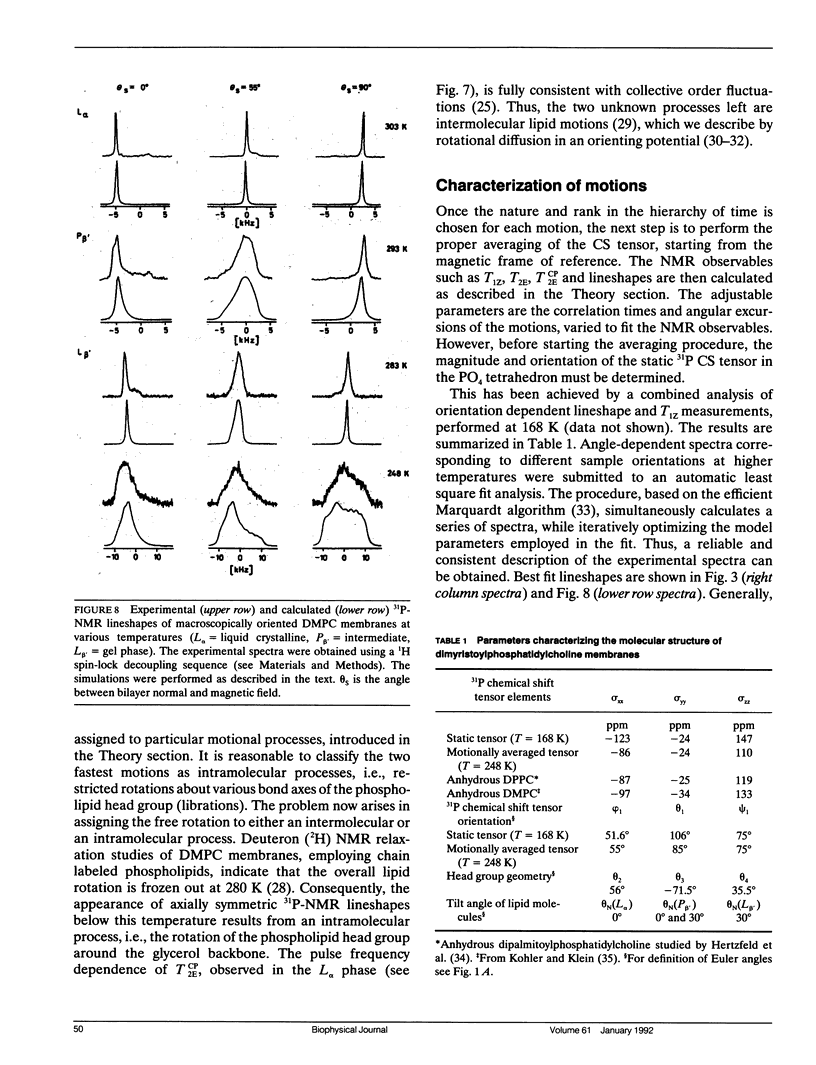
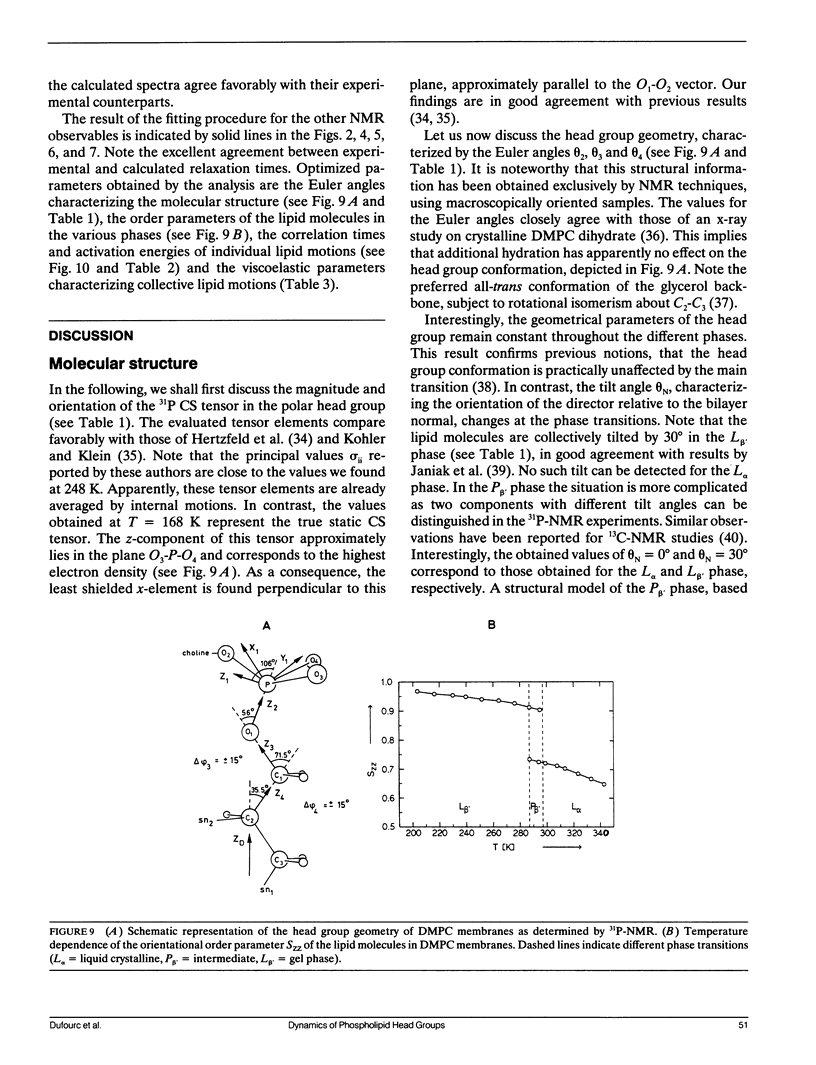
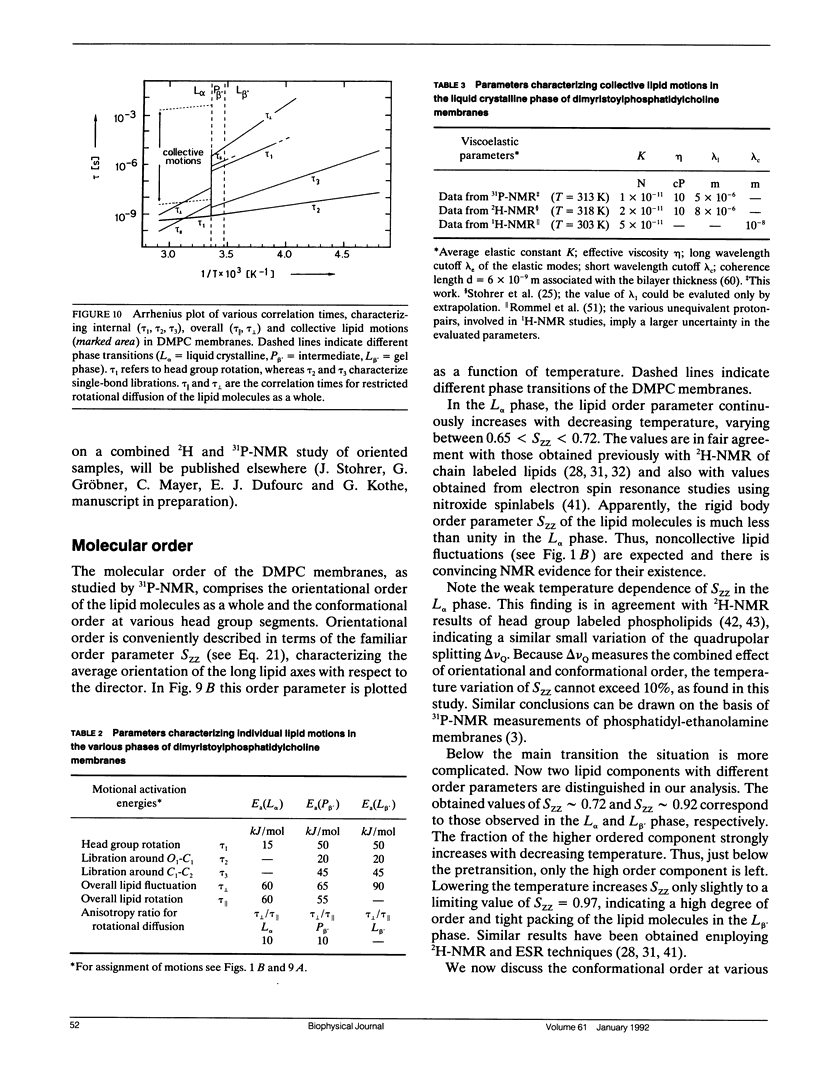
Phospholipid head group dynamics have been studied by pulsed phosphorus-31 nuclear magnetic resonance (31P-NMR) of unoriented and macroscopically aligned dimyristoylphosphatidylcholine model membranes in the temperature range, 203-343 K. Lineshapes and echo intensities have been recorded as a function of interpulse delay times, temperature and macroscopic orientation of the bilayer normal with respect to the magnetic field. The dipolar proton-phosphorus (1H-31P) contribution to the transverse relaxation time, T2E, and to lineshapes was eliminated by means of a proton spin-lock sequence. In case of longitudinal spin relaxation, T1Z, the amount of dipolar coupling was evaluated by measuring the maximum nuclear Overhauser enhancement. Hence, the results could be analyzed by considering chemical shift anisotropy as the only relaxation mechanism. The presence of various minima both in T1Z and T2E temperature plots as well as the angular dependence of these relaxation times allowed description of the dynamics of the phosphate head group in the 31P-NMR time window, by three different motional classes, i.e., intramolecular, intermolecular and collective motions. The intramolecular motions consist of two hindered rotations and one free rotation around the bonds linking the phosphate head group to the glycerol backbone. These motions are the fastest in the hierarchy of time with correlation times varying from less than 10(-12) to 10(-6) s in the temperature range investigated. The intermolecular motions are assigned to phospholipid long axis rotation and fluctuation. They have correlation times ranging from 10(-11) s at high temperatures to 10(-3) s at