Abstract
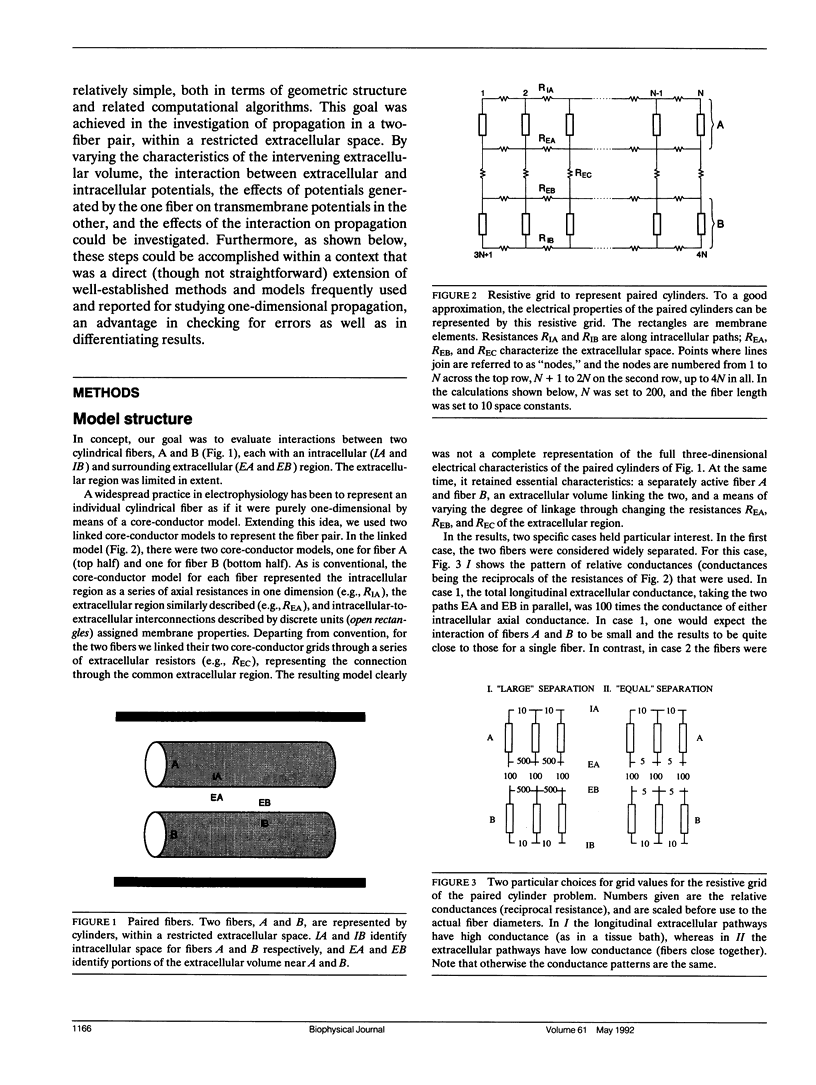
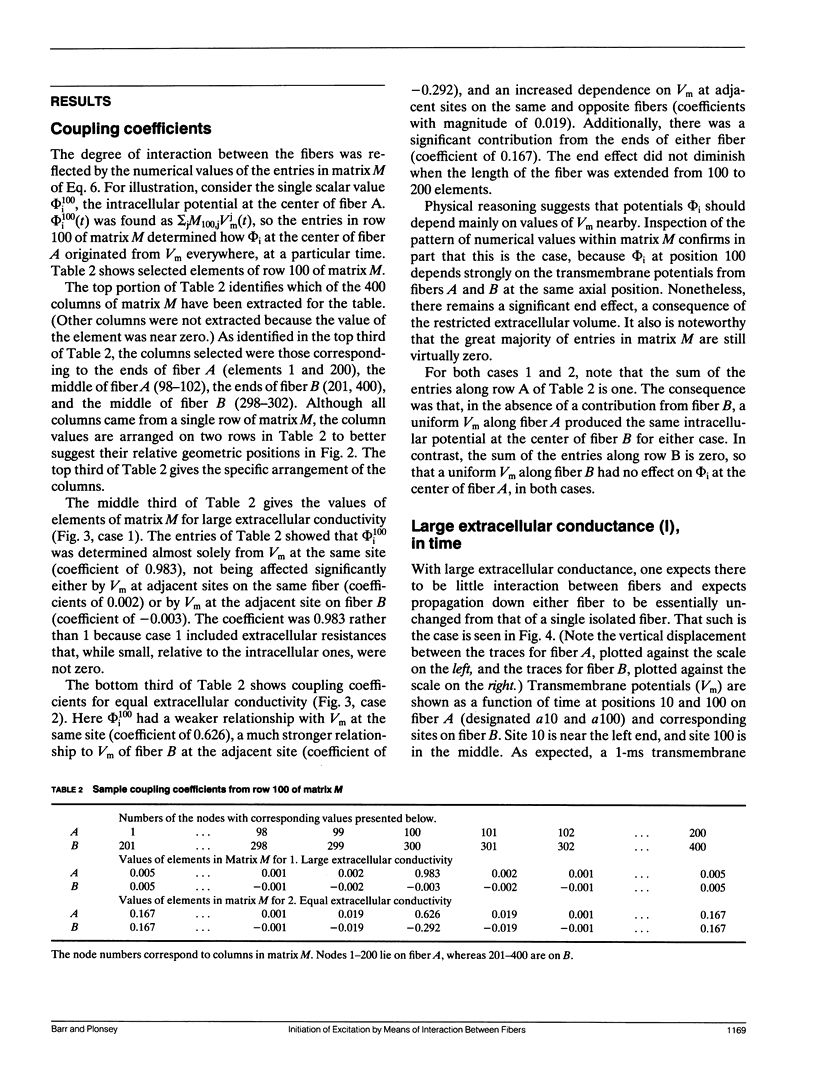
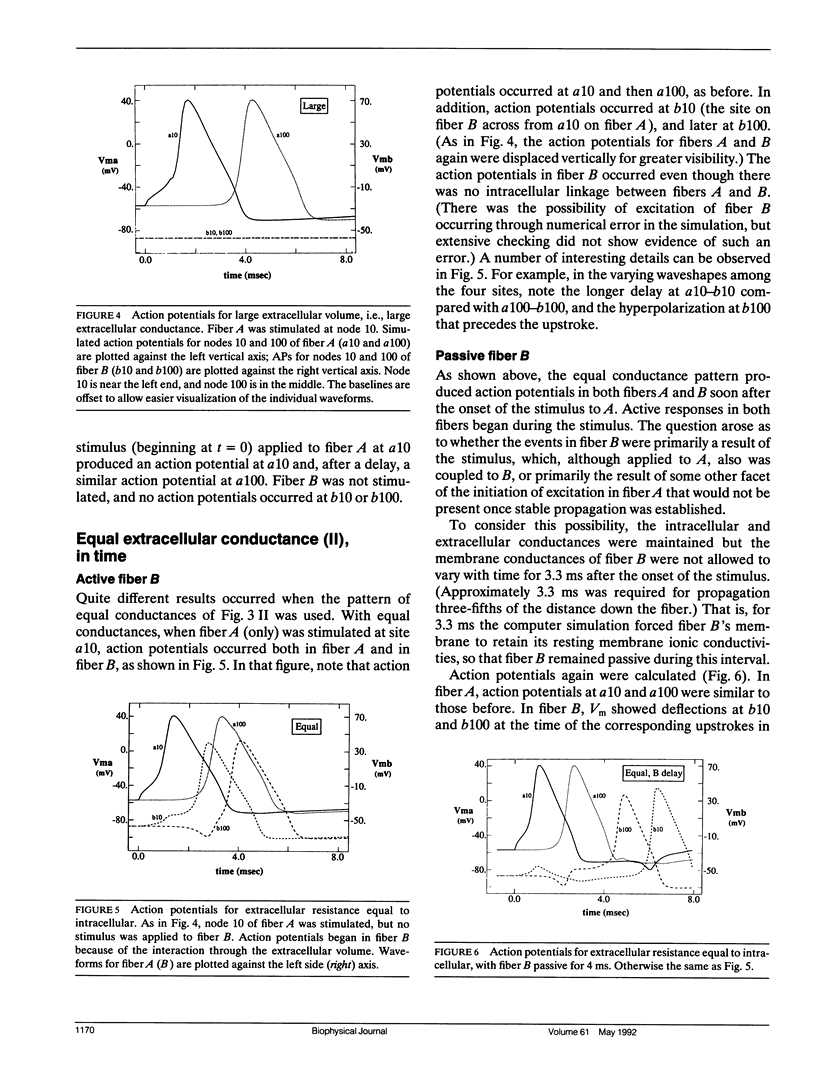
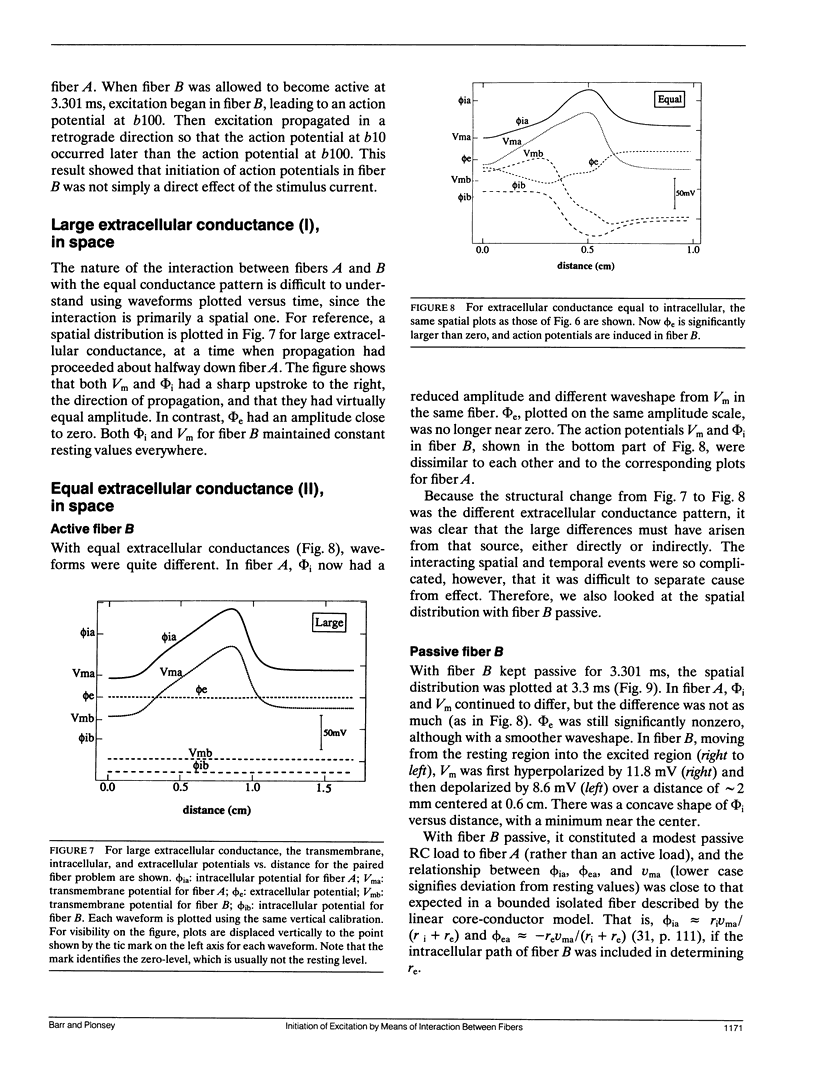
The influence of interstitial or extracellular potentials on propagation usually has been ignored, often through assuming these potentials to be insignificantly different from zero, presumably because both measurements and calculations become much more complex when interstitial interactions are included. This study arose primarily from an interest in cardiac muscle, where it has been well established that substantial interstitial potentials occur in tightly packed structures, e.g., tens of millivolts within the ventricular wall. We analyzed the electrophysiological interaction between two adjacent unmyelinated fibers within a restricted extracellular space. Numerical evaluations made use of two linked core-conductor models and Hodgkin-Huxley membrane properties. Changes in transmembrane potentials induced in the second fiber ranged from nonexistent with large intervening volumes to large enough to initiate excitation when fibers were coupled by interstitial currents through a small interstitial space. With equal interstitial and intracellular longitudinal conductivities and close coupling, the interaction was large enough (induced Vm approximately 20 mV peak-to-peak) that action potentials from one fiber initiated excitation in the other, for the 40-microns radius evaluated. With close coupling but no change in structure, propagation velocity in the first fiber varied from 1.66 mm/ms (when both fibers were simultaneously stimulated) to 2.84 mm/ms (when the second fiber remained passive). Although normal propagation through interstitial interaction is unlikely, the magnitudes of the electrotonic interactions were large and may have a substantial modulating effect on function.