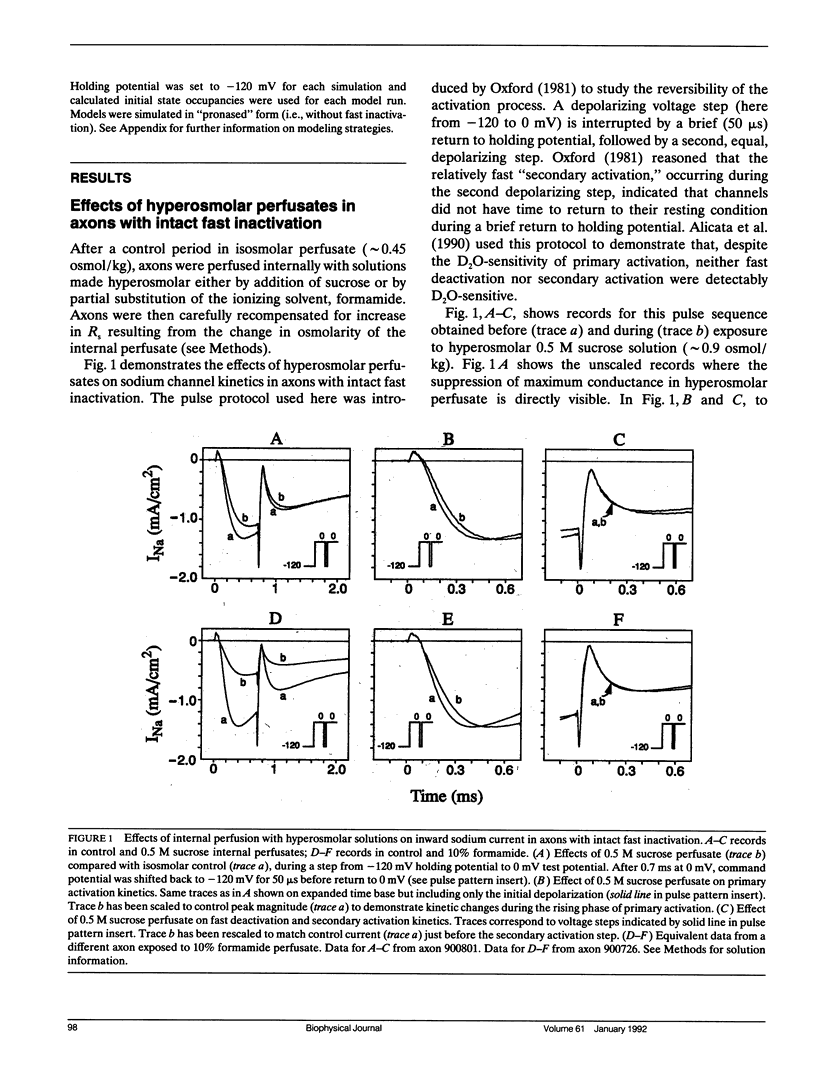
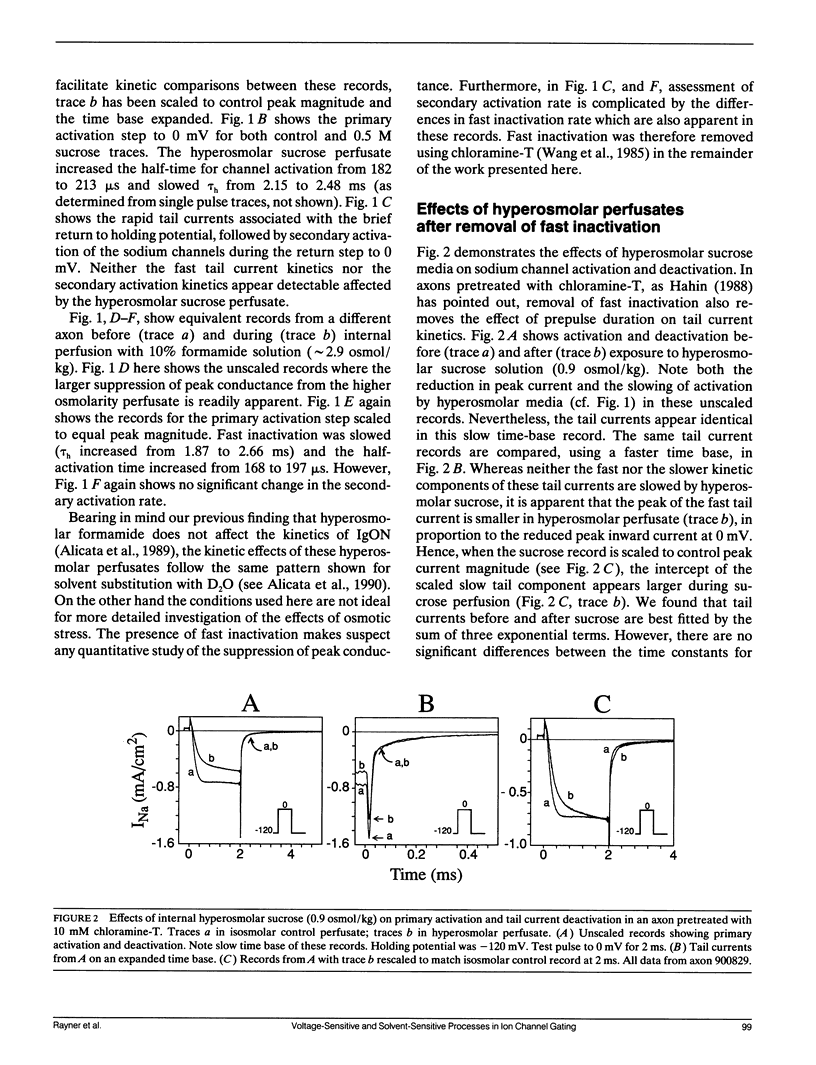
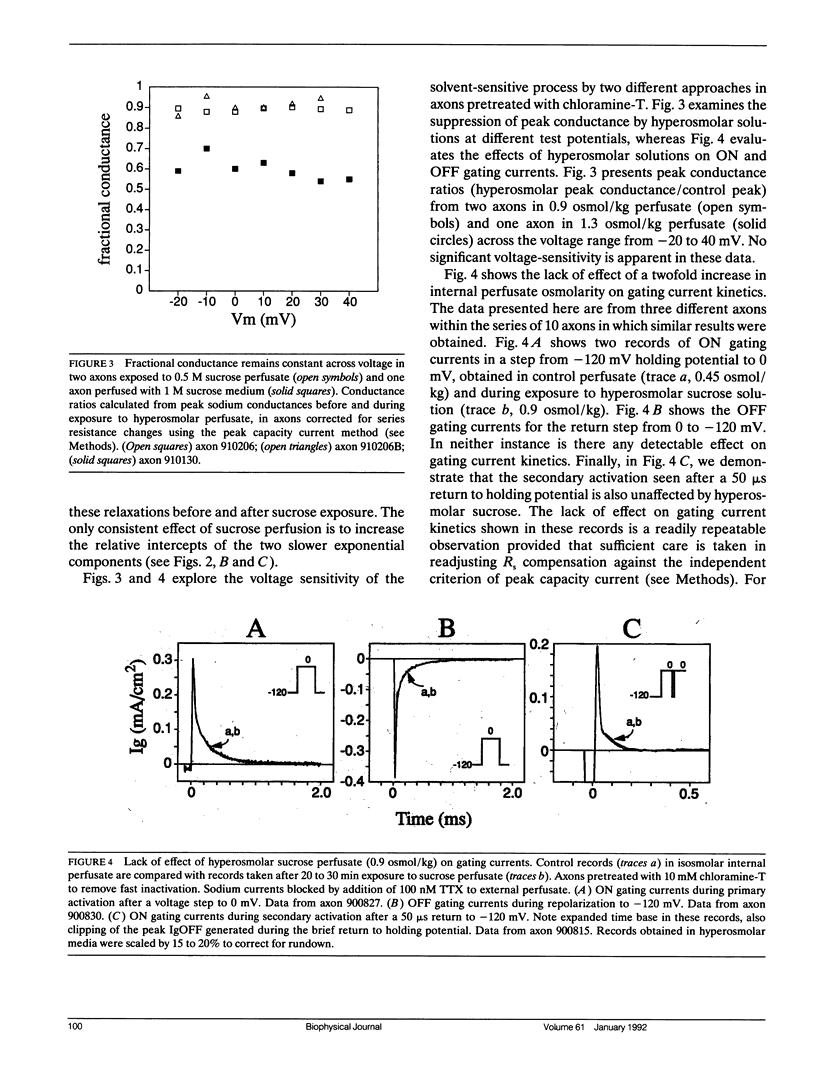
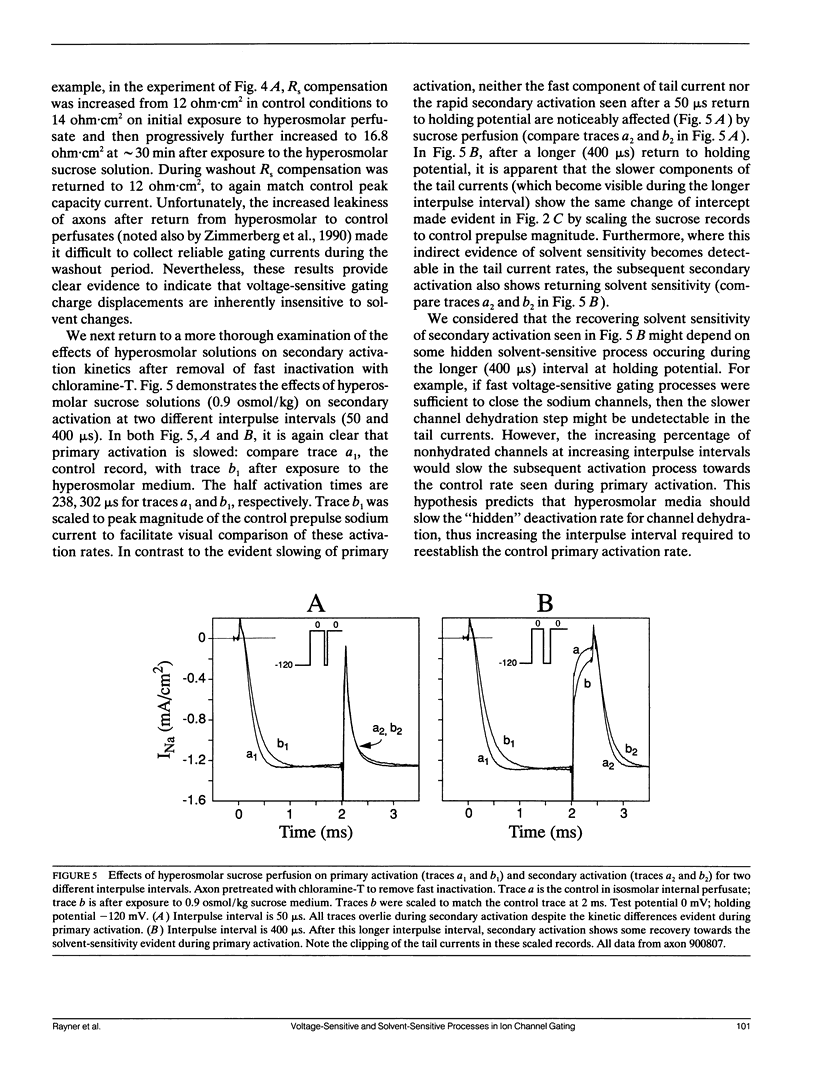
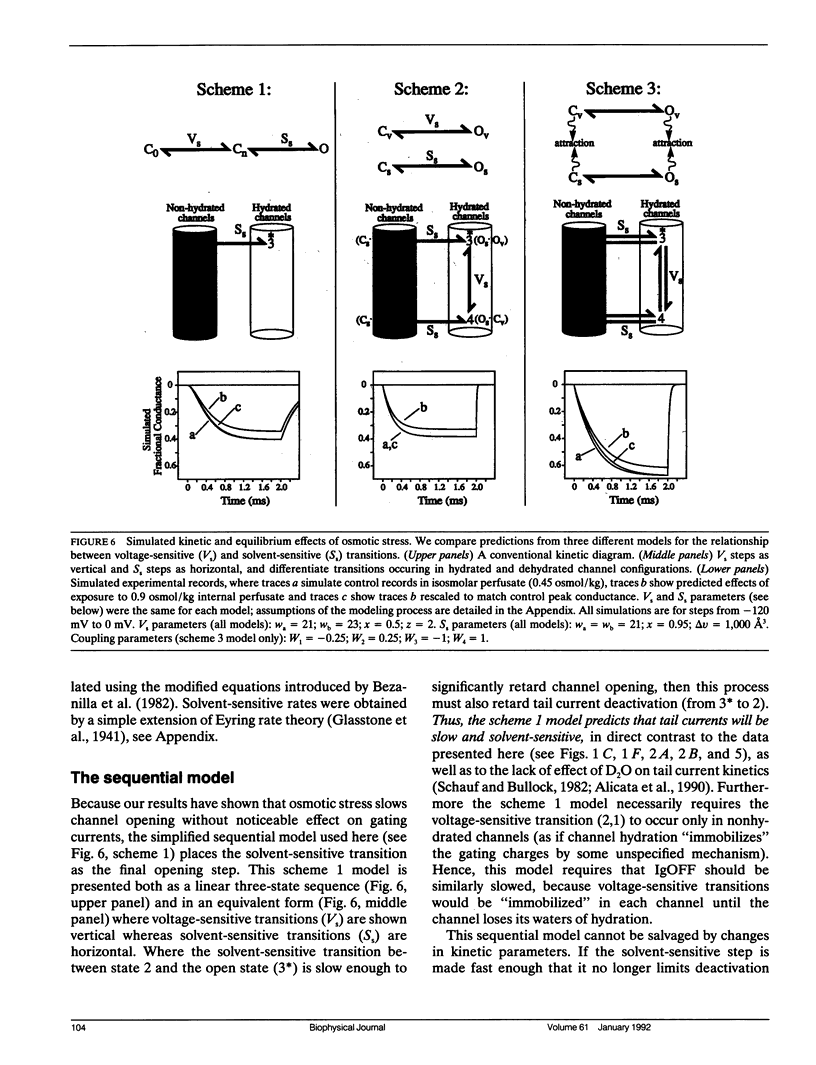
Abstract
Kinetic effects of osmotic stress on sodium ionic and gating currents have been studied in crayfish giant axons after removal of fast inactivation with chloramine-T. Internal perfusion with media made hyperosmolar by addition of formamide or sucrose, reduces peak sodium current (before and after removal of fast inactivation with chloramine-T), increases the half-time for activation, but has no effect on tail current deactivation rate(s). Kinetics of ON and OFF gating currents are not affected by osmotic stress. These results confirm (and extend to sodium channels) the separation of channel gating mechanisms into voltage-sensitive and solvent-sensitive processes recently proposed by Zimmerberg J., F. Bezanilla, and V. A. Parsegian. (1990. Biophys. J. 57:1049-1064) for potassium delayed rectifier channels. Additionally, the kinetic effects produced by hyperosmolar media seem qualitatively similar to the kinetic effects of heavy water substitution in crayfish axons (Alicata, D. A., M. D. Rayner, and J. G. Starkus. 1990. Biophys. J. 57:745-758). However, our observations are incompatible with models in which voltage-sensitive and solvent-sensitive gating processes are presumed to be either (a) strictly sequential or, (b) parallel and independent. We introduce a variant of the parallel