Abstract
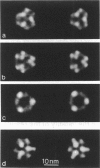
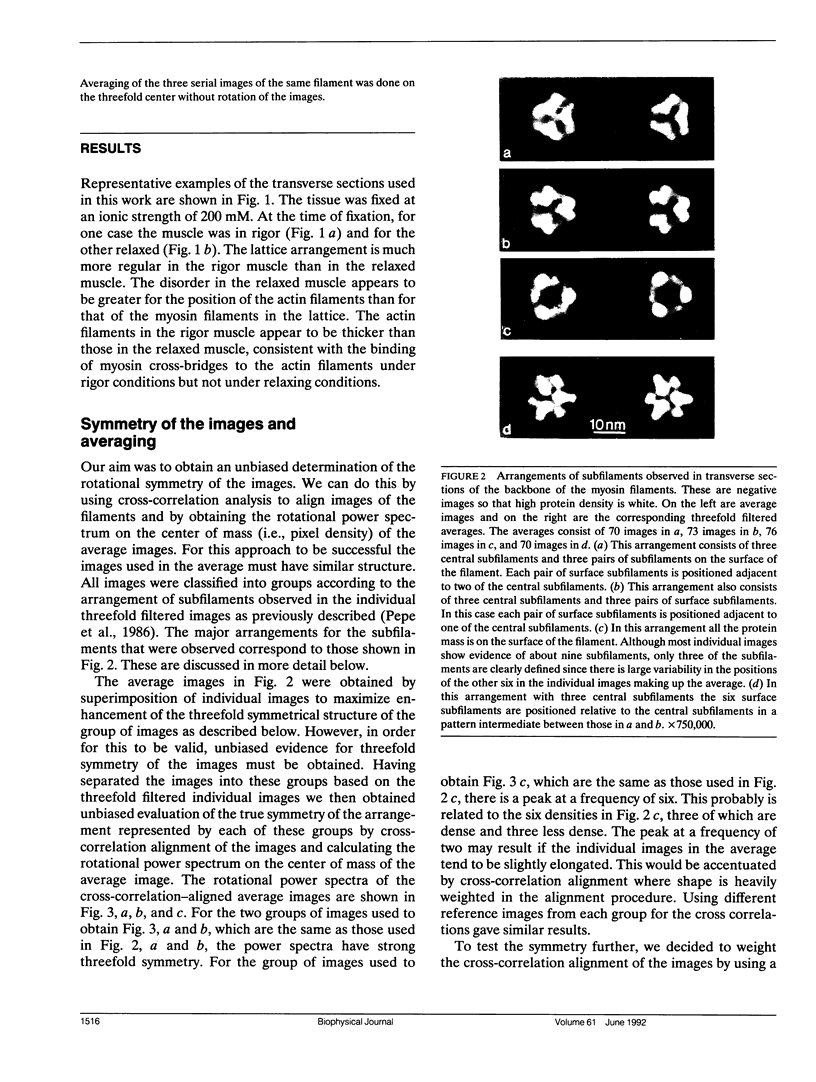
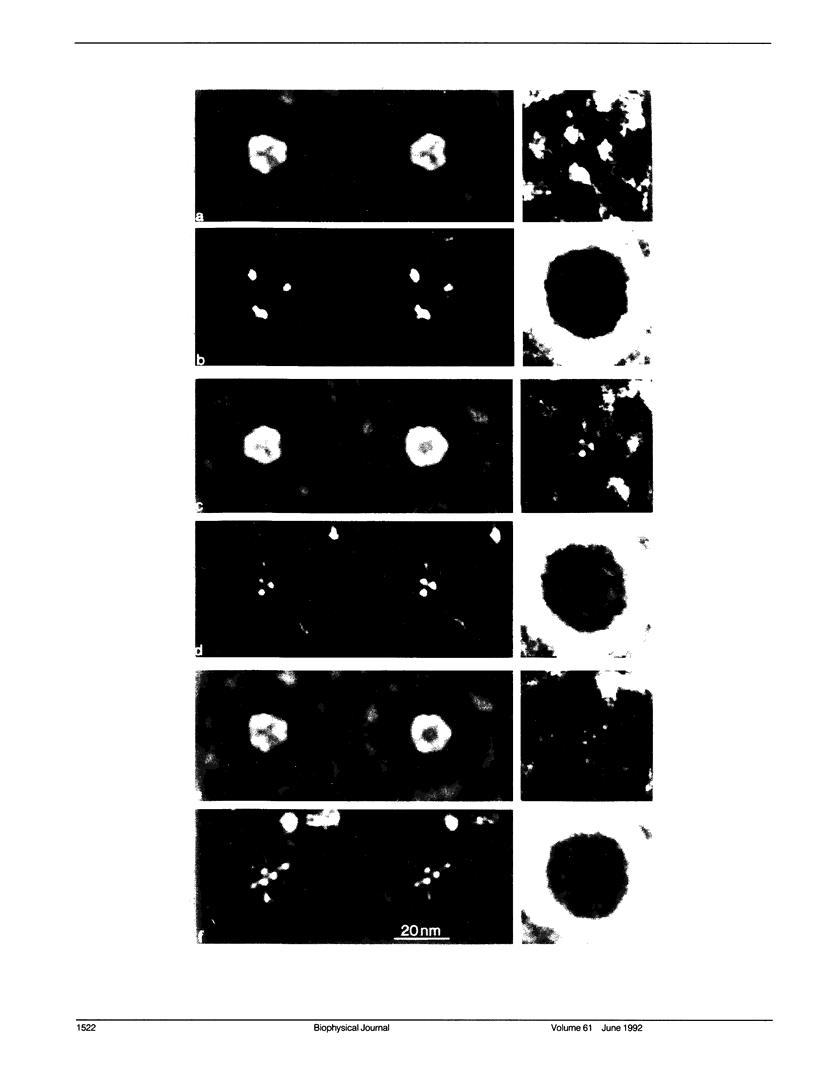
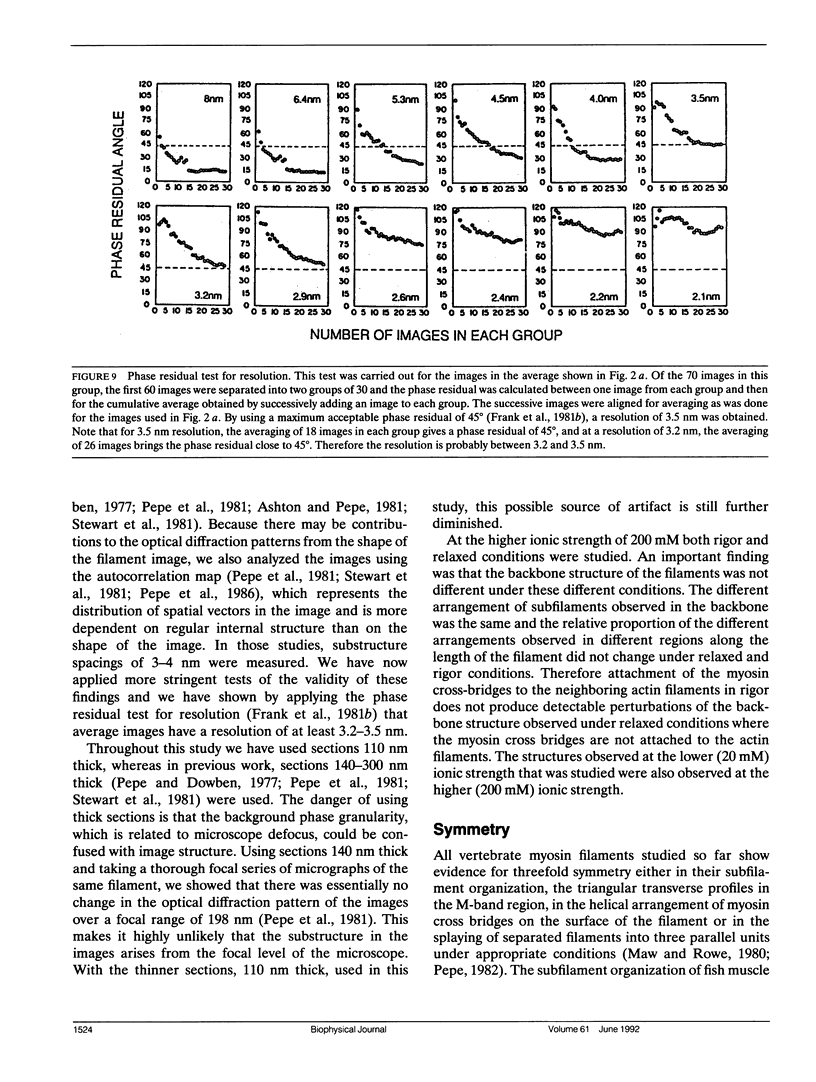
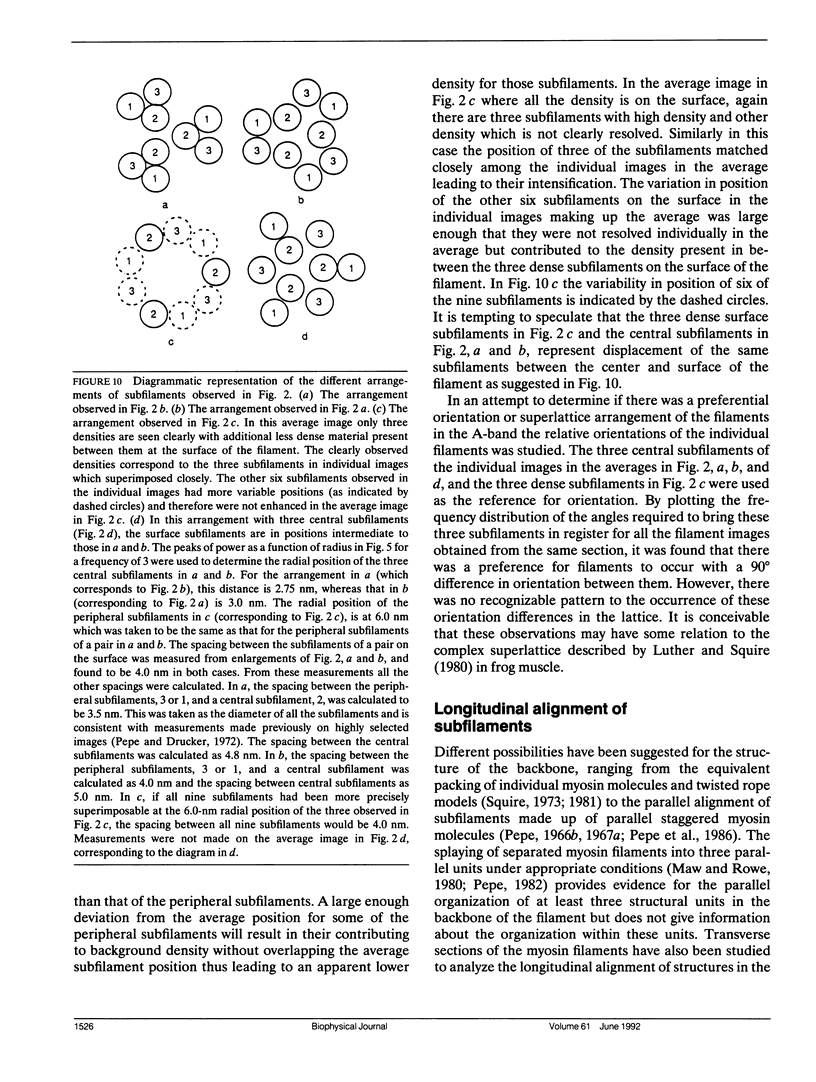
The substructure of the thick filaments of chemically skinned chicken pectoralis muscle was investigated by electron microscopy. Images of transverse sections of the myosin filaments were determined to have threefold symmetry by cross-correlation analysis, which gives an unbiased determination of the rotational symmetry of the images. Resolution, using the phase residual test (Frank et al. 1981. Science [Wash. DC]. 214:1353-1355), was found to be between 3.2 and 3.6 nm. Three arrangements of nine subfilaments in the backbone were found in all regions of the filament at ionic strengths of 20 and 200 mM. In the average images of two of these, there were three dense central subfilaments and three pairs of subfilaments on the surface of the thick filament. In the average image of the third arrangement, all of the protein mass of the nine subfilaments was on the surface of the filament with three of them showing less variation in position than the others. A fourth arrangement appearing to be transitional between two of these was seen often at 200 mM ionic strength and only rarely at 20 mM. On average, the myosin subfilaments were parallel to the long axis of the filament. The different arrangements of subfilaments appear to be randomly distributed among the filaments in a transverse section of the A-band. Relative rotational orientations with respect to the hexagonal filament lattice, using the three densest subfilaments as reference showed a major clustering (32%) of filaments within one 10 degrees spread, a lesser clustering (15%) at 90 degrees to the first, and the remainder scattered thinly over the rest of the 120 degrees range. There was no obvious pattern of distribution of the two predominant orientations that could define a superlattice in the filament lattice.