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr R. C., Plonsey R. Propagation of excitation in idealized anisotropic two-dimensional tissue. Biophys J. 1984 Jun;45(6):1191–1202. doi: 10.1016/S0006-3495(84)84268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. W., Jr, Plonsey R. Fiber interaction in a nerve trunk. Biophys J. 1971 Mar;11(3):281–294. doi: 10.1016/S0006-3495(71)86214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- Drouhard J. P., Roberge F. A. Revised formulation of the Hodgkin-Huxley representation of the sodium current in cardiac cells. Comput Biomed Res. 1987 Aug;20(4):333–350. doi: 10.1016/0010-4809(87)90048-6. [DOI] [PubMed] [Google Scholar]
- Ebihara L., Johnson E. A. Fast sodium current in cardiac muscle. A quantitative description. Biophys J. 1980 Nov;32(2):779–790. doi: 10.1016/S0006-3495(80)85016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Shigeto N., Lieberman M., Johnson E. A. The initial inward current in spherical clusters of chick embryonic heart cells. J Gen Physiol. 1980 Apr;75(4):437–456. doi: 10.1085/jgp.75.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Barcilon V., Mathias R. T. Electrical properties of spherical syncytia. Biophys J. 1979 Jan;25(1):151–180. doi: 10.1016/S0006-3495(79)85283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. S., Korn H. Electrical field effects: their relevance in central neural networks. Physiol Rev. 1989 Jul;69(3):821–863. doi: 10.1152/physrev.1989.69.3.821. [DOI] [PubMed] [Google Scholar]
- Geselowitz D. B., Miller W. T., 3rd A bidomain model for anisotropic cardiac muscle. Ann Biomed Eng. 1983;11(3-4):191–206. doi: 10.1007/BF02363286. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter J. A., Clark J. W., Jr A distributed-parameter model of the myelinated nerve fiber. J Theor Biol. 1991 Feb 7;148(3):345–382. doi: 10.1016/s0022-5193(05)80242-5. [DOI] [PubMed] [Google Scholar]
- Henriquez C. S., Plonsey R. Simulation of propagation along a cylindrical bundle of cardiac tissue--I: Mathematical formulation. IEEE Trans Biomed Eng. 1990 Sep;37(9):850–860. doi: 10.1109/10.58596. [DOI] [PubMed] [Google Scholar]
