Abstract
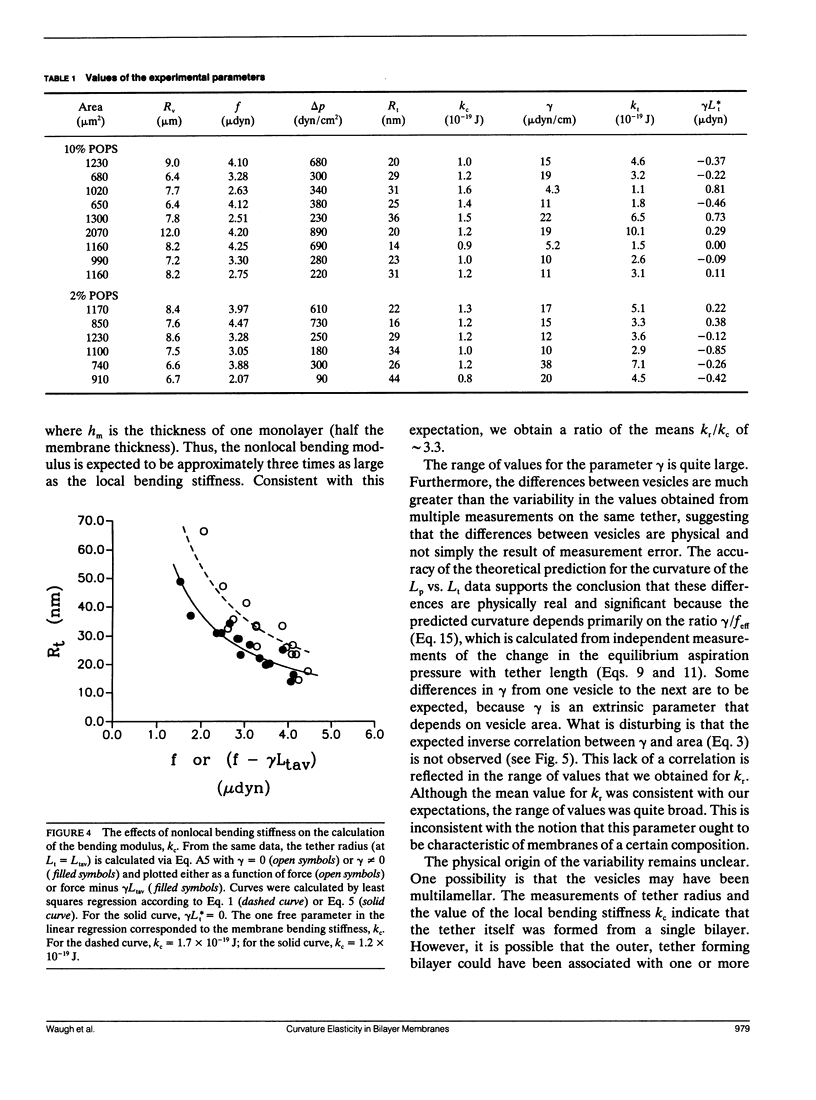
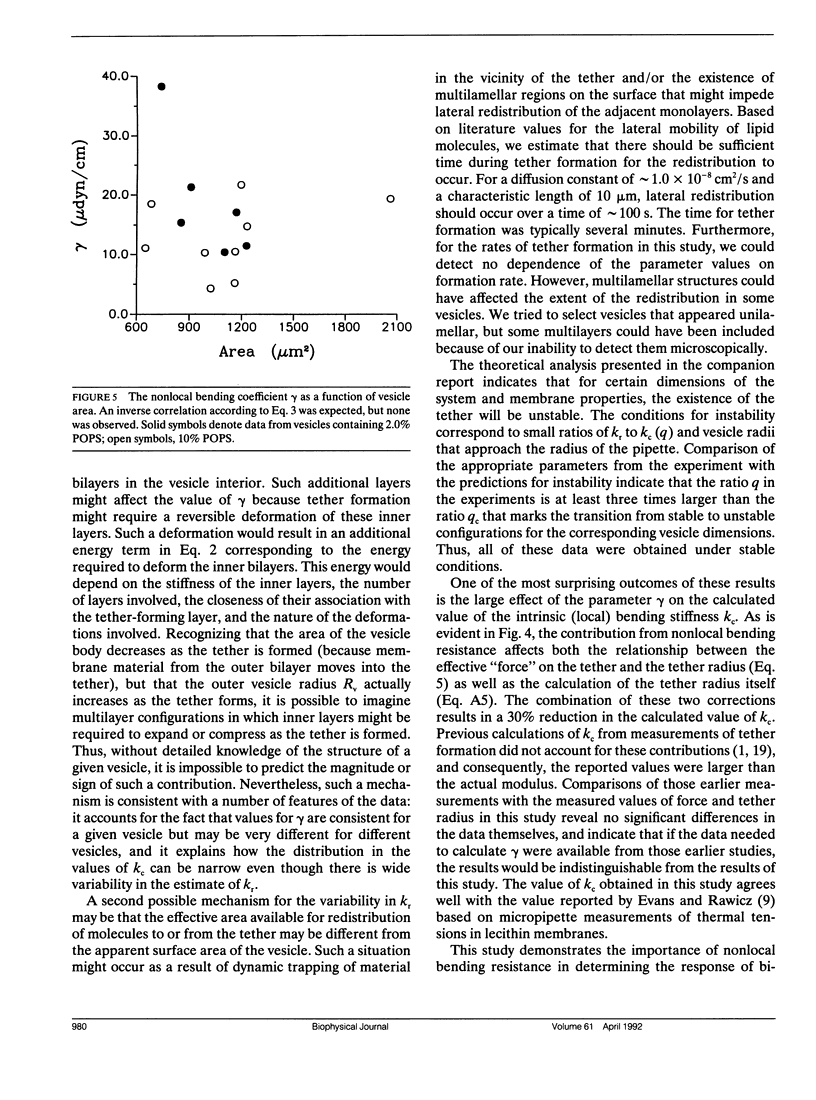
Bilayer membranes exhibit an elastic resistance to changes in curvature. This resistance depends both on the intrinsic stiffness of the constituent monolayers and on the curvature-induced expansion or compression of the monolayers relative to each other. The monolayers are constrained by hydrophobic forces to remain in contact, but they are capable of independent lateral redistribution to minimize the relative expansion or compression of each leaflet. Therefore, the magnitude of the expansion and compression of the monolayers relative to each other depends on the integral of the curvature over the entire membrane capsule. The coefficient characterizing the membrane stiffness resulting from relative expansion is the nonlocal bending modulus kr. Both the intrinsic (local) bending modulus (kc) and the nonlocal bending modulus (kr) can be measured by the formation of thin cylindrical membrane strands (tethers) from giant phospholipid vesicles. Previously, we reported measurements of kc based on measurements of tether radius as a function of force (Song and Waugh, 1991, J. Biomech. Engr. 112:233). Further analysis has revealed that the contribution from the nonlocal bending stiffness can be detected by measuring the change in the aspiration pressure required to establish equilibrium with increasing tether length. Using this approach, we obtain a mean value for the nonlocal bending modulus kr of approximately 4.1 x 10(-19)J. The range of values is broad (1.1-10.1 x 10(-19)J) and could reflect contributions other than simple mechanical equilibrium. Inclusion of the nonlocal bending stiffness in the calculation of kc results in a value for that modulus of approximately 1.20 +/- 0.17 x 10(-19)J, in close agreement with values obtained by other methods.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bo L., Waugh R. E. Determination of bilayer membrane bending stiffness by tether formation from giant, thin-walled vesicles. Biophys J. 1989 Mar;55(3):509–517. doi: 10.1016/S0006-3495(89)82844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic B., Svetina S., Zeks B., Waugh R. E. Role of lamellar membrane structure in tether formation from bilayer vesicles. Biophys J. 1992 Apr;61(4):963–973. doi: 10.1016/S0006-3495(92)81903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. S., Cornell-Bell A. H., Chernjavsky A., Dani J. W., Smith S. J. Tubulovesicular processes emerge from trans-Golgi cisternae, extend along microtubules, and interlink adjacent trans-golgi elements into a reticulum. Cell. 1990 Apr 6;61(1):135–145. doi: 10.1016/0092-8674(90)90221-y. [DOI] [PubMed] [Google Scholar]