Full text
PDF


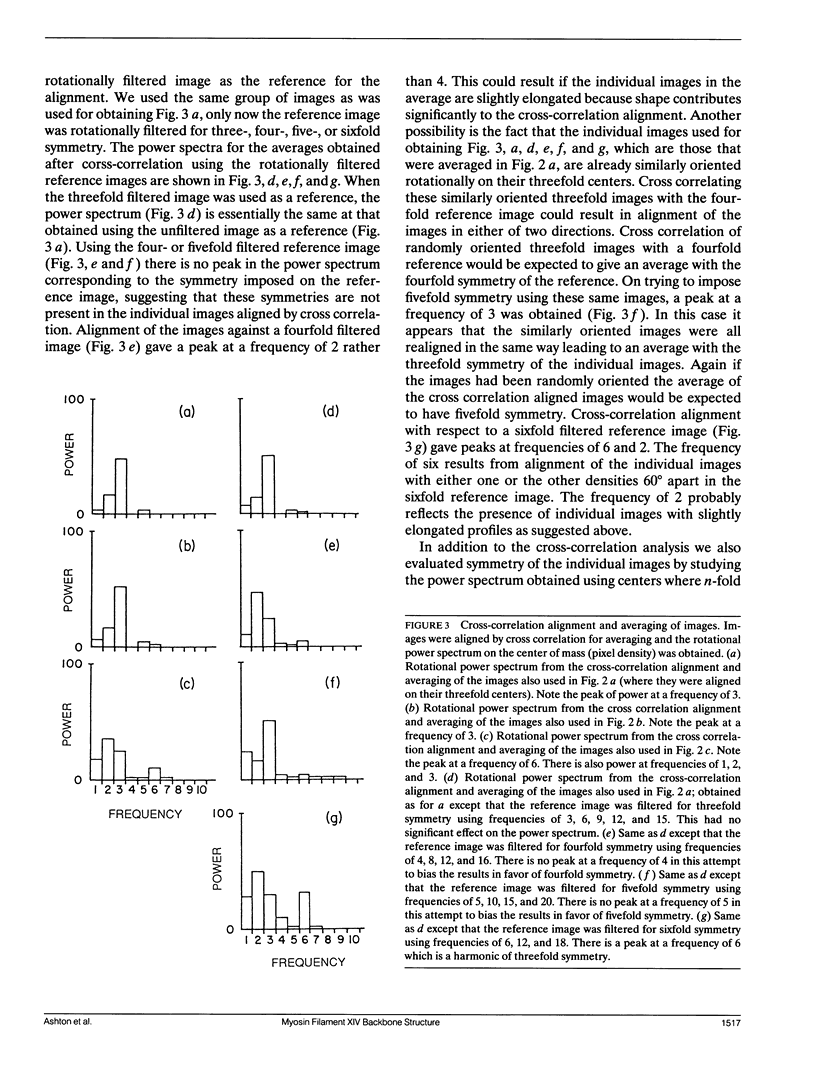
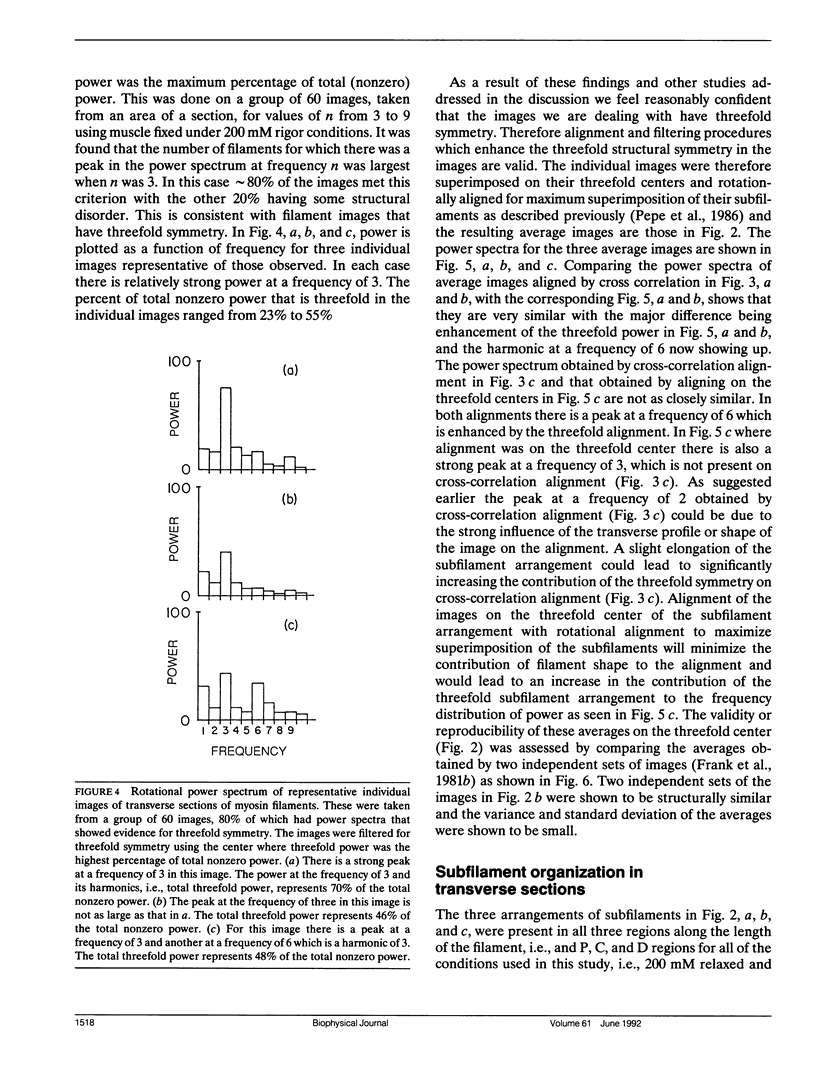
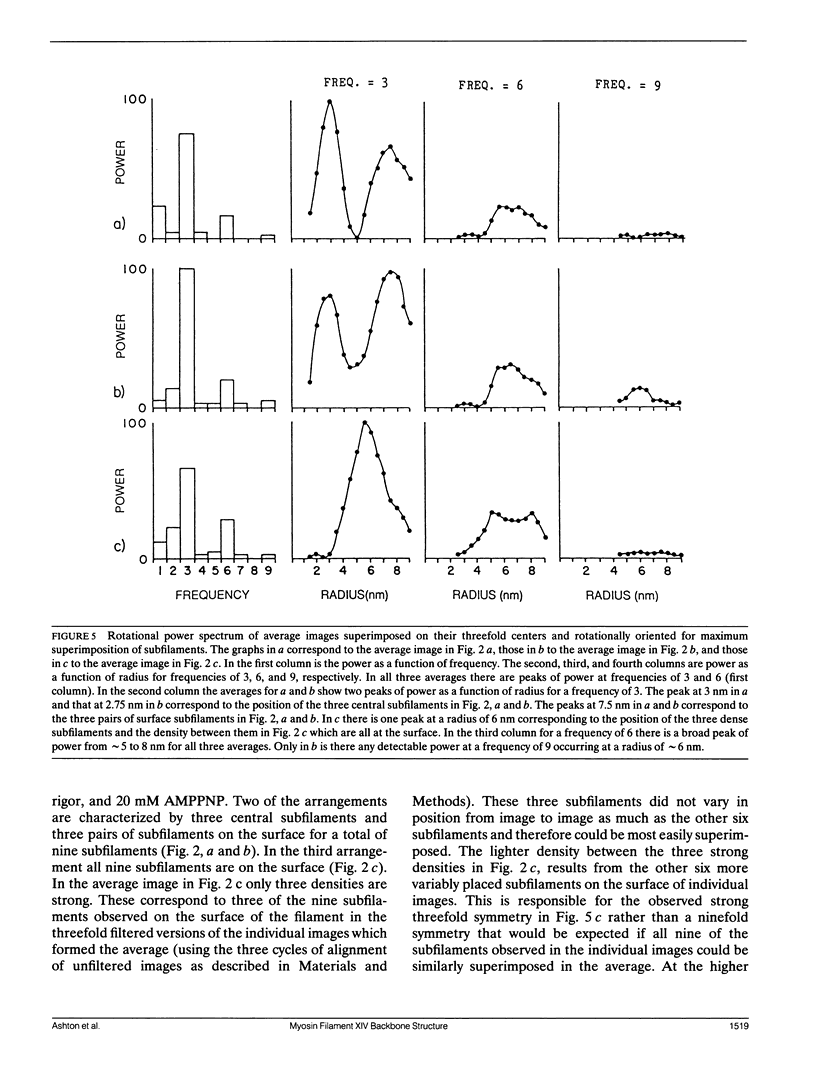

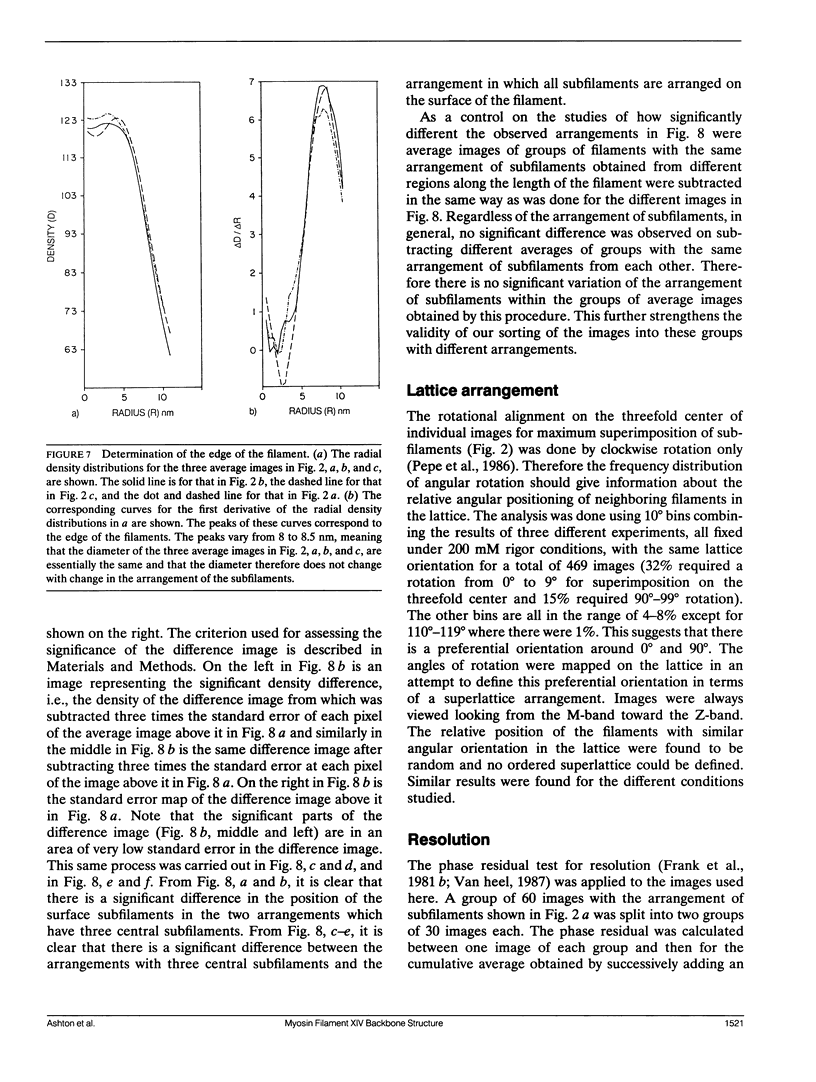




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton F. T., Pepe F. A. The myosin filament. VIII. Preservation of subfilament organization. J Microsc. 1981 Jul;123(Pt 1):93–104. [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Podolsky R. J. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J. 1984 Sep;46(3):299–306. doi: 10.1016/S0006-3495(84)84026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretaudiere J. P., Frank J. Reconstitution of molecule images analysed by correspondence analysis: a tool for structural interpretation. J Microsc. 1986 Oct;144(Pt 1):1–14. doi: 10.1111/j.1365-2818.1986.tb04669.x. [DOI] [PubMed] [Google Scholar]
- Cantino M., Squire J. Resting myosin cross-bridge configuration in frog muscle thick filaments. J Cell Biol. 1986 Feb;102(2):610–618. doi: 10.1083/jcb.102.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R., Offer G. The location of C-protein in rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1976 Mar 16;192(1109):451–461. doi: 10.1098/rspb.1976.0023. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Harmonic analysis of electron microscope images with rotational symmetry. J Mol Biol. 1971 Aug 28;60(1):123–130. doi: 10.1016/0022-2836(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E. Suppression of muscle contraction by vanadate. Mechanical and ligand binding studies on glycerol-extracted rabbit fibers. J Gen Physiol. 