- Henriquez C. S., Plonsey R. Simulation of propagation along a cylindrical bundle of cardiac tissue--II: Results of simulation. IEEE Trans Biomed Eng. 1990 Sep;37(9):861–875. doi: 10.1109/10.58597. [DOI] [PubMed] [Google Scholar]
- Håkanson S. Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery. 1981 Dec;9(6):638–646. doi: 10.1227/00006123-198112000-00005. [DOI] [PubMed] [Google Scholar]
- Joyner R. W., Ramza B. M., Tan R. C., Matsuda J., Do T. T. Effects of tissue geometry on initiation of a cardiac action potential. Am J Physiol. 1989 Feb;256(2 Pt 2):H391–H403. doi: 10.1152/ajpheart.1989.256.2.H391. [DOI] [PubMed] [Google Scholar]
- Katz B., Schmitt O. H. Electric interaction between two adjacent nerve fibres. J Physiol. 1940 Feb 14;97(4):471–488. doi: 10.1113/jphysiol.1940.sp003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber A. G., Riegger C. B. Electrical constants of arterially perfused rabbit papillary muscle. J Physiol. 1987 Apr;385:307–324. doi: 10.1113/jphysiol.1987.sp016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisley S. B., Maruyama T., Buchanan J. W., Jr Interstitial potential during propagation in bathed ventricular muscle. Biophys J. 1991 Mar;59(3):509–515. doi: 10.1016/S0006-3495(91)82267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LING G., GERARD R. W. The normal membrane potential of frog sartorius fibers. J Cell Physiol. 1949 Dec;34(3):383–396. doi: 10.1002/jcp.1030340304. [DOI] [PubMed] [Google Scholar]
- Leon L. J., Roberge F. A. A new cable model formulation based on Green's theorem. Ann Biomed Eng. 1990;18(1):1–17. doi: 10.1007/BF02368414. [DOI] [PubMed] [Google Scholar]
- Markin V. S. Elektricheskoe vzaimodeistvie parallel'nykh nemielinizirovannykh nervnykh volokon. 3. Vzaimodeistvie v puchkakh. Biofizika. 1973 Mar-Apr;18(2):314–321. [PubMed] [Google Scholar]
