Abstract
Src kinase regulation of N-methyl-d-aspartate (NMDA) subtype glutamate receptors in the central nervous system (CNS) has been found to play an important role in processes related to learning and memory, ethanol sensitivity and epilepsy. However, little is known regarding the mechanisms underlying the regulation of Src family kinase activity in the control of NMDA receptors. Here we report that the distal phosphatase domain (D2) of protein tyrosine phosphatase α (PTPα) binds to the PDZ2 domain of post-synaptic density 95 (PSD95). Thus, Src kinase, its activator (PTPα) and substrate (NMDA receptors) are linked by the same scaffold protein, PSD95. Removal of PTPα does not affect the association of Src with NMDA receptors, but turns off the constitutive regulation of NMDA receptors by the kinase. Further more, we found that application of the PTPα catalytic domains (D1 + D2) into neurones enhances NMDA receptor-mediated synaptic responses. Conversely, the blockade of endogenous PTPα inhibits NMDA receptor activity and the induction of long-term potentiation in hippocampal neurones. Thus, PTPα is a novel up-regulator of synaptic strength in the CNS.
Keywords: ligand-gated ion channels/LTP/PSD95/PTPα/Src
Introduction
The reversible phosphorylation of proteins on serine, threonine and tyrosine residues represents a primary post-translational mechanism underlying the regulation of cell functions, including DNA replication, energy metabolism, cell growth and signalling (Thomas and Brugge, 1997). An abundance of data has demonstrated that Src family protein tyrosine kinases (PTKs) may play a central role in the regulation of N-methyl-d-aspartate (NMDA) receptors by multiple signalling pathways (Yu and Salter, 1998; Lu et al., 1999; Huang et al., 2001; Manzerra et al., 2001; Vissel et al., 2001). Recent studies have documented that Src family PTKs may potentiate NMDA receptor functions via both the enhancement of the channel gating and an increase in the number of NMDA receptors on the cell surface (Yu et al., 1997; Grosshans et al., 2001). However, it remains unknown how the activity of Src family PTKs is controlled in the regulation of NMDA receptors.
The Src kinase family is comprised of a total of nine members, at least five of which—Src, Fyn, Lyn, Lck and Yes—are known to be expressed in the central nervous system (CNS) (Thomas and Brugge, 1997; Ali and Salter, 2001). All members of the Src family contain highly homologous regions, including an inhibitory C-terminal sequence, as well as catalytic, SH2 and SH3 domains (Brown and Cooper, 1996). The activity of these PTKs is tightly regulated by the reversible phosphorylation of a C-terminal tyrosine residue (Tyr527 in chicken c-Src) (Cooper et al., 1986; Nada et al., 1991; Brown and Cooper, 1996). Although phosphatases have long been proposed to be responsible for the dephosphorylation of phosphorylated Tyr527, and consequently increase Src family PTK activity (Brown and Cooper, 1996), it has been demonstrated recently via inactivation of the protein tyrosine phosphatase α (PTPα) gene by homologous recombination (Ponniah et al., 1999; Su et al., 1999) that PTPα may act as an endogenous activator of Src family PTKs by dephosphorylating phosphorylated Tyr527 (Zheng et al., 1992; den Hertog et al., 1993).
PTPα belongs to the family of receptor-like PTPs (Neel and Tonks, 1997; den Hertog, 1999), which at present includes seven members discovered in mammalian cells (den Hertog, 1999). Most receptor-like PTPs contain two phosphatase domains, a single transmembrane region and a variable extracellular N-terminus (Neel and Tonks, 1997; den Hertog, 1999). PTPα is highly expressed in the CNS (Sap et al., 1990; Van Vactor, 1998), but the function of this enzyme in the CNS is still poorly understood. To clarify functions of phosphatases in the CNS, we examined the role of PTPα in the regulation of NMDA receptor activity.
Results
PTPα complexes with NMDA receptors by directly binding to the PDZ2 domain of PSD95
In order to determine whether PTPα is a critical factor in the regulation of NMDA receptors, we first set out to determine whether PTPα is an integral component of the NMDA receptor-associated signalling complex in the CNS. We performed co-immunoprecipitation experiments using antibodies recognizing either the NMDA receptor NR1 subunit (which is present in all functional NMDA receptors; Dingledine et al., 1999; Cull-Candy et al., 2001), PTPα or Src from membrane protein extracts of adult rat brain solubilized under non-denaturing conditions. We found that antibodies recognizing either the NMDA receptor NR1 subunit, PTPα or Src could co-precipitate the other two remaining proteins (Figure 1A–C). In contrast, none of these proteins were detected in immunoprecipitates using non-specific IgG (Figure 1A–C). These data indicate that PTPα, Src and the NMDA receptor may associate in the CNS.
