Abstract
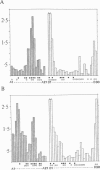
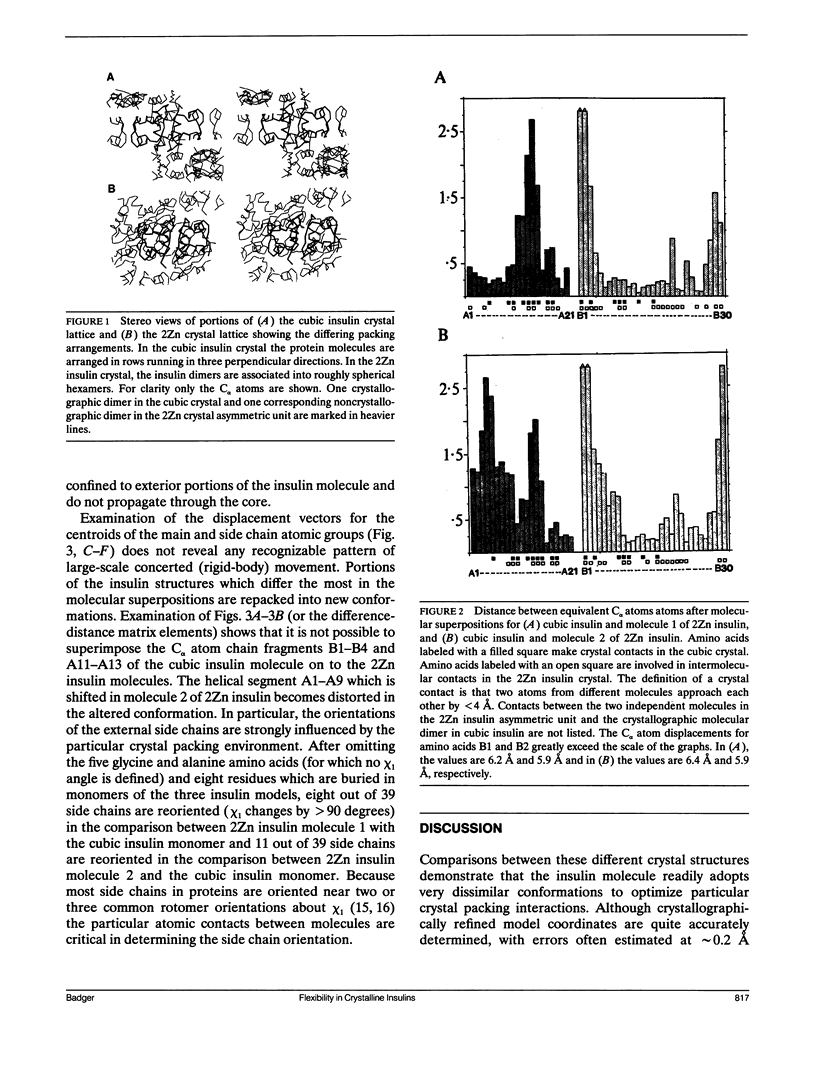
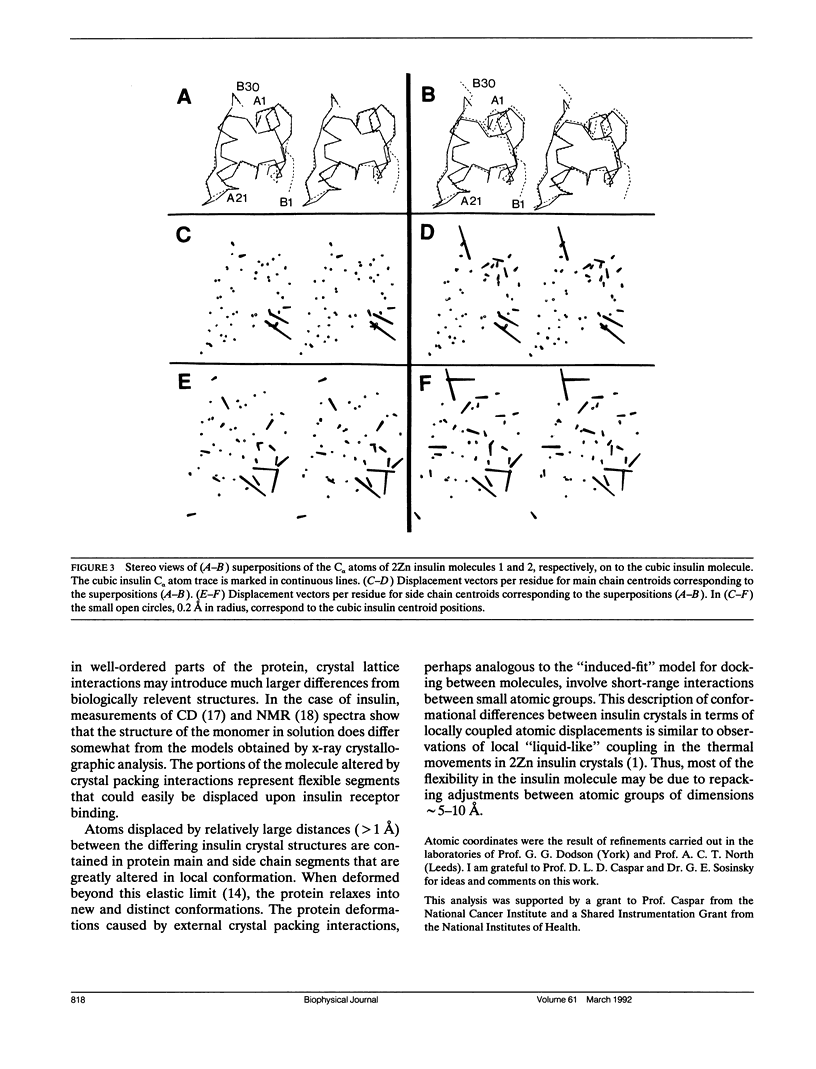
Comparisons of atomic models for chemically identical protein molecules solved in differing crystal environments provide information on flexibility in the protein structure. The structures of five T4 lysozyme proteins in differing crystal environments showed large relative displacements of the two domains with conserved backbone conformations that are connected by a flexible hinge (H. R. Faber and B. W. Matthews. 1990. Nature (Lond.). 348:263-266). In contrast, my comparison of the positions of all the atoms in two crystal forms of insulin shows that the structural changes caused by the differing crystal contacts are contained within nearby amino acids and are not propagated through the core of the insulin molecule. Groups of atoms that are most significantly displaced are not shifted in large rigid units but are repacked into new and distinct conformations. The transmission of displacements through the single domain insulin molecule is, like the movements due to thermal vibrations (D. L. D. Caspar, J. Clarage, D. M. Salunke, M. S. Clarage. 1988. Nature (Lond.). 332:659-662), characterized by short-range interactions between small atomic groups.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger J., Harris M. R., Reynolds C. D., Evans A. C., Dodson E. J., Dodson G. G., North A. C. Structure of the pig insulin dimer in the cubic crystal. Acta Crystallogr B. 1991 Feb 1;47(Pt 1):127–136. doi: 10.1107/s0108768190009570. [DOI] [PubMed] [Google Scholar]
- Baker E. N., Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. M., Hubbard R. E., Isaacs N. W., Reynolds C. D. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 6;319(1195):369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- Bhat T. N., Sasisekharan V., Vijayan M. An analysis of side-chain conformation in proteins. Int J Pept Protein Res. 1979 Feb;13(2):170–184. doi: 10.1111/j.1399-3011.1979.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Clarage J., Salunke D. M., Clarage M. Liquid-like movements in crystalline insulin. Nature. 1988 Apr 14;332(6165):659–662. doi: 10.1038/332659a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Dodson G. G., Hodgkin D. C. Transmission of conformational change in insulin. Nature. 1983 Apr 7;302(5908):500–505. doi: 10.1038/302500a0. [DOI] [PubMed] [Google Scholar]
- Derewenda U., Derewenda Z., Dodson E. J., Dodson G. G., Reynolds C. D., Smith G. D., Sparks C., Swenson D. Phenol stabilizes more helix in a new symmetrical zinc insulin hexamer. Nature. 1989 Apr 13;338(6216):594–596. doi: 10.1038/338594a0. [DOI] [PubMed] [Google Scholar]