low temperatures. The slowest motion affecting the 31P-NMR observables is assigned to viscoelastic modes, i.e., so called order director fluctuations and is only detected at high temperatures, above the main transition in pulse frequency dependent T2ECP experiments. Comprehensive analysis of the phosphate head group dynamics is achieved by a dynamic NMR model based on the stochastic Liouville equation. In addition to correlation times, this analysis provides activation energies and order parameters for the various motions, and a value for the bilayer elastic constant.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cullis P. R., De Kruyff B. 31P NMR studies of unsonicated aqueous dispersions of neutral and acidic phospholipids. Effects of phase transitions, p2H and divalent cations on the motion in the phosphate region of the polar headgroup. Biochim Biophys Acta. 1976 Jul 1;436(3):523–540. doi: 10.1016/0005-2736(76)90438-7. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Bonmatin J. M., Dufourcq J. Membrane structure and dynamics by 2H- and 31P-NMR. Effects of amphipatic peptidic toxins on phospholipid and biological membranes. Biochimie. 1989 Jan;71(1):117–123. doi: 10.1016/0300-9084(89)90141-7. [DOI] [PubMed] [Google Scholar]
- Gally H. U., Niederberger W., Seelig J. Conformation and motion of the choline head group in bilayers of dipalmitoyl-3-sn-phosphatidylcholine. Biochemistry. 1975 Aug 12;14(16):3647–3652. doi: 10.1021/bi00687a021. [DOI] [PubMed] [Google Scholar]
- Ghosh R. 31P and 2H NMR studies of structure and motion in bilayers of phosphatidylcholine and phosphatidylethanolamine. Biochemistry. 1988 Oct 4;27(20):7750–7758. doi: 10.1021/bi00420a025. [DOI] [PubMed] [Google Scholar]
