Abstract
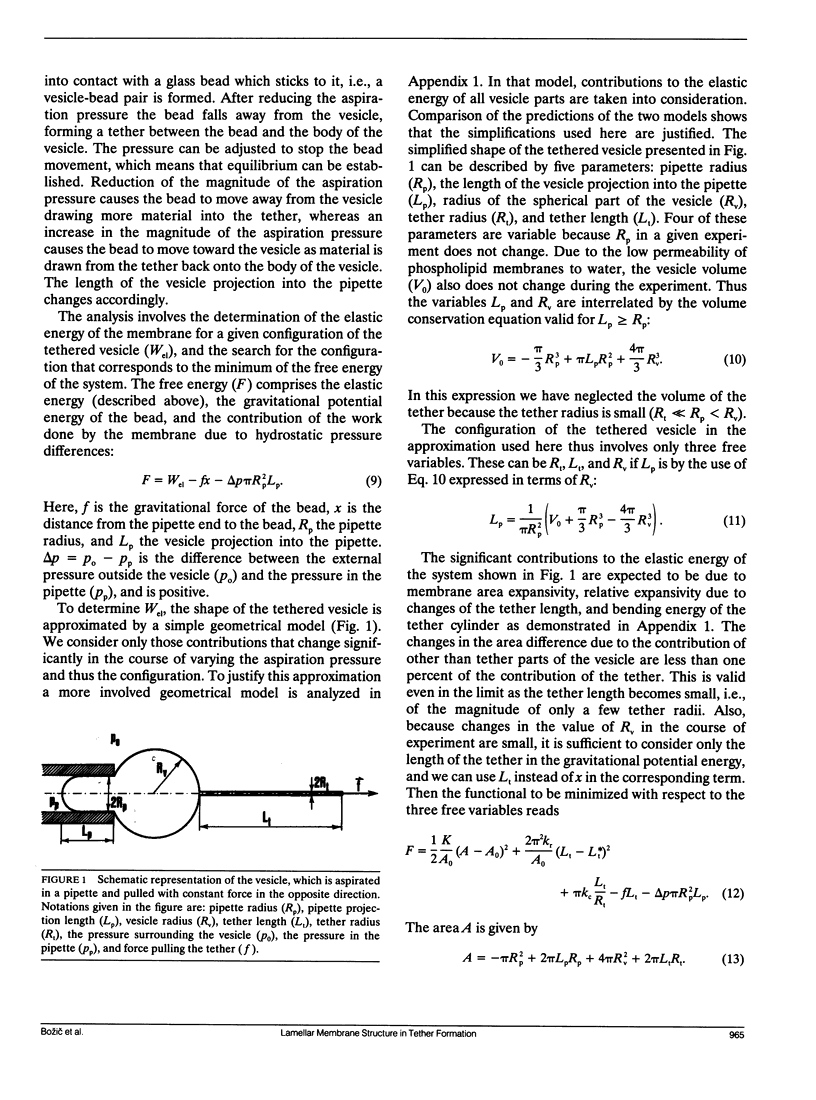
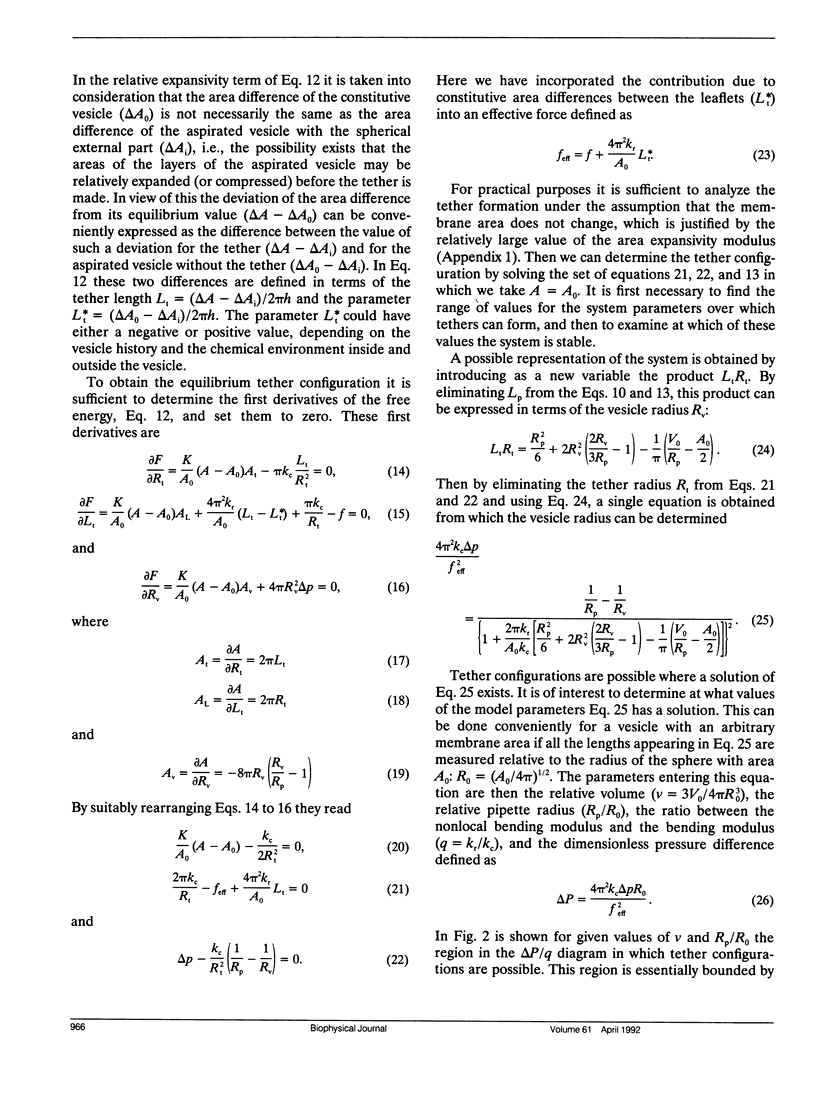
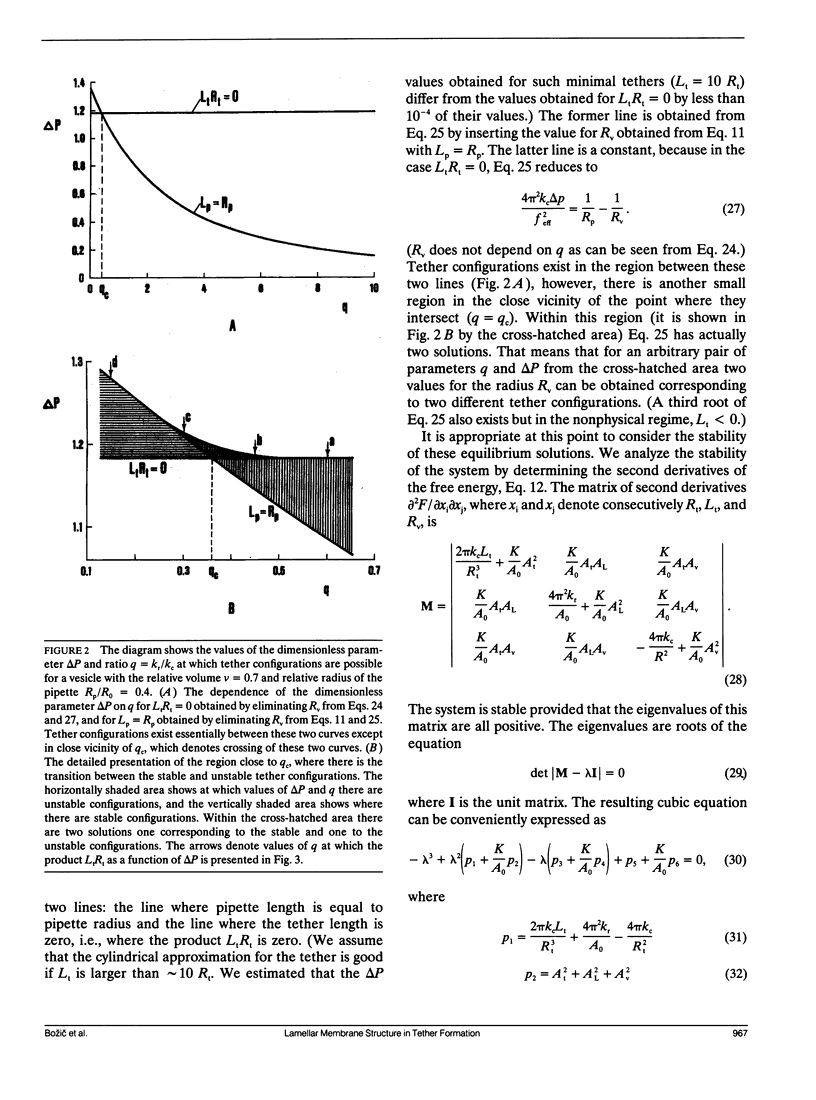
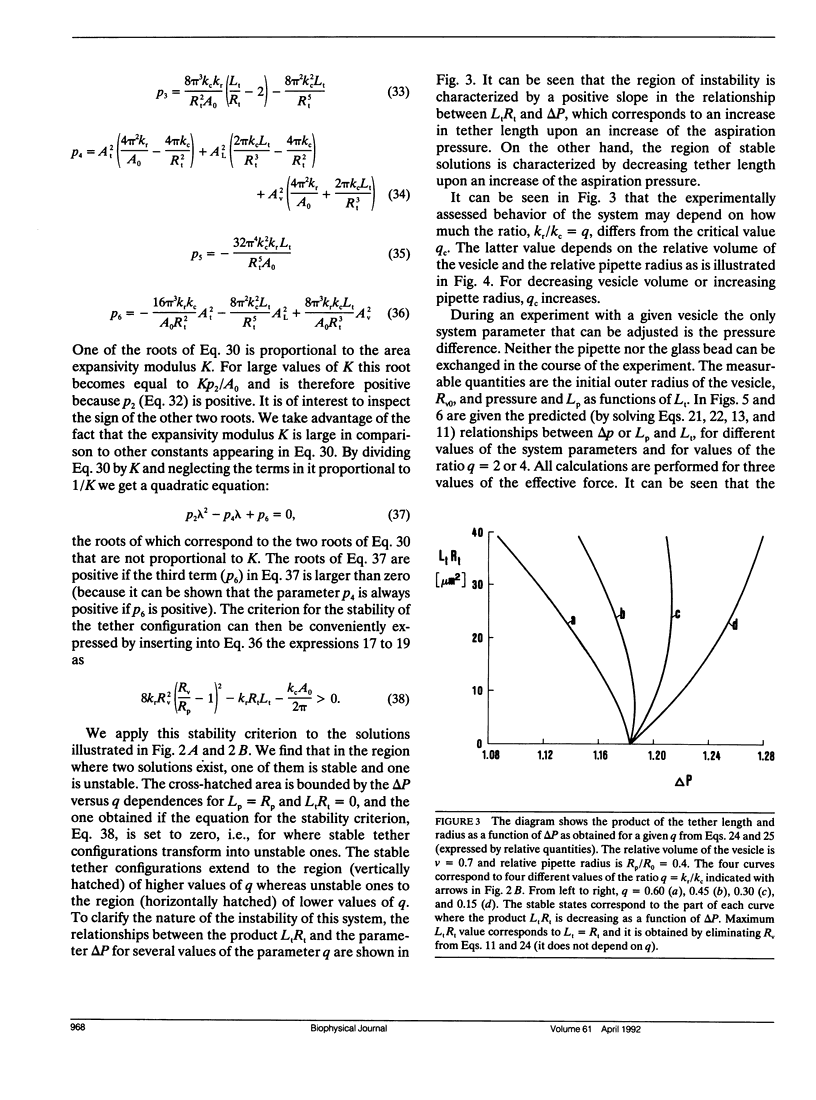
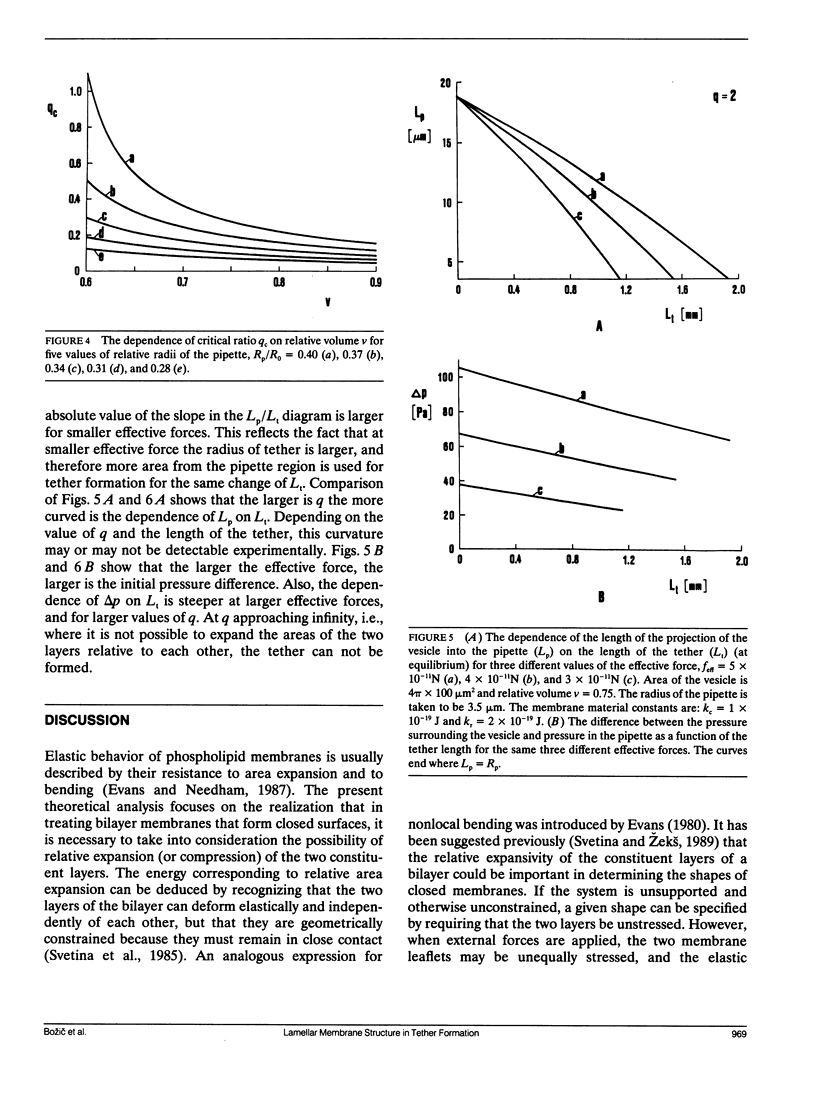
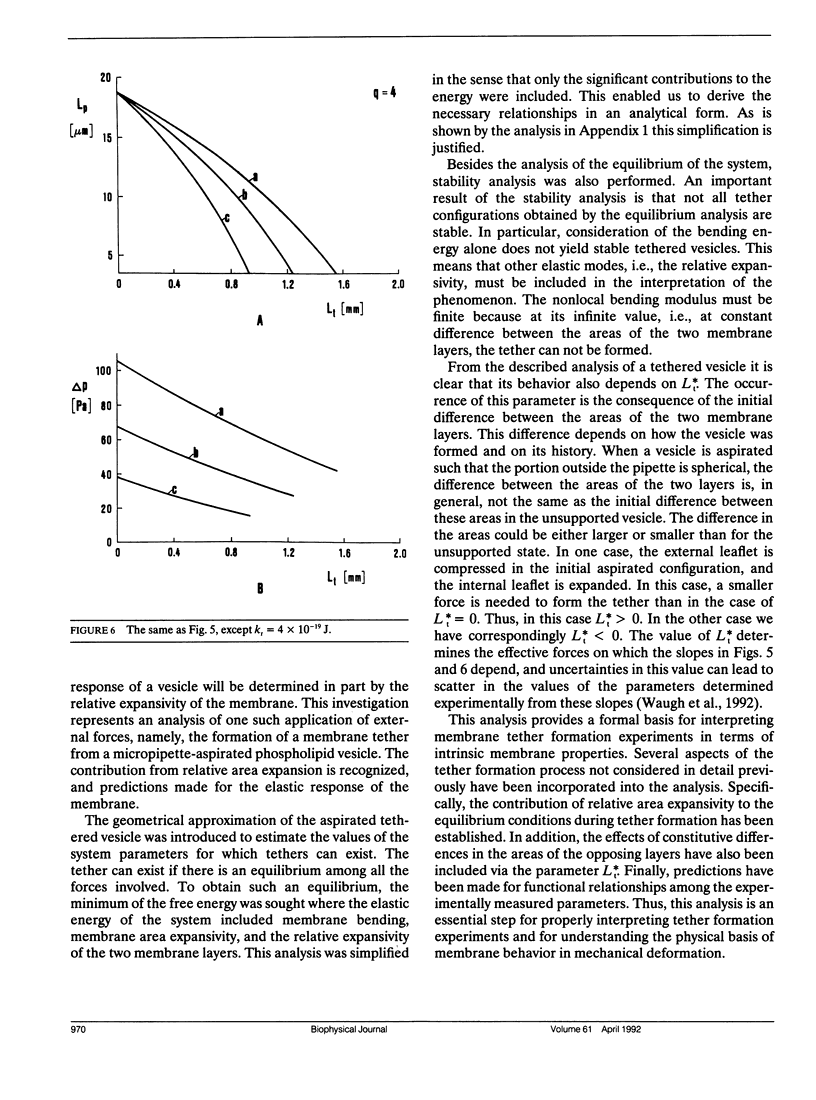
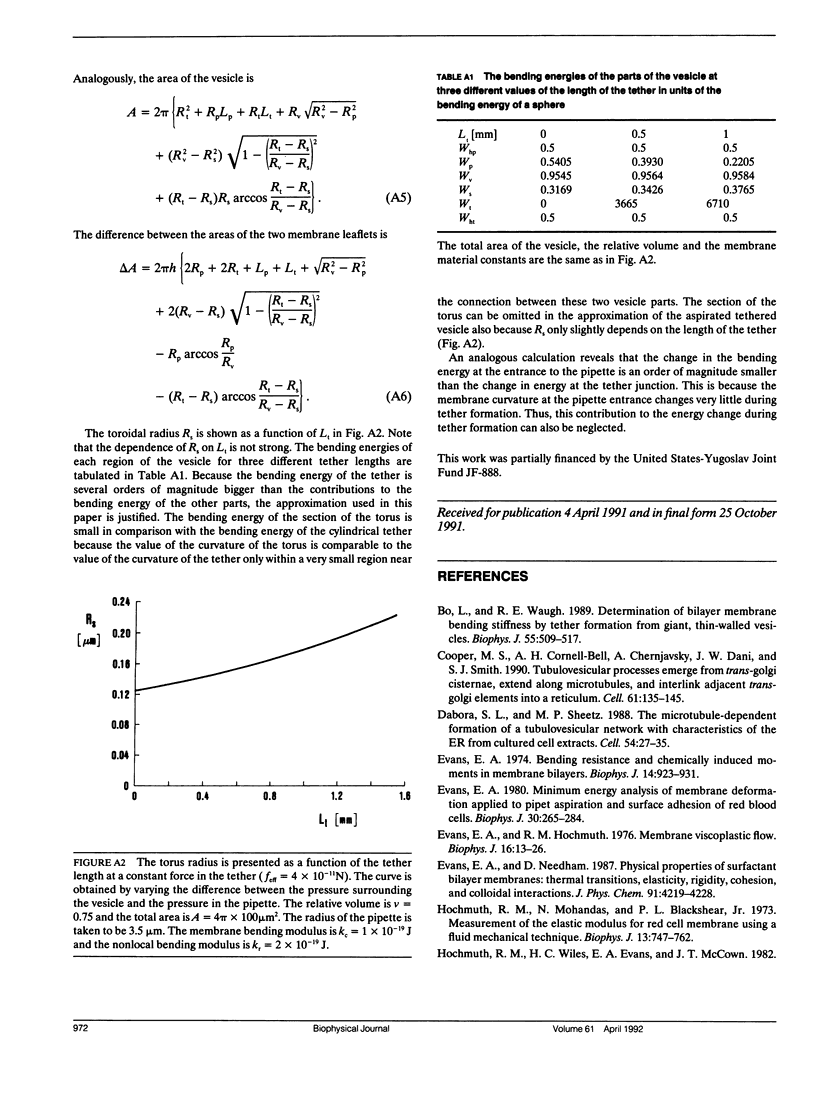
A theoretical analysis is presented of the formation of membrane tethers from micropipette-aspirated phospholipid vesicles. In particular, it is taken into account that the phospholipid membrane is composed of two layers which are in contact but unconnected. The elastic energy of the bilayer is taken to be the sum of contributions from area expansivity, relative expansivity of the two monolayers, and bending. The vesicle is aspirated into a pipette and a constant point force is applied at the opposite side in the direction away from the pipette. The