model which includes explicit coupling between voltage-sensitive and solvent-sensitive processes. Simulations of this model, in which the total coupling energy is as small as 1/10th of kT, demonstrate the characteristic kinetic changes noted in our data.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alicata D. A., Rayner M. D., Starkus J. G. Osmotic and pharmacological effects of formamide on capacity current, gating current, and sodium current in crayfish giant axons. Biophys J. 1989 Feb;55(2):347–353. doi: 10.1016/S0006-3495(89)82811-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alicata D. A., Rayner M. D., Starkus J. G. Sodium channel activation mechanisms. Insights from deuterium oxide substitution. Biophys J. 1990 Apr;57(4):745–758. doi: 10.1016/S0006-3495(90)82595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Taylor R. E., Fernández J. M. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J Gen Physiol. 1982 Jan;79(1):21–40. doi: 10.1085/jgp.79.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahin R. Removal of inactivation causes time-invariant sodium current decays. J Gen Physiol. 1988 Sep;92(3):331–350. doi: 10.1085/jgp.92.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeness S. T., Starkus J. G. Saxitoxin and tetrodotoxin. Electrostatic effects on sodium channel gating current in crayfish axons. Biophys J. 1986 Mar;49(3):629–643. doi: 10.1016/S0006-3495(86)83690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D. A series-parallel model of the voltage-gated sodium channel. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1299):425–432. doi: 10.1098/rspb.1990.0046. [DOI] [PubMed] [Google Scholar]
