Abstract
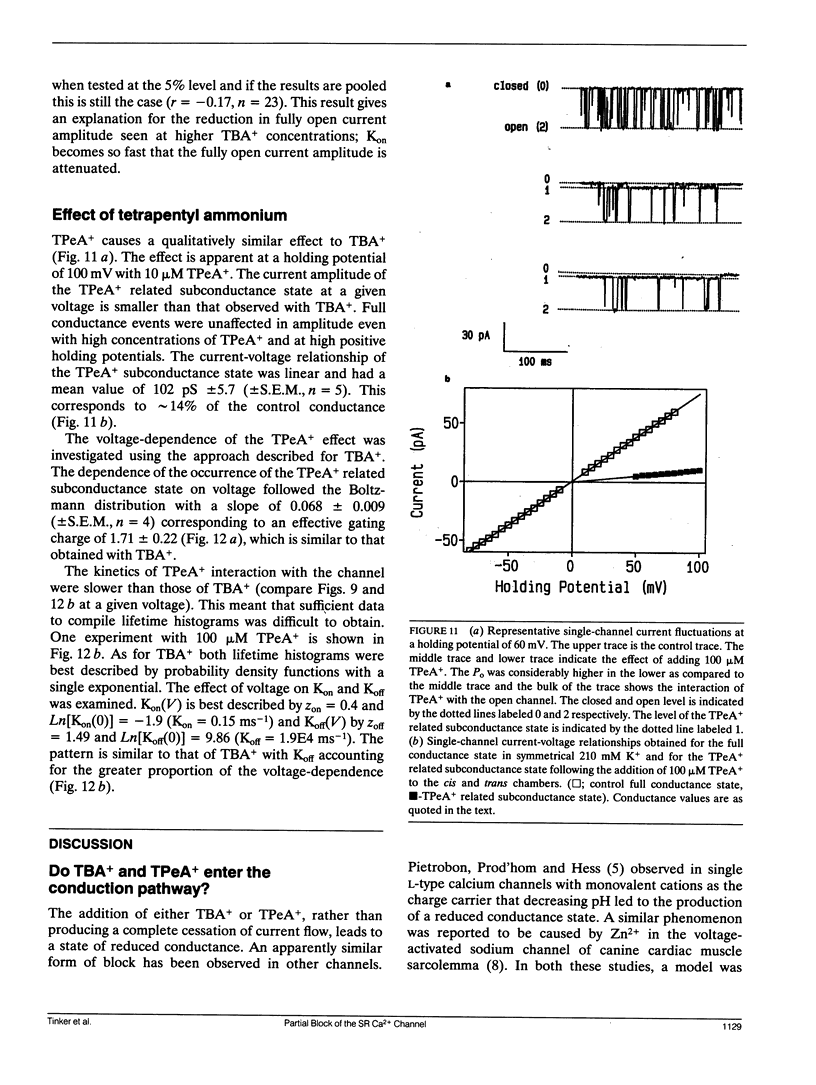
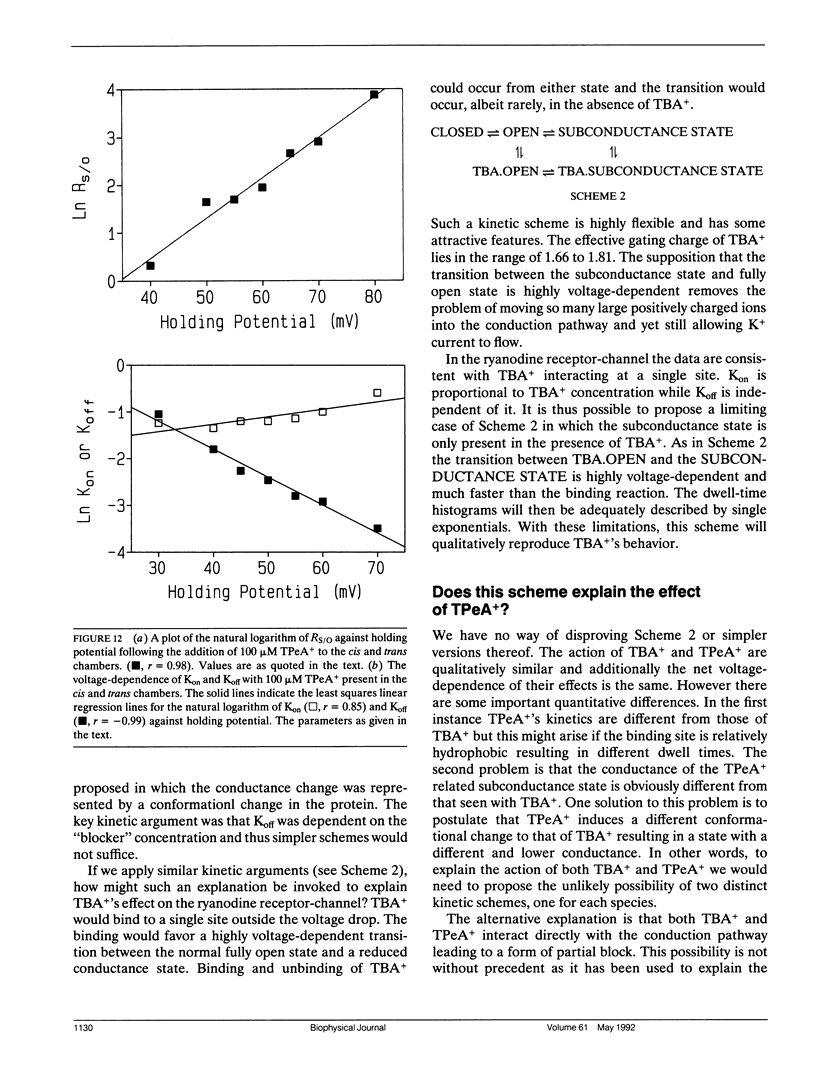
The purified Ca(2+)-release/ryanodine receptor channel of the sheep cardiac muscle sarcoplasmic reticulum (SR) functions as a calcium-activated cation-selective channel under voltage clamp conditions following reconstitution into planar phospholipid bilayers. We have investigated the effect of large tetraalkyl ammonium (TAA) cations, (CnH2n+1)4N+ (n = 4 and 5) on monovalent cation conduction. These cations modify the conductance of the receptor channel at positive holding potentials from the cytosolic side of the channel. Under these conditions, openings are resolved as a mixture of normal full amplitude events and events of reduced conductance. The amplitude of the reduced conductance state is a fixed proportion of the normal open state. As a proportion of all open events, the occurrence of the tetrabutyl ammonium (TBA+) related subconductance state increases with concentration and increasingly positive holding potential. The TBA+ related subconductance state displays similar conduction properties to the unmodified channel; with a linear current-voltage relationship, a similar affinity for K+ and voltage-dependent