Abstract
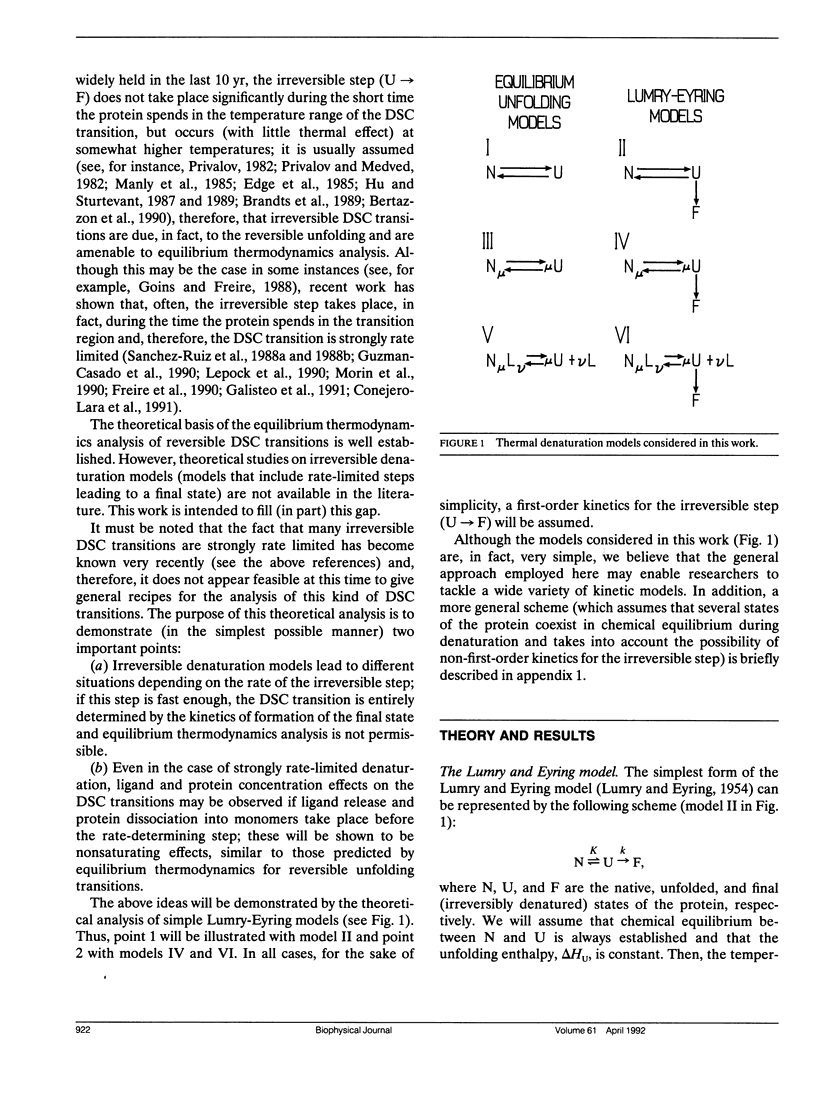
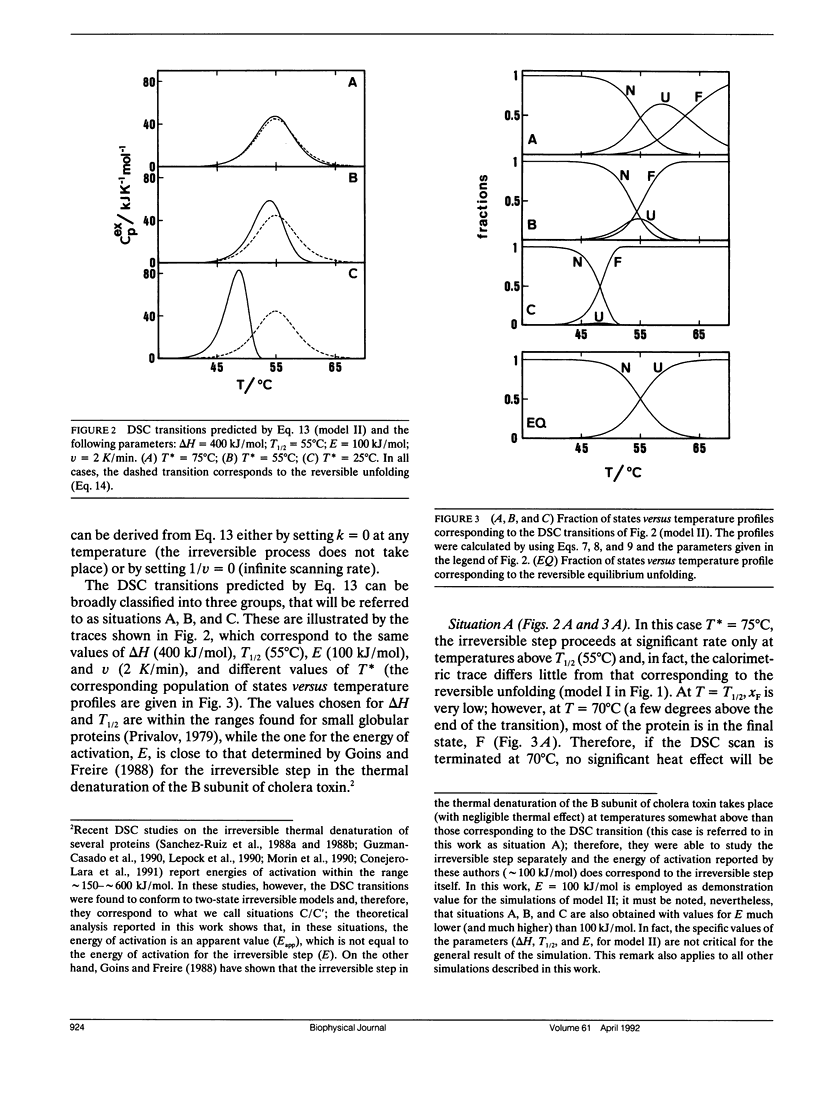
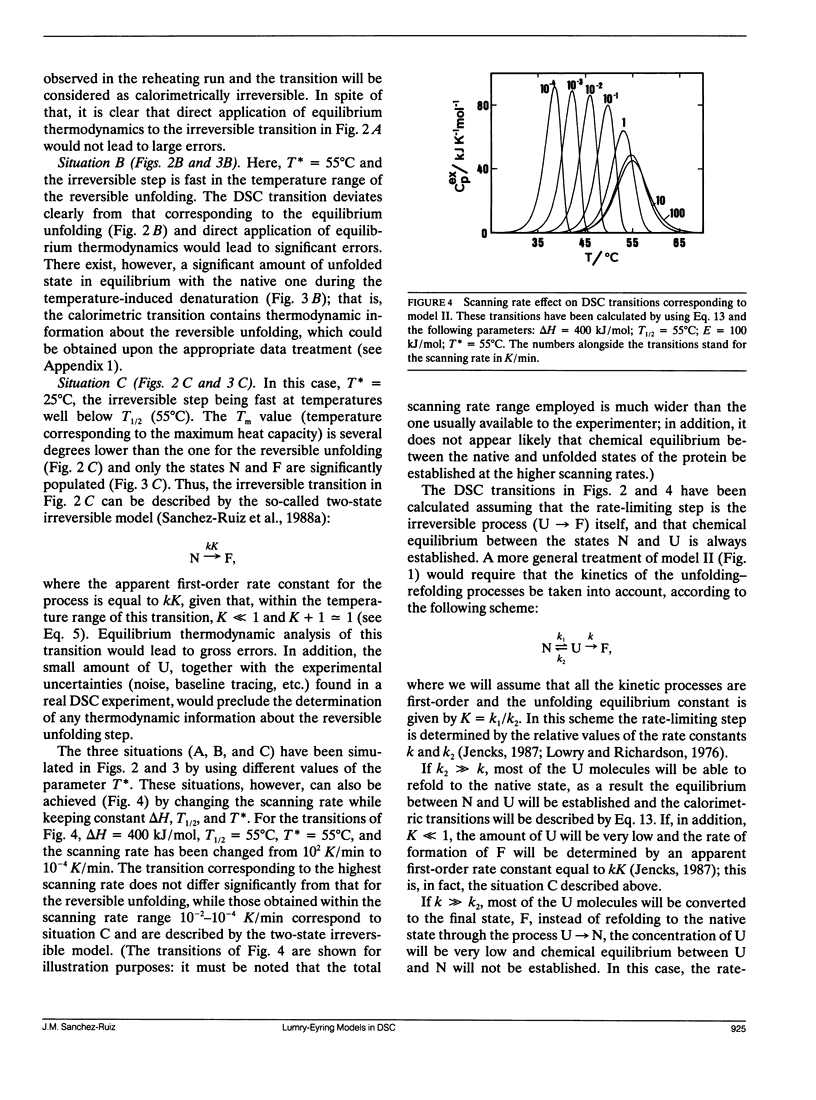
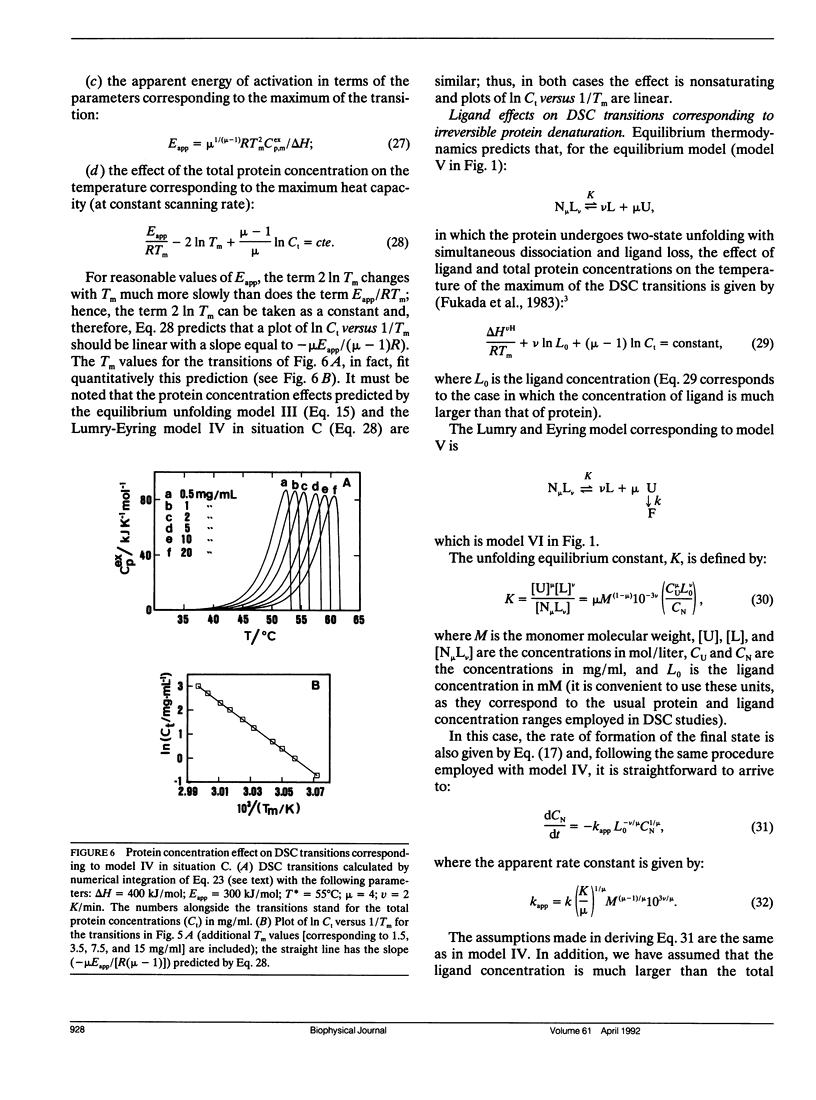
A theoretical analysis of several protein denaturation models (Lumry-Eyring models) that include a rate-limited step leading to an irreversibly denatured state of the protein (the final state) has been carried out. The differential scanning calorimetry transitions predicted for these models can be broadly classified into four groups: situations A, B, C, and C′. (A) The transition is calorimetrically irreversible but the rate-limited, irreversible step takes place with significant rate only at temperatures slightly above those corresponding to the transition. Equilibrium thermodynamics analysis is permissible. (B) The transition is distorted by the occurrence of the rate-limited step; nevertheless, it contains thermodynamic information about the reversible unfolding of the protein, which could be obtained upon the appropriate data treatment. (C) The heat absorption is entirely determined by the kinetics of formation of the final state and no thermodynamic information can be extracted from the calorimetric transition; the rate-determining step is the irreversible process itself. (C′) same as C, but, in this case, the rate-determining step is a previous step in the unfolding pathway. It is shown that ligand and protein concentration effects on transitions corresponding to situation C (strongly rate-limited transitions) are similar to those predicted by equilibrium thermodynamics for simple reversible unfolding models. It has been widely held in recent literature that experimentally observed ligand and protein concentration effects support the applicability of equilibrium thermodynamics to irreversible protein denaturation. The theoretical analysis reported here disfavors this claim.