Abstract
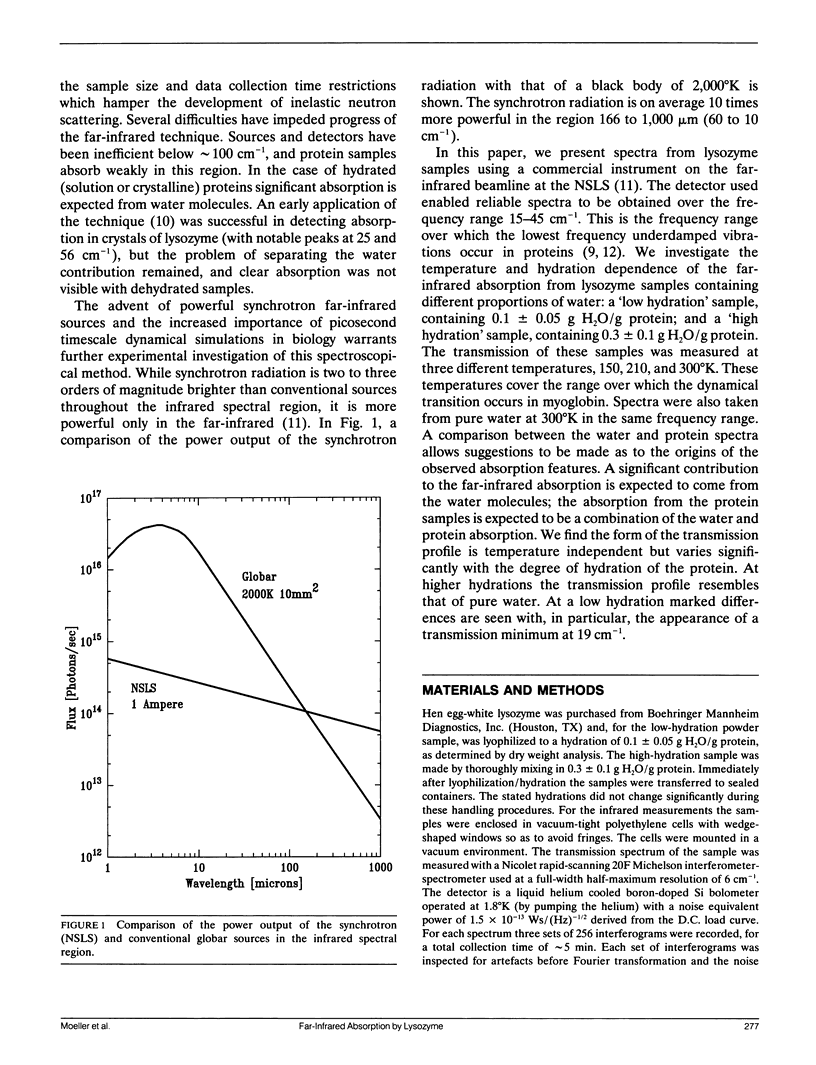
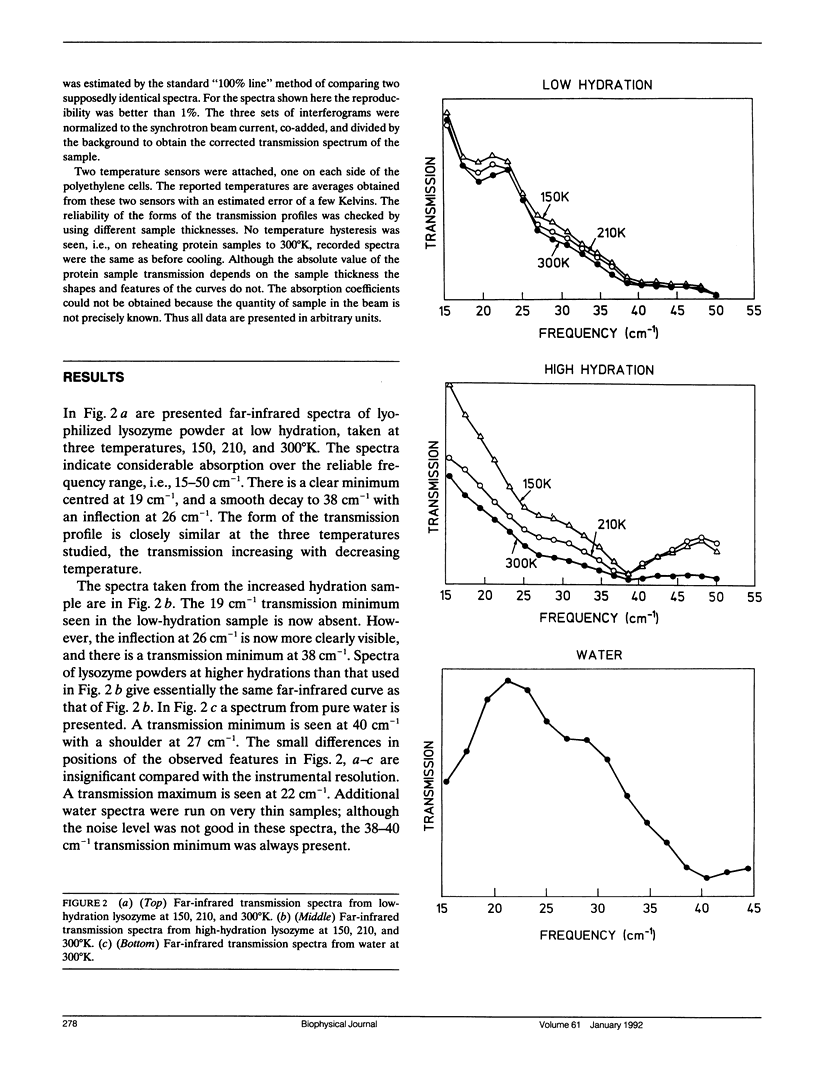
Using the National Synchrotron Light Source (NSLS) at Brookhaven far-infrared absorption in the frequency range 15-45 cm-1 was detected in samples of lysozyme at different hydrations and in water. The absorption is due to the presence of low-frequency (picosecond timescale) motion in the samples, such as are calculated in molecular dynamics simulations. The form of the transmission profile is temperature independent but varies significantly with the degree of hydration of the protein. At higher hydrations the profile resembles closely that of pure water in the region 20-45 cm-1. At a low hydration marked differences are seen with, in particular, the appearance of a transmission minimum at 19 cm-1. The possible origins of the hydration dependence are discussed. The results demonstrate the usefulness of long-wavelength synchrotron radiation for the characterisation of biologically-important low-frequency motions in protein samples.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ataka M., Tanaka S. Far-infrared spectrum of crystalline lysozyme. Biopolymers. 1979 Mar;18(3):507–516. doi: 10.1002/bip.1979.360180303. [DOI] [PubMed] [Google Scholar]
- Cusack S., Doster W. Temperature dependence of the low frequency dynamics of myoglobin. Measurement of the vibrational frequency distribution by inelastic neutron scattering. Biophys J. 1990 Jul;58(1):243–251. doi: 10.1016/S0006-3495(90)82369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack S., Smith J., Finney J., Tidor B., Karplus M. Inelastic neutron scattering analysis of picosecond internal protein dynamics. Comparison of harmonic theory with experiment. J Mol Biol. 1988 Aug 20;202(4):903–908. doi: 10.1016/0022-2836(88)90566-9. [DOI] [PubMed] [Google Scholar]
- Doster W., Cusack S., Petry W. Dynamical transition of myoglobin revealed by inelastic neutron scattering. Nature. 1989 Feb 23;337(6209):754–756. doi: 10.1038/337754a0. [DOI] [PubMed] [Google Scholar]
- Edsall J. T., McKenzie H. A. Water and proteins. II. The location and dynamics of water in protein systems and its relation to their stability and properties. Adv Biophys. 1983;16:53–183. doi: 10.1016/0065-227x(83)90008-4. [DOI] [PubMed] [Google Scholar]
- Elber R., Karplus M. Multiple conformational states of proteins: a molecular dynamics analysis of myoglobin. Science. 1987 Jan 16;235(4786):318–321. doi: 10.1126/science.3798113. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Smith J., Cusack S., Poole P., Finney J. Direct measurement of hydration-related dynamic changes in lysozyme using inelastic neutron scattering spectroscopy. J Biomol Struct Dyn. 1987 Feb;4(4):583–588. doi: 10.1080/07391102.1987.10507662. [DOI] [PubMed] [Google Scholar]
- Smith J., Kuczera K., Karplus M. Dynamics of myoglobin: comparison of simulation results with neutron scattering spectra. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1601–1605. doi: 10.1073/pnas.87.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]