block by TEA+. A method was used to quantify the voltage dependence of the occurrence of the TBA+ effect, which yielded an effective gating charge of 1.66. A second method based on kinetic analysis of the voltage dependence of transitions between the full open state and the TBA+ related subconductance state produced a similar value. In addition, this analysis revealed that the bulk of the voltage-dependence resided in the off rate. TBA+ related subconductance events, expressed as a proportion of all open events, saturated with increasing TBA+ concentration. Kinetic analysis revealed that this could be entirely accounted for by changes in the on rate. Tetrapentyl ammonium (TPeA+) causes a qualitatively similar effect with a subconductance state of lower amplitude. The voltage-dependence of the effect was comparable to that displayed by TBA+. These findings are interpreted as a form of partial block in which more than one large TAA cation binds at the extremity of the voltage drop to produce an electrostatic barrier for ion translocation.
Full text
PDF


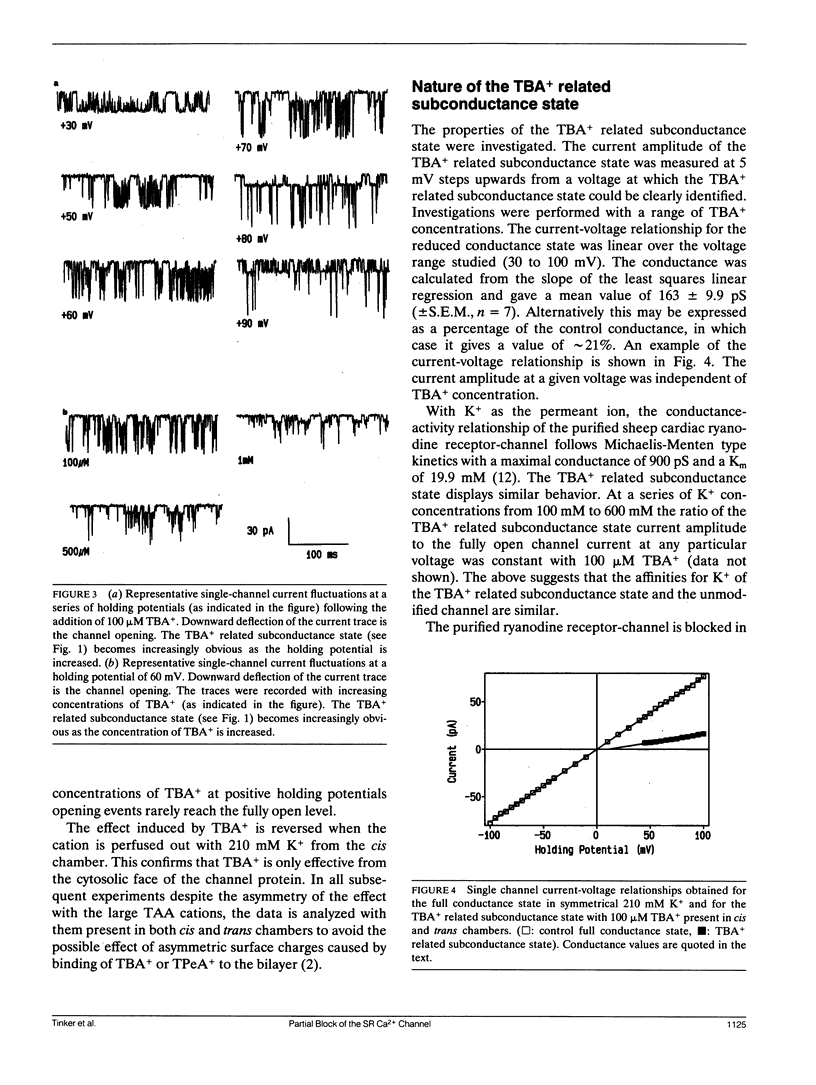
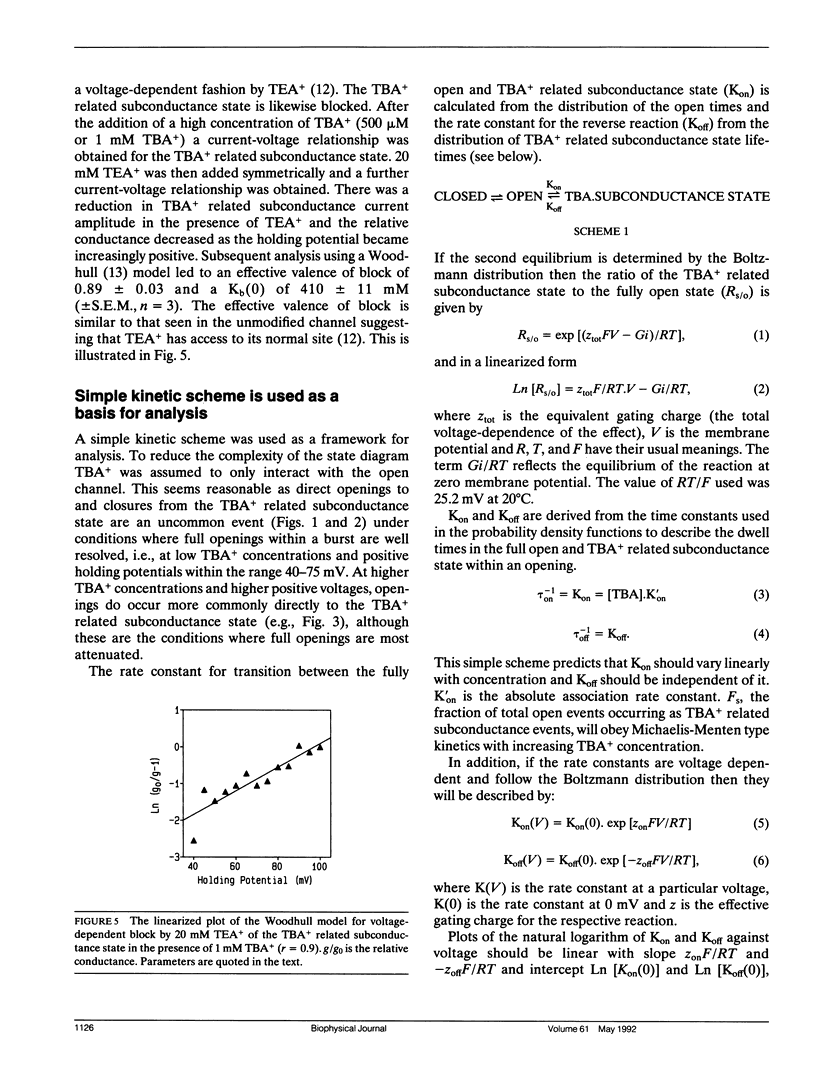
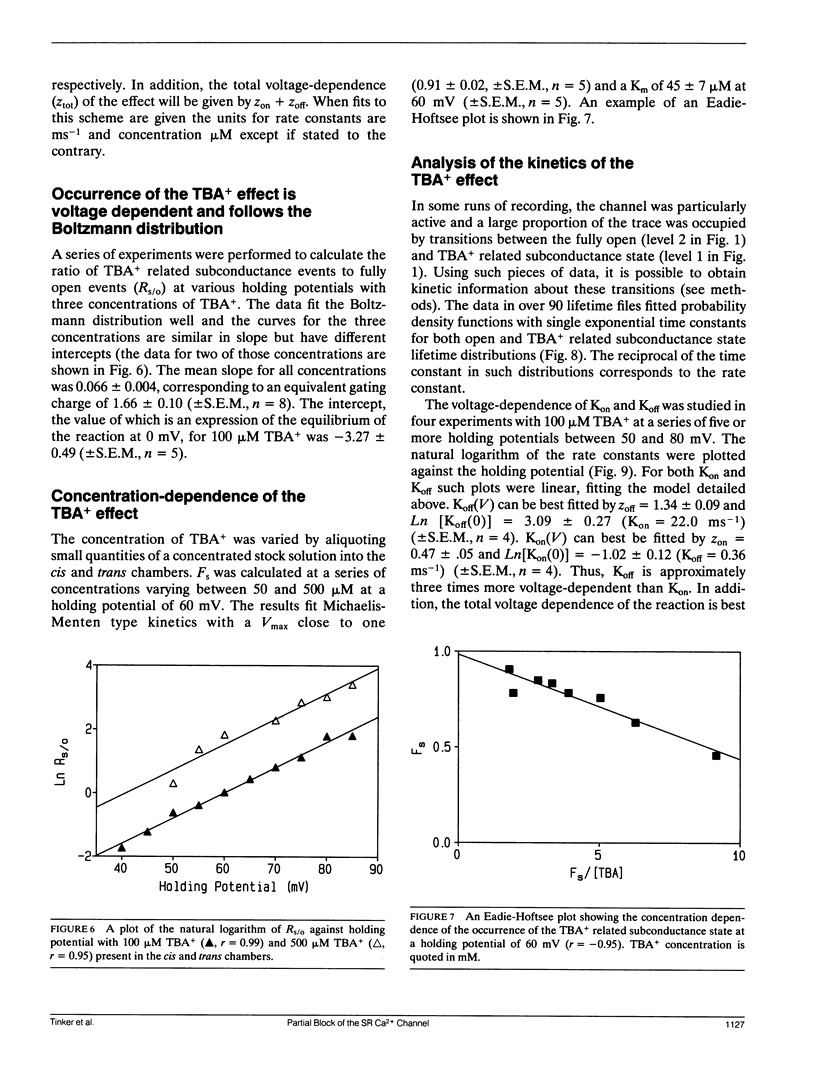
