Abstract
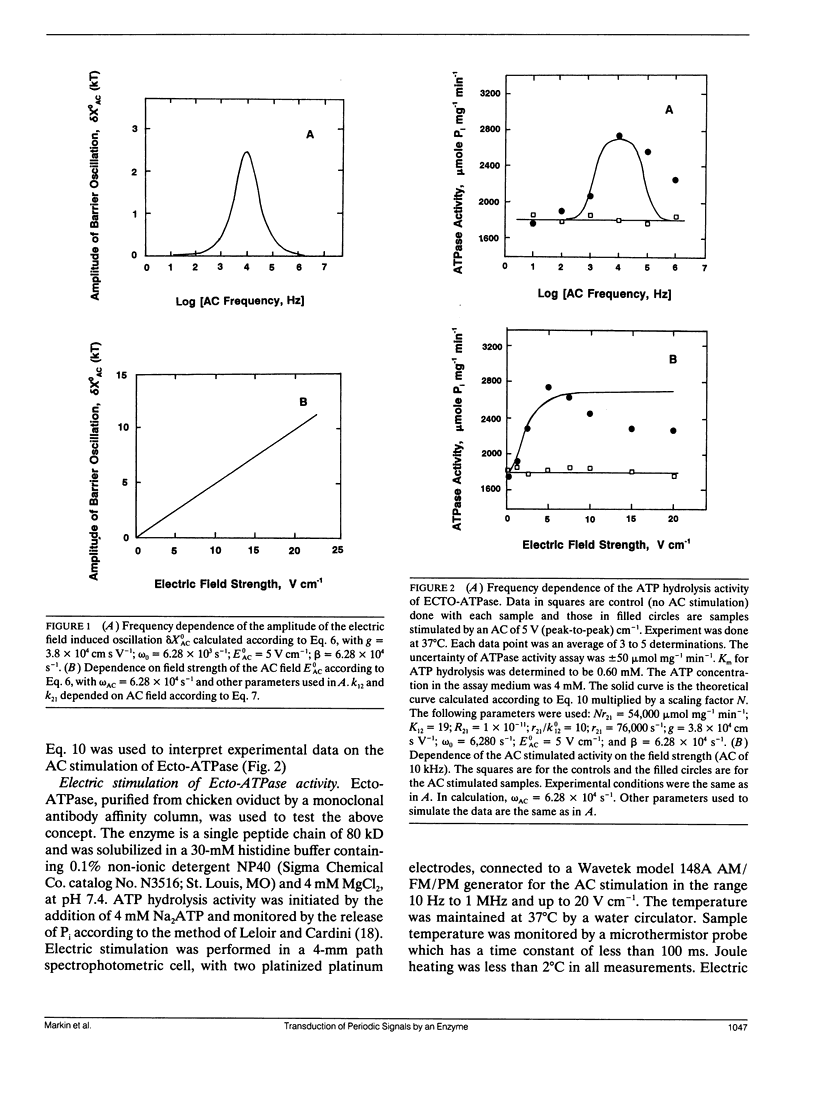
The overall rate of an enzyme catalyzed reaction is determined by the activation barrier of a rate-limiting step. If the barrier is oscillatory due to the intrinsic properties of a fluctuating enzyme, this enzymatic reaction will be influenced by a low level periodic electric field through the resonance transduction between the applied field and the oscillatory activation barrier. The ATP hydrolysis activity of a highly purified, detergent solubilized Ecto-ATPase from chicken oviduct was used to test the above concept. At 37 degrees C, this activity (1,800 mumols mg-1 min-1) was stimulated up to 47% (to 2,650 mumols mg-1 min-1) by an alternating electric field (AC), with a frequency window at 10 kHz. The maximal stimulation occurred at 5.0 V (peak-to-peak) cm-1. The potential drop across the dimension of the enzyme was approximately 10 microV (micelle diameter 20 nm). The activation barrier, or the Arrhenius activation energy, of the ATP splitting was measured to be 30 kT and the maximal barrier oscillation was calculated to be approximately 2.5 kT according to the oscillatory activation barrier (OAB) model. With the optimal AC field, full impact of the electric stimulation could be effected in much less than a second. The OAB model is many orders of magnitude more sensitive for deciphering low level periodic signals than the electroconformational coupling (ECC) model, although the latter has the ability to actively transduce energy while the former does not. By the OAB mechanism, the detecting limit of an external electric field by the ATPase, in a cell 20 micro m in diameter,would be 5 mV cm-1, but could be much lower for other membrane enzymes or receptors (e.g., nV cm-1). We propose that mechanisms similar to the OAB model could explain how a weak electromagnetic field or acoustic noises can exert its effects on an organism or a living cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astumian R. D., Chock P. B., Tsong T. Y., Chen Y. D., Westerhoff H. V. Can free energy be transduced from electric noise? Proc Natl Acad Sci U S A. 1987 Jan;84(2):434–438. doi: 10.1073/pnas.84.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astumian RD, Chock PB, Tsong TY, Westerhoff HV. Effects of oscillations and energy-driven fluctuations on the dynamics of enzyme catalysis and free-energy transduction. Phys Rev A Gen Phys. 1989 Jun 15;39(12):6416–6435. doi: 10.1103/physreva.39.6416. [DOI] [PubMed] [Google Scholar]
