Abstract
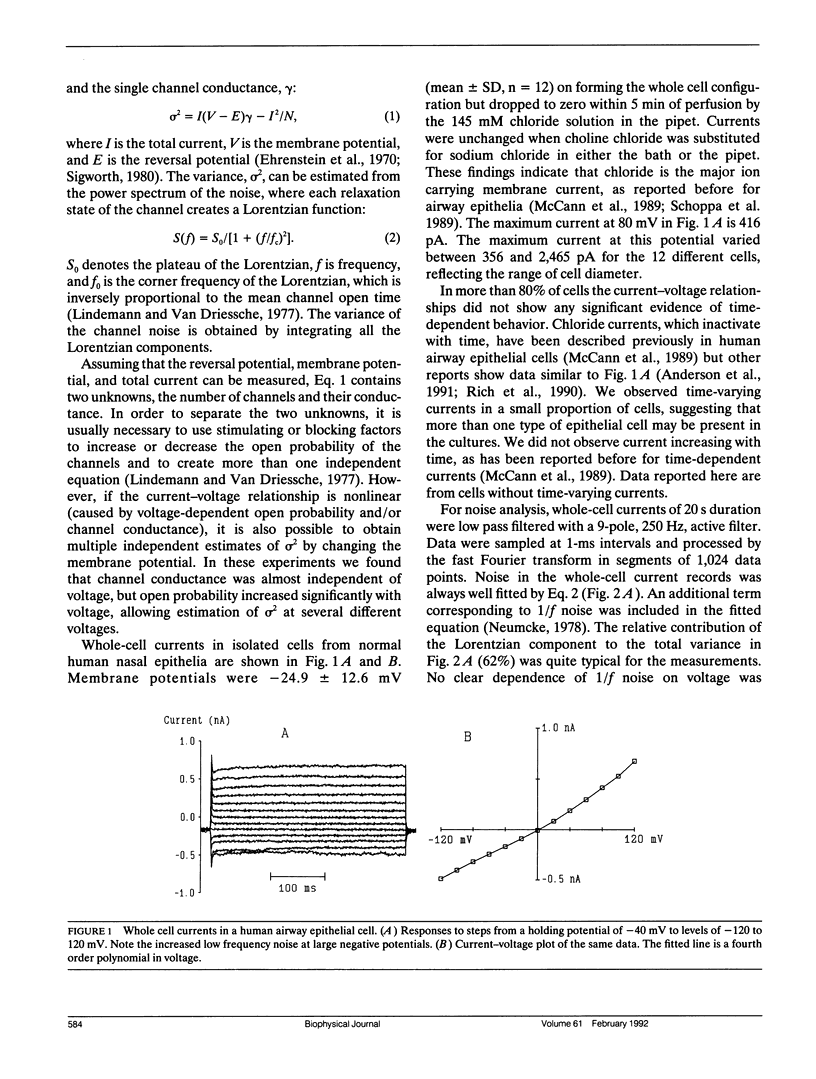
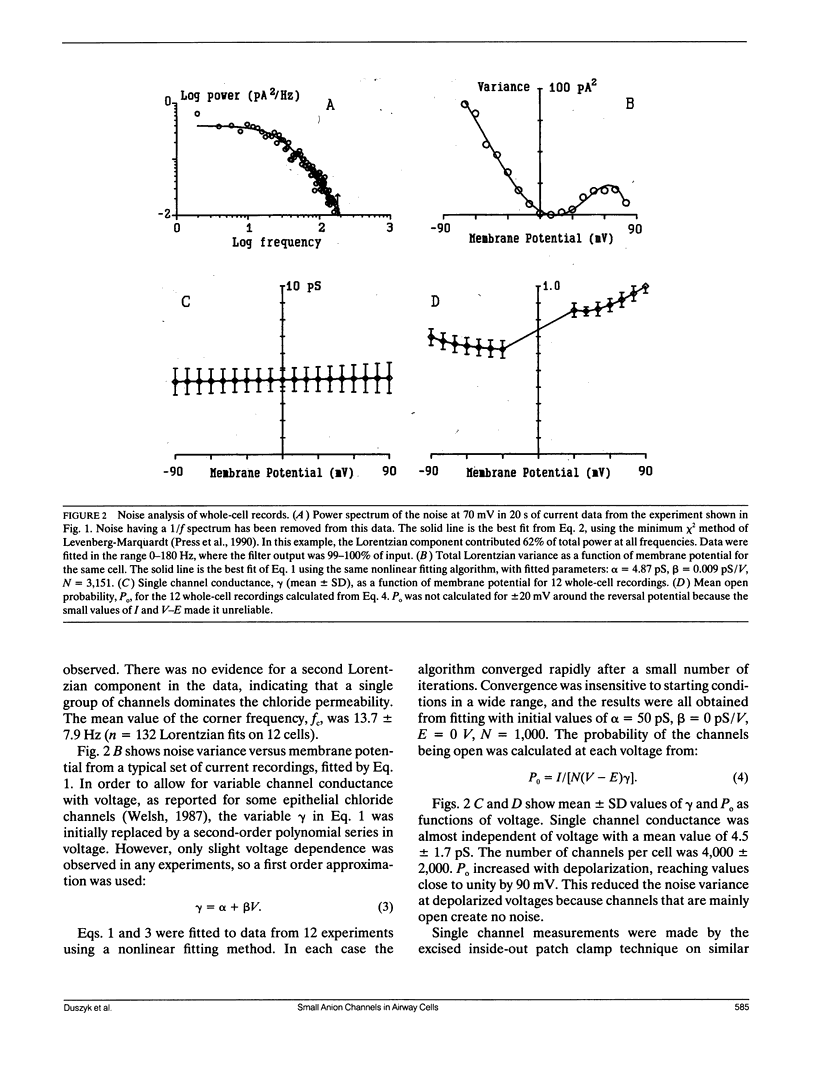
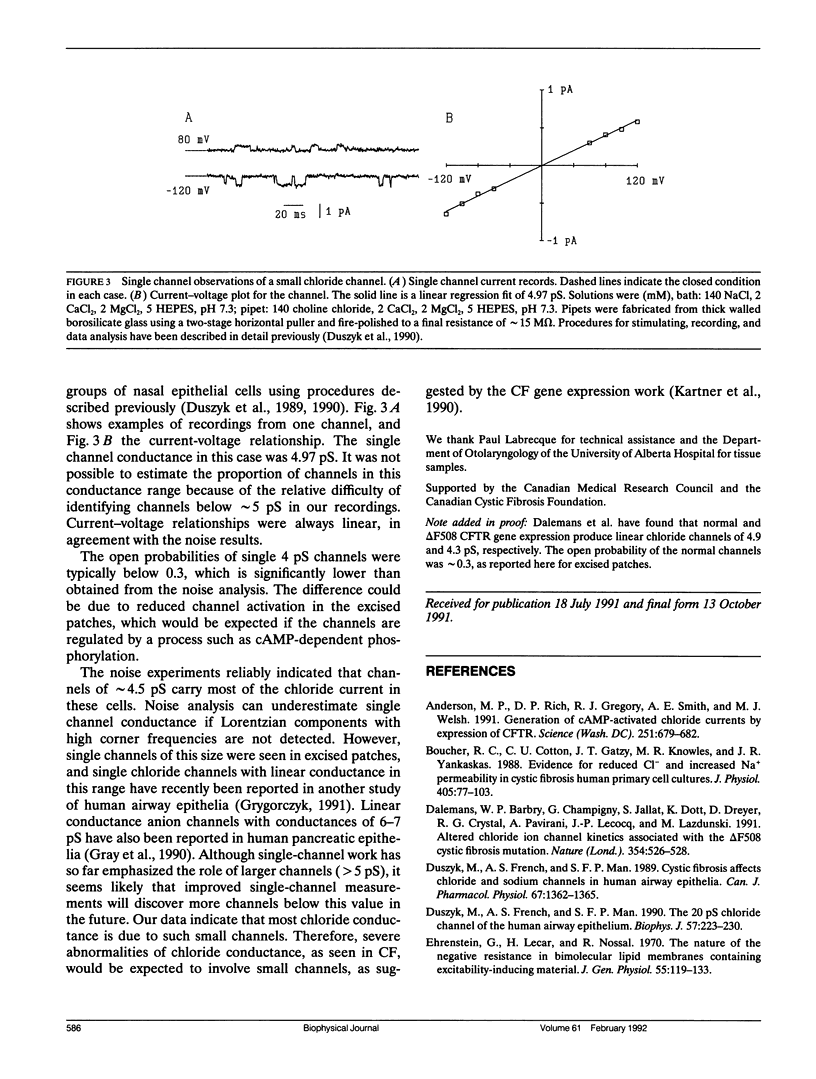
Apical membranes of human airway epithelial cells have significant chloride permeability, which is reduced in cystic fibrosis (CF), causing abnormal electrochemistry and impaired mucociliary clearance. At least four types of chloride channels have been identified in these cells, but their relative roles in total permeability and CF are unclear. Noise analysis was used to measure the conductance of chloride channels in human nasal epithelial cells. The data indicate that channels with a mean conductance of 4.5 pS carry most of the chloride current, and that the mean number of such channels per cell is approximately 4,000. Chloride channels in this conductance range were also seen in single-channel recordings.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Cotton C. U., Gatzy J. T., Knowles M. R., Yankaskas J. R. Evidence for reduced Cl- and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol. 1988 Nov;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991 Dec 19;354(6354):526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Duszyk M., French A. S., Man S. F. Cystic fibrosis affects chloride and sodium channels in human airway epithelia. Can J Physiol Pharmacol. 1989 Oct;67(10):1362–1365. doi: 10.1139/y89-217. [DOI] [PubMed] [Google Scholar]