- Diamond R. On the use of normal modes in thermal parameter refinement: theory and application to the bovine pancreatic trypsin inhibitor. Acta Crystallogr A. 1990 Jun 1;46(Pt 6):425–435. doi: 10.1107/s0108767390002082. [DOI] [PubMed] [Google Scholar]
- Elber R., Karplus M. Multiple conformational states of proteins: a molecular dynamics analysis of myoglobin. Science. 1987 Jan 16;235(4786):318–321. doi: 10.1126/science.3798113. [DOI] [PubMed] [Google Scholar]
- Faber H. R., Matthews B. W. A mutant T4 lysozyme displays five different crystal conformations. Nature. 1990 Nov 15;348(6298):263–266. doi: 10.1038/348263a0. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Hartmann H., Karplus M., Kuntz I. D., Jr, Kuriyan J., Parak F., Petsko G. A., Ringe D., Tilton R. F., Jr, Connolly M. L. Thermal expansion of a protein. Biochemistry. 1987 Jan 13;26(1):254–261. doi: 10.1021/bi00375a035. [DOI] [PubMed] [Google Scholar]
- Janin J., Wodak S. Conformation of amino acid side-chains in proteins. J Mol Biol. 1978 Nov 5;125(3):357–386. doi: 10.1016/0022-2836(78)90408-4. [DOI] [PubMed] [Google Scholar]
- Kundrot C. E., Richards F. M. Crystal structure of hen egg-white lysozyme at a hydrostatic pressure of 1000 atmospheres. J Mol Biol. 1987 Jan 5;193(1):157–170. doi: 10.1016/0022-2836(87)90634-6. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Weis W. I. Rigid protein motion as a model for crystallographic temperature factors. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2773–2777. doi: 10.1073/pnas.88.7.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melberg S. G., Johnson W. C., Jr Changes in secondary structure follow the dissociation of human insulin hexamers: a circular dichroism study. Proteins. 1990;8(3):280–286. doi: 10.1002/prot.340080309. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Davies D. R. Variability of three-dimensional structure in immunoglobulins. Proc Natl Acad Sci U S A. 1975 Mar;72(3):819–823. doi: 10.1073/pnas.72.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M., Lee R. W., Brange J., Dunn M. F. 1H NMR spectrum of the native human insulin monomer. Evidence for conformational differences between the monomer and aggregated forms. J Biol Chem. 1990 Apr 5;265(10):5448–5452. [PubMed] [Google Scholar]
- Smith G. D., Swenson D. C., Dodson E. J., Dodson G. G., Reynolds C. D. Structural stability in the 4-zinc human insulin hexamer. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7093–7097. doi: 10.1073/pnas.81.22.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]