1985 Sep;86(3):305–327. doi: 10.1085/jgp.86.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Computer averaging of electron micrographs of 40S ribosomal subunits. Science. 1981 Dec 18;214(4527):1353–1355. doi: 10.1126/science.7313694. [DOI] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Multivariate statistical analysis of ribosome electron micrographs. L and R lateral views of the 40 S subunit from HeLa cells. J Mol Biol. 1982 Oct 15;161(1):107–133. doi: 10.1016/0022-2836(82)90281-9. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. ELECTRON MICROSCOPE STUDIES ON THE STRUCTURE OF NATURAL AND SYNTHETIC PROTEIN FILAMENTS FROM STRIATED MUSCLE. J Mol Biol. 1963 Sep;7:281–308. doi: 10.1016/s0022-2836(63)80008-x. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Ip W., Heuser J. Direct visualization of the myosin crossbridge helices on relaxed rabbit psoas thick filaments. J Mol Biol. 1983 Nov 25;171(1):105–109. doi: 10.1016/s0022-2836(83)80317-9. [DOI] [PubMed] [Google Scholar]
- Kensler R. W., Stewart M. AN ultrastructural study of cross-bridge arrangement in the frog thigh muscle thick filament. Biophys J. 1986 Jan;49(1):343–351. doi: 10.1016/S0006-3495(86)83647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler R. W., Stewart M. Frog skeletal muscle thick filaments are three-stranded. J Cell Biol. 1983 Jun;96(6):1797–1802. doi: 10.1083/jcb.96.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P. J., Erickson M. A., Rodgers M. E., Beer M., Wiggins J. W. Distribution of mass within native thick filaments of vertebrate skeletal muscle. J Mol Biol. 1986 May 5;189(1):167–177. doi: 10.1016/0022-2836(86)90388-8. [DOI] [PubMed] [Google Scholar]