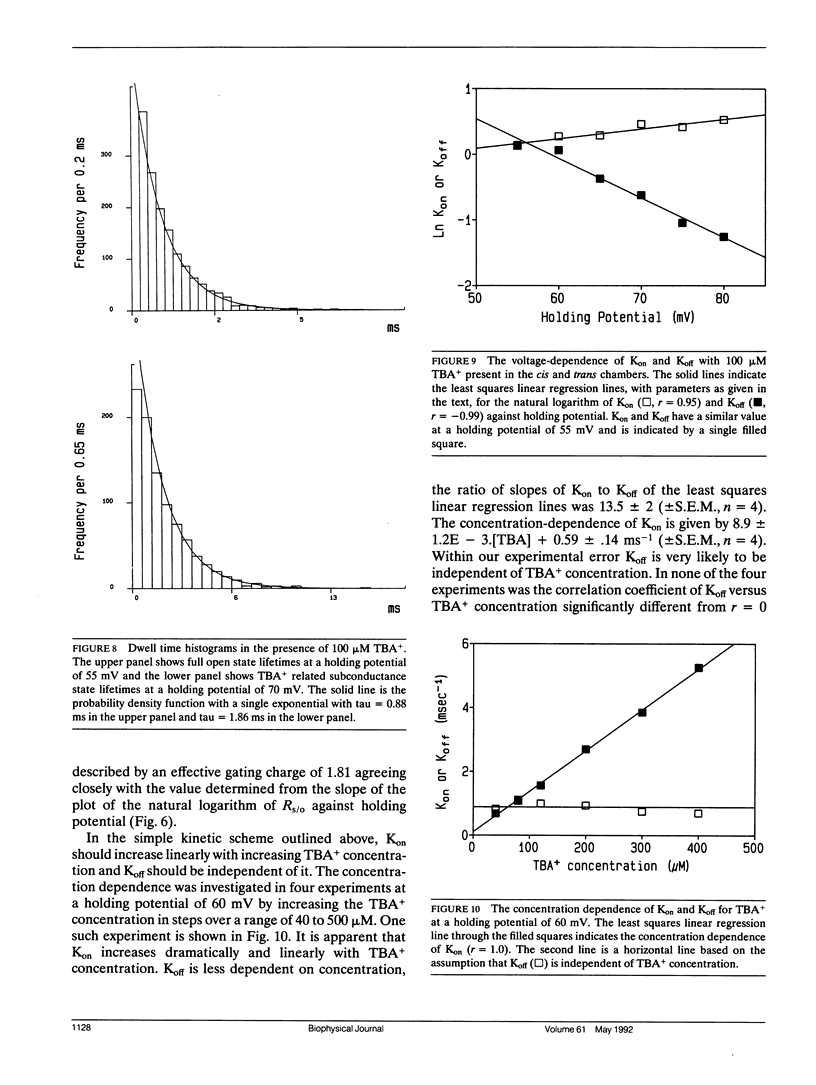


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Green W. N., Andersen O. S. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Jordan P. C. Effect of pore structure on energy barriers and applied voltage profiles. I. Symmetrical channels. Biophys J. 1984 Jun;45(6):1091–1100. doi: 10.1016/S0006-3495(84)84257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. C. Effect of pore structure on energy barriers and applied voltage profiles. II. Unsymmetrical channels. Biophys J. 1984 Jun;45(6):1101–1107. doi: 10.1016/S0006-3495(84)84258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Lindsay A. R., Manning S. D., Williams A. J. Monovalent cation conductance in the ryanodine receptor-channel of sheep cardiac muscle sarcoplasmic reticulum. J Physiol. 1991 Aug;439:463–480. doi: 10.1113/jphysiol.1991.sp018676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay A. R., Williams A. J. Functional characterisation of the ryanodine receptor purified from sheep cardiac muscle sarcoplasmic reticulum. Biochim Biophys Acta. 1991 Apr 26;1064(1):89–102. doi: 10.1016/0005-2736(91)90415-5. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Lai F. A., Rousseau E., Jones R. V., Meissner G. Multiple conductance states of the purified calcium release channel complex from skeletal sarcoplasmic reticulum. Biophys J. 1989 Mar;55(3):415–424. doi: 10.1016/S0006-3495(89)82835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Diffusion-limited ion flow through pores. Biochim Biophys Acta. 1976 Dec 2;455(2):493–509. doi: 10.1016/0005-2736(76)90320-5. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Matsuura H., Noma A. Triple-barrel structure of inwardly rectifying K+ channels revealed by Cs+ and Rb+ block in guinea-pig heart cells. J Physiol. 1989 Jun;413:139–157. doi: 10.1113/jphysiol.1989.sp017646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J Gen Physiol. 1982 May;79(5):869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Miller C., White M. M. Dimeric structure of single chloride channels from Torpedo electroplax. Proc Natl Acad Sci U S A. 1984 May;81(9):2772–2775. doi: 10.1073/pnas.81.9.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D., Prod'hom B., Hess P. Interactions of protons with single open L-type calcium channels. pH dependence of proton-induced current fluctuations with Cs+, K+, and Na+ as permeant ions. J Gen Physiol. 1989 Jul;94(1):1–21. doi: 10.1085/jgp.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeant ion. J Gen Physiol. 1989 Jul;94(1):23–42. doi: 10.1085/jgp.94.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran A., Schild L., Moczydlowski E. Divalent cation selectivity for external block of voltage-dependent Na+ channels prolonged by batrachotoxin. Zn2+ induces discrete substates in cardiac Na+ channels. J Gen Physiol. 1991 Jan;97(1):89–115. doi: 10.1085/jgp.97.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L., Ravindran A., Moczydlowski E. Zn2(+)-induced subconductance events in cardiac Na+ channels prolonged by batrachotoxin. Current-voltage behavior and single-channel kinetics. J Gen Physiol. 1991 Jan;97(1):117–142. doi: 10.1085/jgp.97.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker G. J., Jackson M. B. Curare binding and the curare-induced subconductance state of the acetylcholine receptor channel. Biophys J. 1989 Oct;56(4):795–806. doi: 10.1016/S0006-3495(89)82726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Permeation in potassium channels: implications for channel structure. Annu Rev Biophys Biophys Chem. 1987;16:227–246. doi: 10.1146/annurev.bb.16.060187.001303. [DOI] [PubMed] [Google Scholar]