shape of the vesicle in approximated as a cylindrical projection into the pipette with a hemispherical cap, a spherical section, and a cylindrical tether with a hemispherical cap. The dimensions of the different regions of the vesicle are obtained by minimizing its elastic energy subject to the condition that the volume of the vesicle is fixed. The range of values for the parameters of the system is determined at which the existence of a tether is possible. Stability analysis is performed showing which of these configurations are stable. The importance of the relative expansion and compression of the constituent monolayers is established by recognizing that local bending energy by itself does not stabilize the vesicle geometry, and that in the limit as the relative expansivity modulus becomes infinitely large, a tether cannot be formed. Predictions are made for the functional relationships among experimentally observable quantities. In a companion report, the results of this analysis are applied to experimental measurements of tether formation, and used to calculate values for the membrane material coefficients.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bo L., Waugh R. E. Determination of bilayer membrane bending stiffness by tether formation from giant, thin-walled vesicles. Biophys J. 1989 Mar;55(3):509–517. doi: 10.1016/S0006-3495(89)82844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. S., Cornell-Bell A. H., Chernjavsky A., Dani J. W., Smith S. J. Tubulovesicular processes emerge from trans-Golgi cisternae, extend along microtubules, and interlink adjacent trans-golgi elements into a reticulum. Cell. 1990 Apr 6;61(1):135–145. doi: 10.1016/0092-8674(90)90221-y. [DOI] [PubMed] [Google Scholar]