- Luther P. K., Munro P. M., Squire J. M. Three-dimensional structure of the vertebrate muscle A-band. III. M-region structure and myosin filament symmetry. J Mol Biol. 1981 Oct 5;151(4):703–730. doi: 10.1016/0022-2836(81)90430-7. [DOI] [PubMed] [Google Scholar]
- Luther P. K., Squire J. M. Three-dimensional structure of the vertebrate muscle A-band. II. The myosin filament superlattice. J Mol Biol. 1980 Aug 25;141(4):409–439. doi: 10.1016/0022-2836(80)90254-5. [DOI] [PubMed] [Google Scholar]
- Maw M. C., Rowe A. J. Fraying of A-filaments into three subfilaments. Nature. 1980 Jul 24;286(5771):412–414. doi: 10.1038/286412a0. [DOI] [PubMed] [Google Scholar]
- Pepe F. A., Ashton F. T., Dowben P., Stewart M. The myosin filament. VII Changes in internal structure along the length of the filament. J Mol Biol. 1981 Jan 15;145(2):421–440. doi: 10.1016/0022-2836(81)90213-8. [DOI] [PubMed] [Google Scholar]
- Pepe F. A., Ashton F. T., Street C., Weisel J. The myosin filament. X. Observation of nine subfilaments in transverse sections. Tissue Cell. 1986;18(4):499–508. doi: 10.1016/0040-8166(86)90016-9. [DOI] [PubMed] [Google Scholar]
- Pepe F. A., Dowben P. The myosin filament. V. Intermediate voltage electron microscopy and optical diffraction studies of the substructure. J Mol Biol. 1977 Jun 15;113(1):199–218. doi: 10.1016/0022-2836(77)90050-x. [DOI] [PubMed] [Google Scholar]