- Griffin R. G., Powers L., Pershan P. S. Head-group conformation in phospholipids: a phosphorus-31 nuclear magnetic resonance study of oriented monodomain dipalmitoylphosphatidylcholine bilayers. Biochemistry. 1978 Jul 11;17(14):2718–2722. doi: 10.1021/bi00607a004. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Sundell S. Preferred conformation and dynamics of the glycerol backbone in phospholipids. An NMR and X-ray single-crystal analysis. Biochemistry. 1988 Dec 27;27(26):9166–9174. doi: 10.1021/bi00426a014. [DOI] [PubMed] [Google Scholar]
- Herzfeld J., Griffin R. G., Haberkorn R. A. Phosphorus-31 chemical-shift tensors in barium diethyl phosphate and urea-phosphoric acid: model compounds for phospholipid head-group studies. Biochemistry. 1978 Jul 11;17(14):2711–2718. doi: 10.1021/bi00607a003. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Kar L., Ney-Igner E., Freed J. H. Electron spin resonance and electron-spin-echo study of oriented multilayers of L alpha-dipalmitoylphosphatidylcholine water systems. Biophys J. 1985 Oct;48(4):569–595. doi: 10.1016/S0006-3495(85)83814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. 31P nuclear magnetic resonance chemical shielding tensors of phosphorylethanolamine, lecithin, and related compounds: Applications to head-group motion in model membranes. Biochemistry. 1976 Mar 9;15(5):967–974. doi: 10.1021/bi00650a004. [DOI] [PubMed] [Google Scholar]
- Kohler S. J., Klein M. P. Orientation and dynamics of phospholipid head groups in bilayers and membranes determined from 31P nuclear magnetic resonance chemical shielding tensors. Biochemistry. 1977 Feb 8;16(3):519–526. doi: 10.1021/bi00622a028. [DOI] [PubMed] [Google Scholar]