- Miller W. T., Geselowitz D. B. Simulation studies of the electrocardiogram. I. The normal heart. Circ Res. 1978 Aug;43(2):301–315. doi: 10.1161/01.res.43.2.301. [DOI] [PubMed] [Google Scholar]
- Nelson P. G. Interaction between spinal motoneurons of the cat. J Neurophysiol. 1966 Mar;29(2):275–287. doi: 10.1152/jn.1966.29.2.275. [DOI] [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Current flow patterns in two-dimensional anisotropic bisyncytia with normal and extreme conductivities. Biophys J. 1984 Mar;45(3):557–571. doi: 10.1016/S0006-3495(84)84193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Interstitial potentials and their change with depth into cardiac tissue. Biophys J. 1987 Apr;51(4):547–555. doi: 10.1016/S0006-3495(87)83380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonsey R., Henriquez C., Trayanova N. Extracellular (volume conductor) effect on adjoining cardiac muscle electrophysiology. Med Biol Eng Comput. 1988 Mar;26(2):126–129. doi: 10.1007/BF02442253. [DOI] [PubMed] [Google Scholar]
- Pollard A. E., Barr R. C. Computer simulations of activation in an anatomically based model of the human ventricular conduction system. IEEE Trans Biomed Eng. 1991 Oct;38(10):982–996. doi: 10.1109/10.88444. [DOI] [PubMed] [Google Scholar]
- Ramón F., Moore J. W. Ephaptic transmission in squid giant axons. Am J Physiol. 1978 May;234(5):C162–C169. doi: 10.1152/ajpcell.1978.234.5.C162. [DOI] [PubMed] [Google Scholar]
- Roberge F. A., Boucher L., Vinet A. Model study of the spread of electrotonic potential in cardiac tissue. Med Biol Eng Comput. 1989 Jul;27(4):405–415. doi: 10.1007/BF02441433. [DOI] [PubMed] [Google Scholar]
- Roberts D. E., Hersh L. T., Scher A. M. Influence of cardiac fiber orientation on wavefront voltage, conduction velocity, and tissue resistivity in the dog. Circ Res. 1979 May;44(5):701–712. doi: 10.1161/01.res.44.5.701. [DOI] [PubMed] [Google Scholar]