- Pepe F. A., Drucker B. The myosin filament. III. C-protein. J Mol Biol. 1975 Dec 25;99(4):609–617. doi: 10.1016/s0022-2836(75)80175-6. [DOI] [PubMed] [Google Scholar]
- Pepe F. A., Drucker B. The myosin filament. IV. Observation of the internal structural arrangement. J Cell Biol. 1972 Feb;52(2):255–260. doi: 10.1083/jcb.52.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe F. A. Some aspects of the structural organization of the myofibril as revealed by antibody--staining methods. J Cell Biol. 1966 Mar;28(3):505–525. doi: 10.1083/jcb.28.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe F. A. The myosin filament. I. Structural organization from antibody staining observed in electron microscopy. J Mol Biol. 1967 Jul 28;27(2):203–225. doi: 10.1016/0022-2836(67)90016-2. [DOI] [PubMed] [Google Scholar]
- Pepe F. A. The myosin filament. II. Interaction between myosin and actin filaments observed using antibody staining in fluorescent and electron microscopy. J Mol Biol. 1967 Jul 28;27(2):227–236. doi: 10.1016/0022-2836(67)90017-4. [DOI] [PubMed] [Google Scholar]
- Sjöström M., Squire J. M. Fine structure of the A-band in cryo-sections. The structure of the A-band of human skeletal muscle fibres from ultra-thin cryo-sections negatively stained. J Mol Biol. 1977 Jan 5;109(1):49–68. doi: 10.1016/s0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]