- Meves H. The effect of holding potential on the asymmetry currents in squid gaint axons. J Physiol. 1974 Dec;243(3):847–867. doi: 10.1113/jphysiol.1974.sp010780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M. Channel protein engineering. An approach to the identification of molecular determinants of function in voltage-gated and ligand-regulated channel proteins. Ion Channels. 1990;2:1–31. [PubMed] [Google Scholar]
- Oiki S., Danho W., Montal M. Channel protein engineering: synthetic 22-mer peptide from the primary structure of the voltage-sensitive sodium channel forms ionic channels in lipid bilayers. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2393–2397. doi: 10.1073/pnas.85.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Lee B., Parsegian V. A. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution: hydration forces between parallel DNA double helices. Proc Natl Acad Sci U S A. 1984 May;81(9):2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner M. D., Starkus J. G. The steady-state distribution of gating charge in crayfish giant axons. Biophys J. 1989 Jan;55(1):1–19. doi: 10.1016/S0006-3495(89)82775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben P. C., Starkus J. G., Rayner M. D. Holding potential affects the apparent voltage-sensitivity of sodium channel activation in crayfish giant axons. Biophys J. 1990 Nov;58(5):1169–1181. doi: 10.1016/S0006-3495(90)82458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanley B. E., Hanck D. A., Chay T., Fozzard H. A. Kinetic analysis of single sodium channels from canine cardiac Purkinje cells. J Gen Physiol. 1990 Mar;95(3):411–437. doi: 10.1085/jgp.95.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Bullock J. O. Modifications of sodium channel gating in Myxicola giant axons by deuterium oxide, temperature, and internal cations. Biophys J. 1979 Aug;27(2):193–208. doi: 10.1016/S0006-3495(79)85211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Bullock J. O. Solvent substitution as a probe of channel gating in Myxicola. Differential effects of D2O on some components of membrane conductance. Biophys J. 1980 May;30(2):295–305. doi: 10.1016/S0006-3495(80)85095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Bullock J. O. Solvent substitution as a probe of channel gating in Myxicola. Effects of D2O on kinetic properties of drugs that occlude channels. Biophys J. 1982 Feb;37(2):441–452. doi: 10.1016/S0006-3495(82)84690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P. Ionic conductance changes in voltage clamped crayfish axons at low pH. J Gen Physiol. 1974 Dec;64(6):666–690. doi: 10.1085/jgp.64.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P., Starkus J. C., Lo M. V., Peracchia C. The periaxonal space of crayfish giant axons. J Gen Physiol. 1983 Aug;82(2):221–244. doi: 10.1085/jgp.82.