- Chen Y. Free energy transduction in membrane transport systems induced by externally imposed fluctuations in ligand concentrations. Biochim Biophys Acta. 1987 Sep 3;902(3):307–316. doi: 10.1016/0005-2736(87)90199-4. [DOI] [PubMed] [Google Scholar]
- Knox B. E., Devreotes P. N., Goldbeter A., Segel L. A. A molecular mechanism for sensory adaptation based on ligand-induced receptor modification. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2345–2349. doi: 10.1073/pnas.83.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Liu D. S., Astumian R. D., Tsong T. Y. Activation of Na+ and K+ pumping modes of (Na,K)-ATPase by an oscillating electric field. J Biol Chem. 1990 May 5;265(13):7260–7267. [PubMed] [Google Scholar]
- Markin V. S., Tsong T. Y. Frequency and concentration windows for the electric activation of a membrane active transport system. Biophys J. 1991 Jun;59(6):1308–1316. doi: 10.1016/S0006-3495(91)82345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin V. S., Tsong T. Y. Reversible mechanosensitive ion pumping as a part of mechanoelectrical transduction. Biophys J. 1991 Jun;59(6):1317–1324. doi: 10.1016/S0006-3495(91)82346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Activation of electrogenic Rb+ transport of (Na,K)-ATPase by an electric field. J Biol Chem. 1984 Jun 10;259(11):7155–7162. [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Stimulation of a ouabain-sensitive Rb+ uptake in human erthrocytes with an external electric field. J Membr Biol. 1983;74(3):191–201. doi: 10.1007/BF02332123. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Astumian R. D. Electroconformational coupling: how membrane-bound ATPase transduces energy from dynamic electric fields. Annu Rev Physiol. 1988;50:273–290. doi: 10.1146/annurev.ph.50.030188.001421. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Deciphering the language of cells. Trends Biochem Sci. 1989 Mar;14(3):89–92. doi: 10.1016/0968-0004(89)90127-8. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electrical modulation of membrane proteins: enforced conformational oscillations and biological energy and signal transductions. Annu Rev Biophys Biophys Chem. 1990;19:83–106. doi: 10.1146/annurev.bb.19.060190.000503. [DOI] [PubMed] [Google Scholar]
- Weaver J. C., Astumian R. D. The response of living cells to very weak electric fields: the thermal noise limit. Science. 1990 Jan 26;247(4941):459–462. doi: 10.1126/science.2300806. [DOI] [PubMed] [Google Scholar]
- Zhou T, Moss F, Jung P. Escape-time distributions of a periodically modulated bistable system with noise. Phys Rev A. 1990 Sep 15;42(6):3161–3169. doi: 10.1103/physreva.42.3161. [DOI] [PubMed] [Google Scholar]