- Lange A., Marsh D., Wassmer K. H., Meier P., Kothe G. Electron spin resonance study of phospholipid membranes employing a comprehensive line-shape model. Biochemistry. 1985 Jul 30;24(16):4383–4392. doi: 10.1021/bi00337a020. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. I. Lipid phase transitions. Biochim Biophys Acta. 1977 Aug 9;472(2):237–281. doi: 10.1016/0304-4157(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Milburn M. P., Jeffrey K. R. Dynamics of the phosphate group in phospholipid bilayers. A 31P angular dependent nuclear spin relaxation time study. Biophys J. 1989 Sep;56(3):543–549. doi: 10.1016/S0006-3495(89)82701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn M. P., Jeffrey K. R. Dynamics of the phosphate group in phospholipid bilayers. A 31P nuclear relaxation time study. Biophys J. 1987 Nov;52(5):791–799. doi: 10.1016/S0006-3495(87)83273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J., Gally H. Investigation of phosphatidylethanolamine bilayers by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 1976 Nov 30;15(24):5199–5204. doi: 10.1021/bi00669a001. [DOI] [PubMed] [Google Scholar]
- Seelig J., Tamm L., Hymel L., Fleischer S. Deuterium and phosphorus nuclear magnetic resonance and fluorescence depolarization studies of functional reconstituted sarcoplasmic reticulum membrane vesicles. Biochemistry. 1981 Jun 23;20(13):3922–3932. doi: 10.1021/bi00516a040. [DOI] [PubMed] [Google Scholar]
- Watnick P. I., Dea P., Chan S. I. Characterization of the transverse relaxation rates in lipid bilayers. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2082–2086. doi: 10.1073/pnas.87.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittebort R. J., Schmidt C. F., Griffin R. G. Solid-state carbon-13 nuclear magnetic resonance of the lecithin gel to liquid-crystalline phase transition. Biochemistry. 1981 Jul 7;20(14):4223–4228. doi: 10.1021/bi00517a042. [DOI] [PubMed] [Google Scholar]