- Dabora S. L., Sheetz M. P. The microtubule-dependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell. 1988 Jul 1;54(1):27–35. doi: 10.1016/0092-8674(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Bending resistance and chemically induced moments in membrane bilayers. Biophys J. 1974 Dec;14(12):923–931. doi: 10.1016/S0006-3495(74)85959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoplastic flow. Biophys J. 1976 Jan;16(1):13–26. doi: 10.1016/S0006-3495(76)85659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. Minimum energy analysis of membrane deformation applied to pipet aspiration and surface adhesion of red blood cells. Biophys J. 1980 May;30(2):265–284. doi: 10.1016/S0006-3495(80)85093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Mohandas N., Blackshear P. L., Jr Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophys J. 1973 Aug;13(8):747–762. doi: 10.1016/S0006-3495(73)86021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Wiles H. C., Evans E. A., McCown J. T. Extensional flow of erythrocyte membrane from cell body to elastic tether. II. Experiment. Biophys J. 1982 Jul;39(1):83–89. doi: 10.1016/S0006-3495(82)84493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Waugh R. E. Bilayer membrane bending stiffness by tether formation from mixed PC-PS lipid vesicles. J Biomech Eng. 1990 Aug;112(3):235–240. doi: 10.1115/1.2891178. [DOI] [PubMed] [Google Scholar]
- Svetina S., Zeks B. Membrane bending energy and shape determination of phospholipid vesicles and red blood cells. Eur Biophys J. 1989;17(2):101–111. doi: 10.1007/BF00257107. [DOI] [PubMed] [Google Scholar]
- Waugh R. E., Hochmuth R. M. Mechanical equilibrium of thick, hollow, liquid membrane cylinders. Biophys J. 1987 Sep;52(3):391–400. doi: 10.1016/S0006-3495(87)83227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E., Song J., Svetina S., Zeks B. Local and nonlocal curvature elasticity in bilayer membranes by tether formation from lecithin vesicles. Biophys J. 1992 Apr;61(4):974–982. doi: 10.1016/S0006-3495(92)81904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Surface viscosity measurements from large bilayer vesicle tether formation. I. Analysis. Biophys J. 1982 Apr;38(1):19–27. doi: 10.1016/S0006-3495(82)84526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Surface viscosity measurements from large bilayer vesicle tether formation. II. Experiments. Biophys J. 1982 Apr;38(1):29–37. doi: 10.1016/S0006-3495(82)84527-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


