Abstract
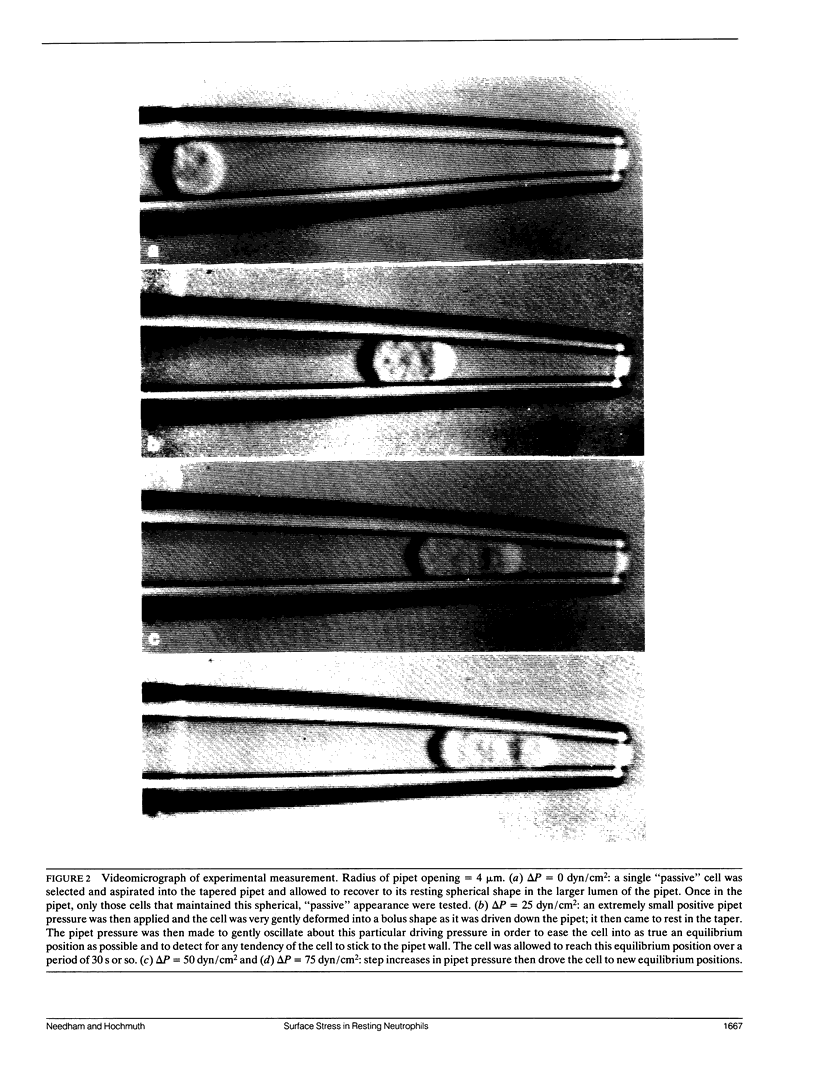
The simplest parameterized model of the "passive" or "resting receptive" neutrophil views the cell as being composed of an outer cortex surrounding an essentially liquid-like highly viscous cytoplasm. This cortex has been measured to maintain a small persistent tension of approximately 0.035 dyn/cm (Evans and Yeung. 1989. Biophys. J. 56:151-160) and is responsible for recovering the spherical shape of the cell after large deformation. The origin of the cortical tension is at present unknown, but speculations are that it may be an active process related to the sensitivity of a given cell to external stimulation and the "passive-active" transition. In order to characterize further this feature of the neutrophil we have used a new micropipet manipulation method to give a sensitive measure of the surface stress as a function of the surface area dilation of the highly ruffled cellular membrane. In the experiment, a single cell is driven down a tapered pipet in a series equilibrium deformation positions. Each equilibrium position represents a balance between the stress in the membrane and the pressure drop across the cell. For most cells that seemed to be "passive," as judged by their spherical appearance and lack of pseudopod activity, area dilations of approximately 30% were accompanied by only a small increase in the membrane tension, indicative of a very small apparent elastic area expansion modulus (approximately 0.04 dyn/cm). Extrapolations back to zero area dilation gave a value for the tension in the resting membrane of 0.024 +/- 0.003 dyn/cm, in close agreement with earlier measures. A few cells showed virtually no change in cortical tension and fit the persistent cortical tension model of Evans and Yeung (1989. Biophys. J. 56:151-160). However, other cells that also appeared "passive," as judged by their spherical appearance, had membrane tensions that increased as the apparent surface area was increased. Thus, the postulated,persistent "cortical tension" does not appear to be a unique and constant parameter for all cells as the membrane area is dilated.This measurement of membrane tension could represent a sensitive indication of the first stages of cell activation and the"passive-active" transition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Evans E., Kukan B. Passive material behavior of granulocytes based on large deformation and recovery after deformation tests. Blood. 1984 Nov;64(5):1028–1035. [PubMed] [Google Scholar]
- Evans E., Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989 Jul;56(1):151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D., Armstrong M., Hatchell D. L., Nunn R. S. Rapid deformation of "passive" polymorphonuclear leukocytes: the effects of pentoxifylline. J Cell Physiol. 1989 Sep;140(3):549–557. doi: 10.1002/jcp.1041400321. [DOI] [PubMed] [Google Scholar]
- Needham D., Hochmuth R. M. Rapid flow of passive neutrophils into a 4 microns pipet and measurement of cytoplasmic viscosity. J Biomech Eng. 1990 Aug;112(3):269–276. doi: 10.1115/1.2891184. [DOI] [PubMed] [Google Scholar]
- Needham D., Nunn R. S. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990 Oct;58(4):997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Sung K. L., Tözeren H., Skalak R., Chien S. Passive mechanical properties of human leukocytes. Biophys J. 1981 Oct;36(1):243–256. doi: 10.1016/S0006-3495(81)84726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheterline P., Rickard J. E., Boothroyd B., Richards R. C. Phorbol ester induces rapid actin assembly in neutrophil leucocytes independently of changes in [Ca2+]i and pHi. J Muscle Res Cell Motil. 1986 Oct;7(5):405–412. doi: 10.1007/BF01753583. [DOI] [PubMed] [Google Scholar]
- Sheterline P., Rickard J. E., Richards R. C. Involvement of the cortical actin filament network of neutrophil leucocytes during phagocytosis. Biochem Soc Trans. 1984 Dec;12(6):983–987. doi: 10.1042/bst0120983. [DOI] [PubMed] [Google Scholar]
- Sung K. L., Dong C., Schmid-Schönbein G. W., Chien S., Skalak R. Leukocyte relaxation properties. Biophys J. 1988 Aug;54(2):331–336. doi: 10.1016/S0006-3495(88)82963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Son-Tay R., Needham D., Yeung A., Hochmuth R. M. Time-dependent recovery of passive neutrophils after large deformation. Biophys J. 1991 Oct;60(4):856–866. doi: 10.1016/S0006-3495(91)82119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosbeck K., Tobias P., Mueller H., Allen R. A., Arfors K. E., Ulevitch R. J., Sklar L. A. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J Leukoc Biol. 1990 Feb;47(2):97–104. doi: 10.1002/jlb.47.2.97. [DOI] [PubMed] [Google Scholar]
- Wallace P. J., Wersto R. P., Packman C. H., Lichtman M. A. Chemotactic peptide-induced changes in neutrophil actin conformation. J Cell Biol. 1984 Sep;99(3):1060–1065. doi: 10.1083/jcb.99.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]