- Duszyk M., French A. S., Man S. F. The 20-pS chloride channel of the human airway epithelium. Biophys J. 1990 Feb;57(2):223–230. doi: 10.1016/S0006-3495(90)82525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Lecar H., Nossal R. The nature of the negative resistance in bimolecular lipid membranes containing excitability-inducing material. J Gen Physiol. 1970 Jan;55(1):119–133. doi: 10.1085/jgp.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Pollard C. E., Harris A., Coleman L., Greenwell J. R., Argent B. E. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990 Nov;259(5 Pt 1):C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochim Biophys Acta. 1988 Oct 11;947(3):521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Lindemann B., Van Driessche W. Sodium-specific membrane channels of frog skin are pores: current fluctuations reveal high turnover. Science. 1977 Jan 21;195(4275):292–294. doi: 10.1126/science.299785. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Li M., Welsh M. J. Identification and regulation of whole-cell chloride currents in airway epithelium. J Gen Physiol. 1989 Dec;94(6):1015–1036. doi: 10.1085/jgp.94.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson M. A., Dormer R. L. Molecular and cellular biology of cystic fibrosis. Mol Aspects Med. 1991;12(1):1–81. doi: 10.1016/0098-2997(91)90015-e. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neumcke B. 1/f noise in membranes. Biophys Struct Mech. 1978 Jul 12;4(3):179–199. doi: 10.1007/BF02426084. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Schoppa N., Shorofsky S. R., Jow F., Nelson D. J. Voltage-gated chloride currents in cultured canine tracheal epithelial cells. J Membr Biol. 1989 Apr;108(1):73–90. doi: 10.1007/BF01870427. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker R. L., Frizzell R. A., Dwyer T. M., Farley J. M. Single chloride channel currents from canine tracheal epithelial cells. Biochim Biophys Acta. 1986 Jun 26;858(2):235–242. doi: 10.1016/0005-2736(86)90328-7. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. C., French A. S. Non-ligand-activated chloride channels of skeletal muscle and epithelia. Prog Biophys Mol Biol. 1989;54(1):59–79. doi: 10.1016/0079-6107(89)90009-6. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Zweifach A. Recent advances in the characterization of epithelial ionic channels. Biochim Biophys Acta. 1987 Apr 27;906(1):1–31. doi: 10.1016/0304-4157(87)90003-7. [DOI] [PubMed] [Google Scholar]