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandts J. F., Hu C. Q., Lin L. N., Mos M. T. A simple model for proteins with interacting domains. Applications to scanning calorimetry data. Biochemistry. 1989 Oct 17;28(21):8588–8596. doi: 10.1021/bi00447a048. [DOI] [PubMed] [Google Scholar]
- Conejero-Lara F., Mateo P. L., Aviles F. X., Sanchez-Ruiz J. M. Effect of Zn2+ on the thermal denaturation of carboxypeptidase B. Biochemistry. 1991 Feb 26;30(8):2067–2072. doi: 10.1021/bi00222a010. [DOI] [PubMed] [Google Scholar]
- Edge V., Allewell N. M., Sturtevant J. M. High-resolution differential scanning calorimetric analysis of the subunits of Escherichia coli aspartate transcarbamoylase. Biochemistry. 1985 Oct 8;24(21):5899–5906. doi: 10.1021/bi00342a032. [DOI] [PubMed] [Google Scholar]
- Freire E., van Osdol W. W., Mayorga O. L., Sanchez-Ruiz J. M. Calorimetrically determined dynamics of complex unfolding transitions in proteins. Annu Rev Biophys Biophys Chem. 1990;19:159–188. doi: 10.1146/annurev.bb.19.060190.001111. [DOI] [PubMed] [Google Scholar]
- Fukada H., Sturtevant J. M., Quiocho F. A. Thermodynamics of the binding of L-arabinose and of D-galactose to the L-arabinose-binding protein of Escherichia coli. J Biol Chem. 1983 Nov 10;258(21):13193–13198. [PubMed] [Google Scholar]
- Galisteo M. L., Mateo P. L., Sanchez-Ruiz J. M. Kinetic study on the irreversible thermal denaturation of yeast phosphoglycerate kinase. Biochemistry. 1991 Feb 26;30(8):2061–2066. doi: 10.1021/bi00222a009. [DOI] [PubMed] [Google Scholar]