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc C. K., Aldrich R. W. Gating of single non-Shaker A-type potassium channels in larval Drosophila neurons. J Gen Physiol. 1990 Jul;96(1):135–165. doi: 10.1085/jgp.96.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Heggeness S. T., Rayner M. D. Kinetic analysis of sodium channel block by internal methylene blue in pronased crayfish giant axons. Biophys J. 1984 Aug;46(2):205–218. doi: 10.1016/S0006-3495(84)84014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel activation in the squid giant axon. Steady state properties. J Gen Physiol. 1985 Jan;85(1):65–82. doi: 10.1085/jgp.85.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel gating currents. Origin of the rising phase. J Gen Physiol. 1987 Apr;89(4):521–540. doi: 10.1085/jgp.89.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Brodwick M. S., Eaton D. C. Removal of sodium channel inactivation in squid axon by the oxidant chloramine-T. J Gen Physiol. 1985 Aug;86(2):289–302. doi: 10.1085/jgp.86.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Alterations in activation gating of single Shaker A-type potassium channels by the Sh5 mutation. J Neurosci. 1990 Jun;10(6):1799–1810. doi: 10.1523/JNEUROSCI.10-06-01799.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Bezanilla F., Parsegian V. A. Solute inaccessible aqueous volume changes during opening of the potassium channel of the squid giant axon. Biophys J. 1990 May;57(5):1049–1064. doi: 10.1016/S0006-3495(90)82623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]