- Squire J. M. General model of myosin filament structure. 3. Molecular packing arrangements in myosin filaments. J Mol Biol. 1973 Jun 25;77(2):291–323. doi: 10.1016/0022-2836(73)90337-9. [DOI] [PubMed] [Google Scholar]
- Stewart M., Ashton F. T., Lieberson R., Pepe F. A. The myosin filament. IX. Determination of subfilament positions by computer processing of electron micrographs. J Mol Biol. 1981 Dec 5;153(2):381–392. doi: 10.1016/0022-2836(81)90284-9. [DOI] [PubMed] [Google Scholar]
- Stewart M., Kensler R. W. Arrangement of myosin heads in relaxed thick filaments from frog skeletal muscle. J Mol Biol. 1986 Dec 20;192(4):831–851. doi: 10.1016/0022-2836(86)90032-x. [DOI] [PubMed] [Google Scholar]
- Varriano-Marston E., Franzini-Armstrong C., Haselgrove J. C. The structure and disposition of crossbridges in deep-etched fish muscle. J Muscle Res Cell Motil. 1984 Aug;5(4):363–386. doi: 10.1007/BF00818256. [DOI] [PubMed] [Google Scholar]
- Wachsberger P., Lampson L., Pepe F. A. Non-uniform staining of myofibril a bands by a monoclonal antibody to skeletal muscle myosin S1 heavy chain. Tissue Cell. 1983;15(3):341–349. doi: 10.1016/0040-8166(83)90067-8. [DOI] [PubMed] [Google Scholar]
- Wray J. S. Structure of the backbone in myosin filaments of muscle. Nature. 1979 Jan 4;277(5691):37–40. doi: 10.1038/277037a0. [DOI] [PubMed] [Google Scholar]
- Zingsheim H. P., Barrantes F. J., Frank J., Hänicke W., Neugebauer D. C. Direct structural localization of two toxin-recognition sites on an ACh receptor protein. Nature. 1982 Sep 2;299(5878):81–84. doi: 10.1038/299081a0. [DOI] [PubMed] [Google Scholar]