- Goins B., Freire E. Thermal stability and intersubunit interactions of cholera toxin in solution and in association with its cell-surface receptor ganglioside GM1. Biochemistry. 1988 Mar 22;27(6):2046–2052. doi: 10.1021/bi00406a035. [DOI] [PubMed] [Google Scholar]
- Guzmán-Casado M., Parody-Morreale A., Mateo P. L., Sánchez-Ruiz J. M. Differential scanning calorimetry of lobster haemocyanin. Eur J Biochem. 1990 Feb 22;188(1):181–185. doi: 10.1111/j.1432-1033.1990.tb15386.x. [DOI] [PubMed] [Google Scholar]
- Hu C. Q., Sturtevant J. M. A differential scanning calorimetric study of the binding of sulfate ion and of Cibacron blue F3GA to yeast phosphoglycerate kinase. Biochemistry. 1989 Jan 24;28(2):813–818. doi: 10.1021/bi00428a060. [DOI] [PubMed] [Google Scholar]
- Hu C. Q., Sturtevant J. M. Thermodynamic study of yeast phosphoglycerate kinase. Biochemistry. 1987 Jan 13;26(1):178–182. doi: 10.1021/bi00375a025. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Rodahl A. M., Zhang C., Heynen M. L., Waters B., Cheng K. H. Thermal denaturation of the Ca2(+)-ATPase of sarcoplasmic reticulum reveals two thermodynamically independent domains. Biochemistry. 1990 Jan 23;29(3):681–689. doi: 10.1021/bi00455a013. [DOI] [PubMed] [Google Scholar]
- Manly S. P., Matthews K. S., Sturtevant J. M. Thermal denaturation of the core protein of lac repressor. Biochemistry. 1985 Jul 16;24(15):3842–3846. doi: 10.1021/bi00336a004. [DOI] [PubMed] [Google Scholar]
