Abstract
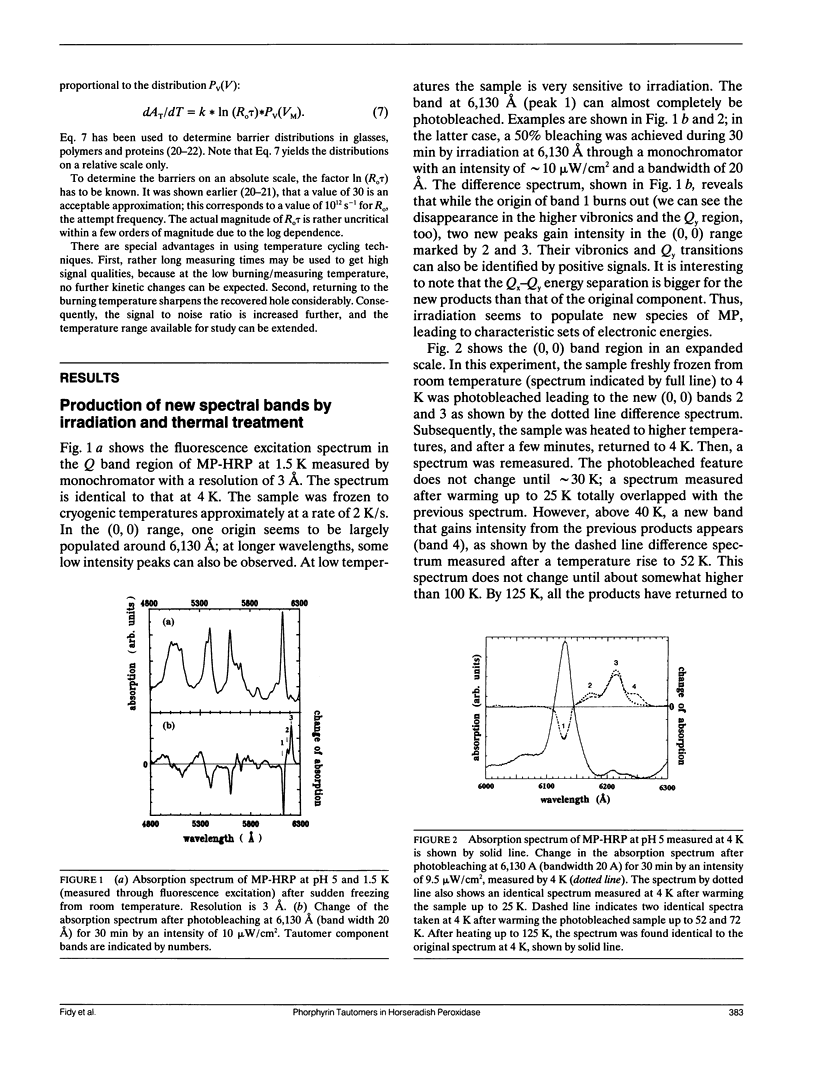
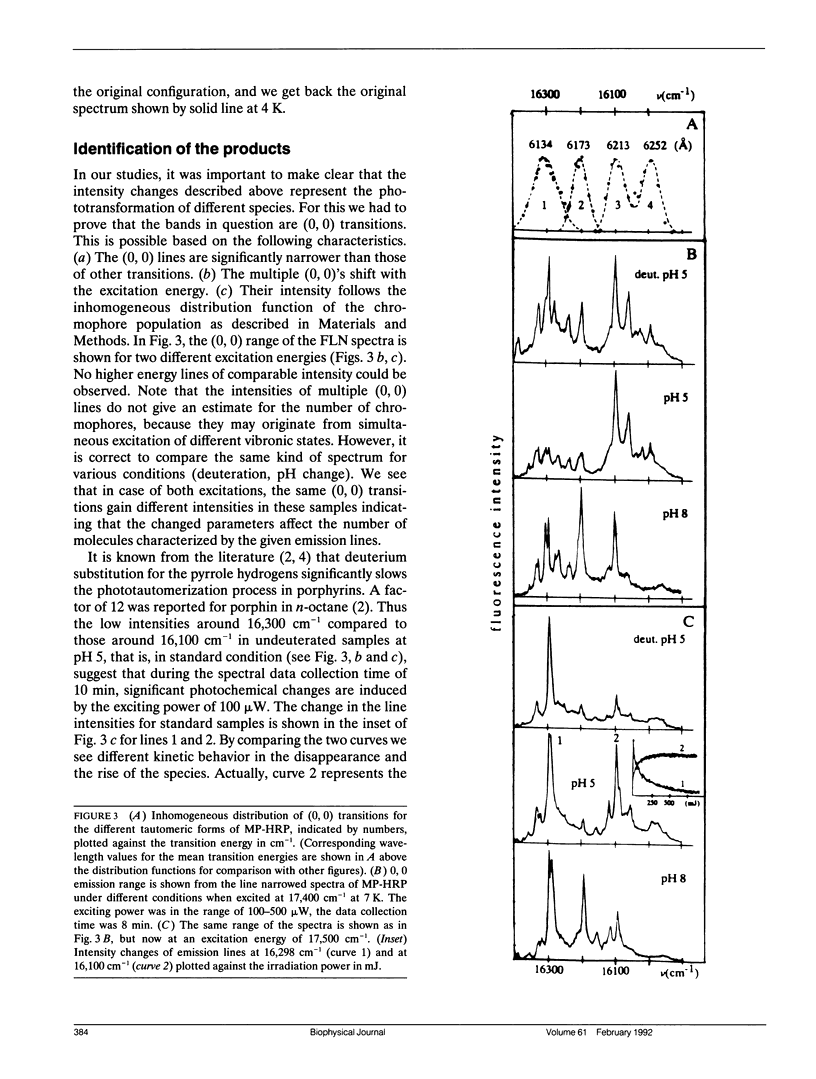
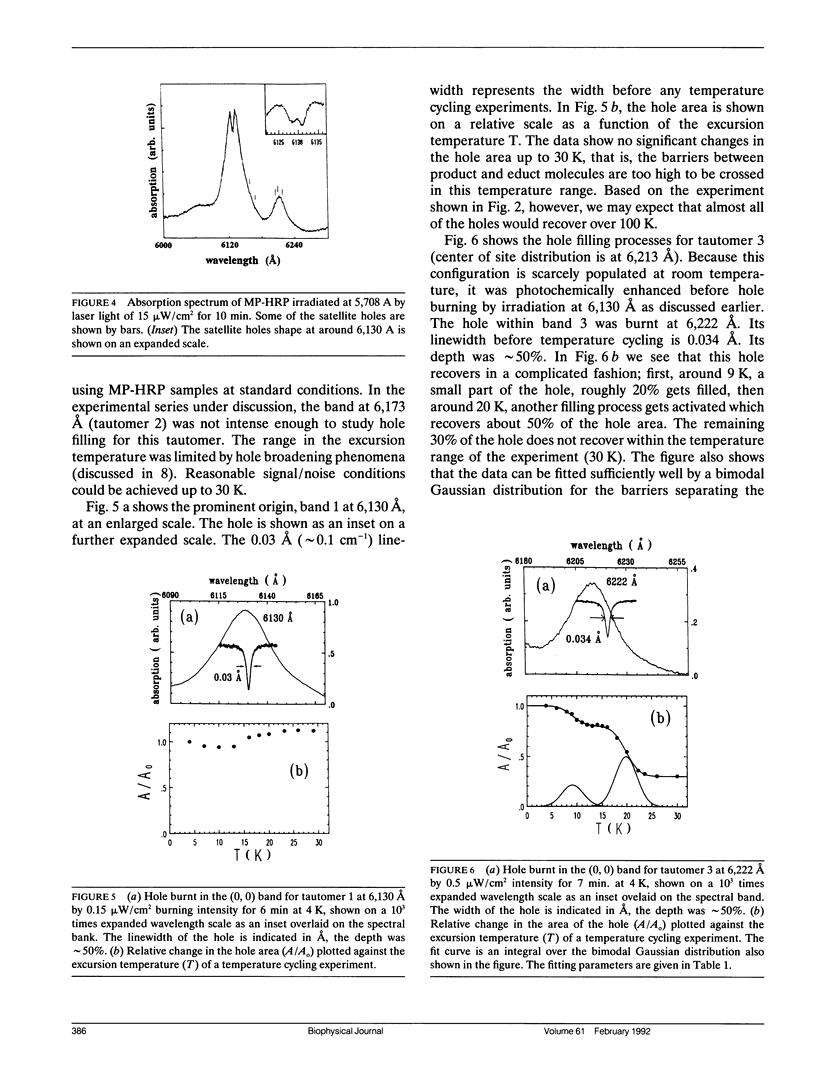
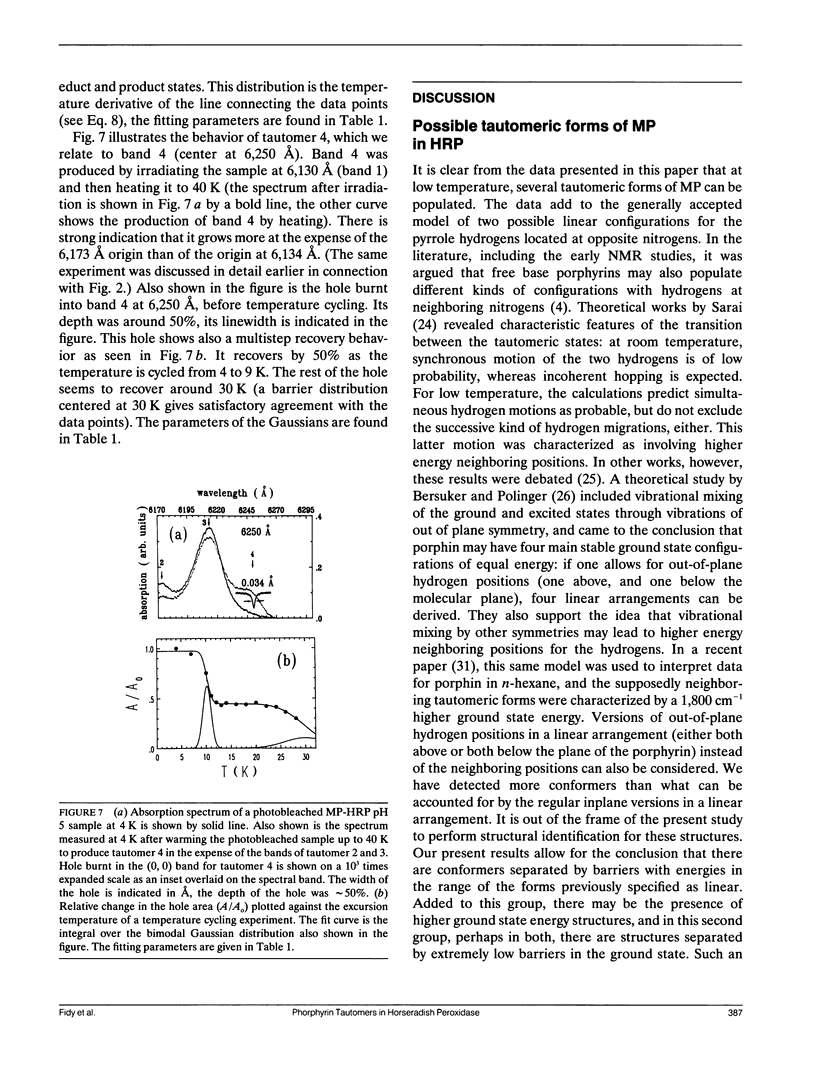
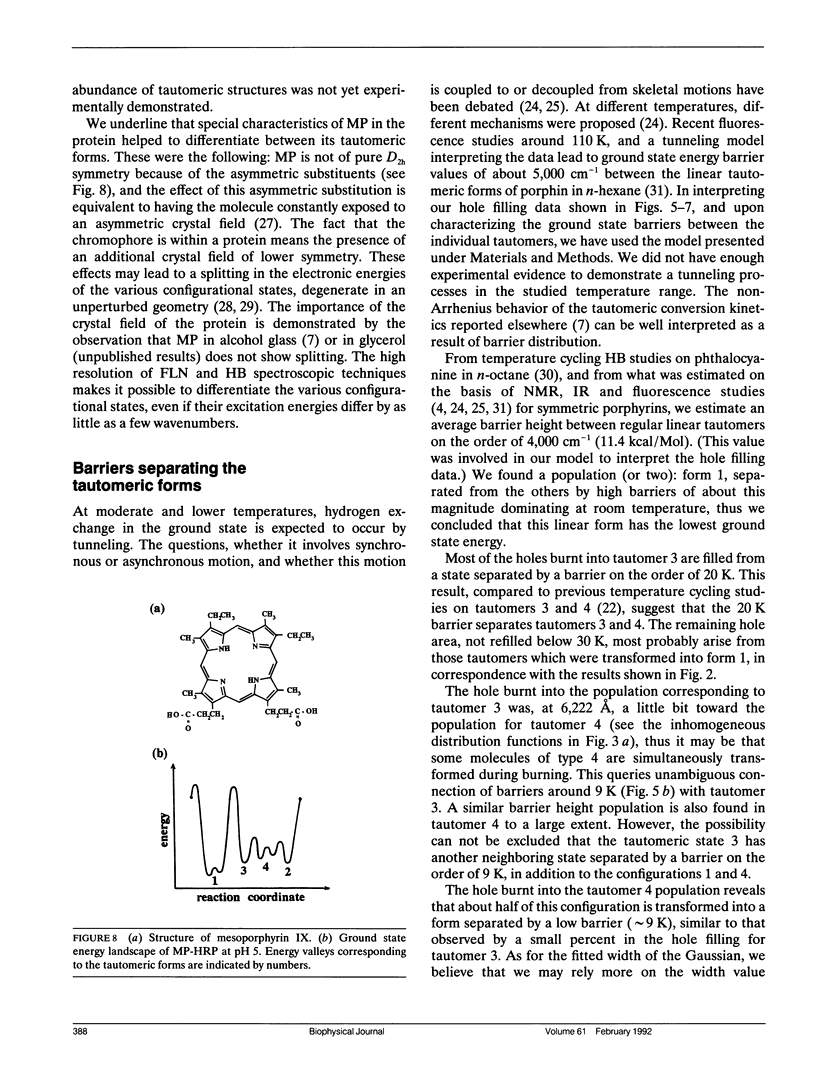
Mesoporphyrin IX substituted horseradish peroxidase was studied by fluorescence line narrowing and hole burning techniques at cryogenic temperatures. The spectral data reveal that four pyrrole tautomeric configurations of the chromophore are populated within the protein under the influence of irradiation and/or thermal treatment, and the existence of a fifth and a sixth tautomeric configuration is also likely. The relative population of the tautomers changes upon deuterium substitution through modification of the phototransition rate, and also depends on pH, which changes the protonation of neighboring amino acids in the heme pocket. The energy separation of the origins of the tautomers is approximately 100 cm-1. The distribution of barrier heights separating the different tautomeric forms in the ground