- Dabora S. L., Sheetz M. P. The microtubule-dependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell. 1988 Jul 1;54(1):27–35. doi: 10.1016/0092-8674(88)90176-6. [DOI] [PubMed] [Google Scholar]
- Evans E. A. Bending resistance and chemically induced moments in membrane bilayers. Biophys J. 1974 Dec;14(12):923–931. doi: 10.1016/S0006-3495(74)85959-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Skalak R. Mechanics and thermodynamics of biomembranes: part 2. CRC Crit Rev Bioeng. 1979 Nov;3(4):331–418. [PubMed] [Google Scholar]
- Evans E, Rawicz W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett. 1990 Apr 23;64(17):2094–2097. doi: 10.1103/PhysRevLett.64.2094. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Blocked lipid exchange in bilayers and its possible influence on the shape of vesicles. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):510–515. doi: 10.1515/znc-1974-9-1010. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Lee C., Chen L. B. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988 Jul 1;54(1):37–46. doi: 10.1016/0092-8674(88)90177-8. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- Schneider M. B., Jenkins J. T., Webb W. W. Thermal fluctuations of large cylindrical phospholipid vesicles. Biophys J. 1984 May;45(5):891–899. doi: 10.1016/S0006-3495(84)84235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servuss R. M., Harbich W., Helfrich W. Measurement of the curvature-elastic modulus of egg lecithin bilayers. Biochim Biophys Acta. 1976 Jul 15;436(4):900–903. doi: 10.1016/0005-2736(76)90422-3. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Waugh R. E. Bilayer membrane bending stiffness by tether formation from mixed PC-PS lipid vesicles. J Biomech Eng. 1990 Aug;112(3):235–240. doi: 10.1115/1.2891178. [DOI] [PubMed] [Google Scholar]
- Svetina S., Zeks B. Membrane bending energy and shape determination of phospholipid vesicles and red blood cells. Eur Biophys J. 1989;17(2):101–111. doi: 10.1007/BF00257107. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. Synaptic vesicles. Curr Opin Cell Biol. 1989 Aug;1(4):655–659. doi: 10.1016/0955-0674(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Torbet J., Wilkins M. H. X-ray diffraction studies of lecithin bilayers. J Theor Biol. 1976 Oct 21;62(2):447–458. doi: 10.1016/0022-5193(76)90129-6. [DOI] [PubMed] [Google Scholar]
- Waugh R. E., Hochmuth R. M. Mechanical equilibrium of thick, hollow, liquid membrane cylinders. Biophys J. 1987 Sep;52(3):391–400. doi: 10.1016/S0006-3495(87)83227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilschut J. Intracellular membrane fusion. Curr Opin Cell Biol. 1989 Aug;1(4):639–647. doi: 10.1016/0955-0674(89)90028-8. [DOI] [PubMed] [Google Scholar]