- Morin P. E., Diggs D., Freire E. Thermal stability of membrane-reconstituted yeast cytochrome c oxidase. Biochemistry. 1990 Jan 23;29(3):781–788. doi: 10.1021/bi00455a028. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Medved L. V. Domains in the fibrinogen molecule. J Mol Biol. 1982 Aug 25;159(4):665–683. doi: 10.1016/0022-2836(82)90107-3. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins. Proteins which do not present a single cooperative system. Adv Protein Chem. 1982;35:1–104. [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Thermodynamic problems of protein structure. Annu Rev Biophys Biophys Chem. 1989;18:47–69. doi: 10.1146/annurev.bb.18.060189.000403. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Freire E. Linked thermal and solute perturbation analysis of cooperative domain interactions in proteins. Structural stability of diphtheria toxin. Biochemistry. 1990 Sep 18;29(37):8677–8683. doi: 10.1021/bi00489a024. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ruiz J. M., Lopez-Lacomba J. L., Mateo P. L., Vilanova M., Serra M. A., Aviles F. X. Analysis of the thermal unfolding of porcine procarboxypeptidase A and its functional pieces by differential scanning calorimetry. Eur J Biochem. 1988 Sep 1;176(1):225–230. doi: 10.1111/j.1432-1033.1988.tb14272.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ruiz J. M., López-Lacomba J. L., Cortijo M., Mateo P. L. Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry. 1988 Mar 8;27(5):1648–1652. doi: 10.1021/bi00405a039. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sturtevant J. M. Thermal denaturation of streptomyces subtilisin inhibitor, subtilisin BPN', and the inhibitor-subtilisin complex. Biochemistry. 1981 Oct 13;20(21):6185–6190. doi: 10.1021/bi00524a042. [DOI] [PubMed] [Google Scholar]