state and their distribution was determined by temperature cycling hole burning. The distributions can be approximated by Gaussians. The experiment directly yields the distributions on a relative temperature scale, and a model is presented to transform the barrier heights into energy values. It is suggested that the energies for the tautomers are split partially due to the protein crystal field and that the trapping of the tautomeric forms is the consequence of interactions with neighboring amino acids within the heme pocket.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angiolillo P. J., Leigh J. S., Jr, Vanderkooi J. M. Resolved fluorescence emission spectra of iron-free cytochrome c. Photochem Photobiol. 1982 Aug;36(2):133–137. doi: 10.1111/j.1751-1097.1982.tb04354.x. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Berendzen J., Braunstein D. Temperature-derivative spectroscopy: a tool for protein dynamics. Proc Natl Acad Sci U S A. 1990 Jan;87(1):1–5. doi: 10.1073/pnas.87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet J. E., Jullian C., Jameson D. M. Oxygen diffusion through horseradish peroxidase. Photochem Photobiol. 1990 Apr;51(4):487–489. doi: 10.1111/j.1751-1097.1990.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Fidy J., Paul K. G., Vanderkooi J. M. Differences in the binding of aromatic substrates to horseradish peroxidase revealed by fluorescence line narrowing. Biochemistry. 1989 Sep 19;28(19):7531–7541. doi: 10.1021/bi00445a006. [DOI] [PubMed] [Google Scholar]
- Köhler W, Friedrich J. Distribution of barrier heights in amorphous organic materials. Phys Rev Lett. 1987 Nov 9;59(19):2199–2202. doi: 10.1103/PhysRevLett.59.2199. [DOI] [PubMed] [Google Scholar]
- Köhler W, Friedrich J, Scheer H. Conformational barriers in low-temperature proteins and glasses. Phys Rev A Gen Phys. 1988 Jan 15;37(2):660–662. doi: 10.1103/physreva.37.660. [DOI] [PubMed] [Google Scholar]
- Köhler W, Zollfrank J, Friedrich J. Thermal irreversibility in optically labeled low-temperature glasses. Phys Rev B Condens Matter. 1989 Mar 15;39(8):5414–5424. doi: 10.1103/physrevb.39.5414. [DOI] [PubMed] [Google Scholar]
- Stein D. L. A model of protein conformational substates. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3670–3672. doi: 10.1073/pnas.82.11.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Teklu Y., Storm C. B. Nitrogen-hydrogen tautomerism in porphyrins and chlorins. J Am Chem Soc. 1972 Mar 8;94(5):1745–1746. doi: 10.1021/ja00760a056. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J. M., Moy V. T., Maniara G., Koloczek H., Paul K. G. Site-selected fluorescence spectra of porphyrin derivatives of heme proteins. Biochemistry. 1985 Dec 31;24(27):7931–7935. doi: 10.1021/bi00348a013. [DOI] [PubMed] [Google Scholar]
- Zollfrank J., Friedrich J., Vanderkooi J. M., Fidy J. Conformational relaxation of a low-temperature protein as probed by photochemical hole burning. Horseradish peroxidase. Biophys J. 1991 Feb;59(2):305–312. doi: 10.1016/S0006-3495(91)82224-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