- Roth B. J. The electrical potential produced by a strand of cardiac muscle: a bidomain analysis. Ann Biomed Eng. 1988;16(6):609–637. doi: 10.1007/BF02368018. [DOI] [PubMed] [Google Scholar]
- Sanders D. B. Ephaptic transmission in hemifacial spasm: a single-fiber EMG study. Muscle Nerve. 1989 Aug;12(8):690–694. doi: 10.1002/mus.880120810. [DOI] [PubMed] [Google Scholar]
- Sepulveda N. G., Roth B. J., Wikswo J. P., Jr Current injection into a two-dimensional anisotropic bidomain. Biophys J. 1989 May;55(5):987–999. doi: 10.1016/S0006-3495(89)82897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach M. S., Barr R. C. Ventricular intramural and epicardial potential distributions during ventricular activation and repolarization in the intact dog. Circ Res. 1975 Aug;37(2):243–257. doi: 10.1161/01.res.37.2.243. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Miller W. T., 3rd, Geselowitz D. B., Barr R. C., Kootsey J. M., Johnson E. A. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res. 1981 Jan;48(1):39–54. doi: 10.1161/01.res.48.1.39. [DOI] [PubMed] [Google Scholar]
- Suenson M. Ephaptic impulse transmission between ventricular myocardial cells in vitro. Acta Physiol Scand. 1984 Mar;120(3):445–455. doi: 10.1111/j.1748-1716.1984.tb07405.x. [DOI] [PubMed] [Google Scholar]
- Tan R. C., Joyner R. W. Electrotonic influences on action potentials from isolated ventricular cells. Circ Res. 1990 Nov;67(5):1071–1081. doi: 10.1161/01.res.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Vander Ark C. R., Reynolds E. W., Jr An experimental study of propagated electrical activity in the canine heart. Circ Res. 1970 Apr;26(4):451–460. doi: 10.1161/01.res.26.4.451. [DOI] [PubMed] [Google Scholar]
- Wikswo J. P., Jr, Wisialowski T. A., Altemeier W. A., Balser J. R., Kopelman H. A., Roden D. M. Virtual cathode effects during stimulation of cardiac muscle. Two-dimensional in vivo experiments. Circ Res. 1991 Feb;68(2):513–530. doi: 10.1161/01.res.68.2.513. [DOI] [PubMed] [Google Scholar]