Fig. 1. PTPα, Src and the NMDA receptor form a complex in the CNS. The lane immediately left of (A) was loaded with solubilized membrane protein (20 µg, SM) which was prepared from rat brain tissues and used for immunoprecipitation shown in (A–C). (A) Immuno precipitates with non-specific IgG (mouse) and an antibody against NMDA NR1 subunit (mouse IgG) were loaded in the lanes (left to right) of the gel. Approximately 88 ± 4% (mean ± SE, n = 4) of the NR1 subunit protein was precipitated from SM with the anti-NR1 antibody. (B) Immunoprecipitates with non-specific IgG (mouse) and an antibody against Src (mouse IgG) were loaded in the lanes (left to right) of the gel. (C) Immunoprecipitates with non-specific IgG (rabbit) and an antibody against PTPα (rabbit IgG) (Harder et al., 1998) were loaded in the lanes (left to right) of the gel. The filters shown in (A–C) were stripped sequentially and immunoblotted with the antibodies against PTPα, NR1 and Src as indicated next to the arrows. (D) Immunoprecipitates from three consecutive immunoprecipitations with anti-PSD95 antibody (mouse IgG) were loaded in the lanes (left to right) of the gel. (E) Immunodepletion of PSD95 significantly reduced the amount of PTPα co-precipitated with NMDA receptors. The left blots in (E) show the effect of PSD95 immunodepletion on PTPα association with NMDA receptors. NR1 immunoprecipitates from brain lysates without PSD95 immunodepletion were loaded in the left lane of the gel. The middle lane was loaded with NR1 immunoprecipitates from the supernatant after immunoprecipitation was performed twice with PSD95 antibodies. PSD95 immunoprecipitates were loaded in the right lane. The right blots in (E) show the effect of immunoprecipitation with non-specific IgG (mouse) on PTPα association with NMDA receptors. NR1 immunoprecipitates from brain lysates without IgG immunoprecipitation were loaded in the left lane of the gel. The middle lane of the gel was loaded with NR1 immunoprecipitates from the supernatant after two consecutive immunoprecipitations with non- specific IgG. IgG immunoprecipitates were loaded in the right lane. The filters were stripped sequentially and immunoblotted with antibodies against proteins as indicated next to the arrows. (F) The bar graph shows the mean ratios (±SE, four experiments) of band intensity of co-precipitated PTPα versus that of NR1 subunit protein detected in NR1 immunoprecipitates. P < 0.05 (t-test). All experiments shown in this figure were repeated >3 times. Numbers to the left of the blots indicate the position and size (kDa) of molecular mass markers. IP, immunoprecipitation.
Recently, mechanisms underlying the association of Src family PTKs, Src and Fyn, with NMDA receptors have been investigated in depth by other groups (Tezuka et al., 1999; Hajdur et al., 2001). It is found that Src and Fyn may bind directly to the PDZ domain-containing protein, post-synaptic density 95 (PSD95), and thereby associate with NMDA receptors (Tezuka et al., 1999; Hajdur et al., 2001). In order to get an indication as to how the Src family PTK activator PTPα associates with NMDA receptors, we examined whether PSD95 may also be involved in the association of PTPα with NMDA receptors. For this purpose, we first investigated whether the association of PTPα with NMDA receptors was affected by a PSD95 depletion. PSD95 immunoprecipitation was conducted twice to deplete PSD95 from the rat brain lysates, after which very little (<3%) PSD95 was found remaining in the supernatant (Figure 1D). The left blots of Figure 1E show an example of PSD95 depletion experiments. PSD95 antibodies co-precipitated both the NMDA NR1 subunit and PTPα (the right lane of the left blots). After PSD95 immunoprecipitation, the supernatant was subjected to immunoprecipitation with anti-NR1 antibodies. The resultant precipitates were loaded in the middle lane of the gel. Compared with the amount of PTPα co-precipitated with NR1 antibodies from rat brain lysates without the initial PSD95 immunodepletion (the left lane of the left blots in Figure 1E), there was much less PTPα detected in NR1 immunoprecipitates after the PSD95 depletion (the middle lane of the left blots in Figure 1E). As a control, immunoprecipitation performed twice with non-specific IgG produced no significant change in PTPα association with NMDA receptors (the right blots of Figure 1E). The fact that the ratio of band intensity of co-precipitated PTPα versus that of NR1 subunit protein detected in NR1 immunoprecipitates was significantly reduced by PSD95 depletion (Figure 1F) strongly suggests that PTPα may associate mainly with those NMDA receptors that are also associated with PSD95.
It is known that the PDZ1 and PDZ2 domains of PSD95 may bind to the C-terminus of NMDA NR2 subunits (Kornau et al., 1995; Sheng and Sala, 2001). To identify whether PSD95 may act as a linker to recruit PTPα into the NMDA receptor complex, we examined whether PSD95 expression is sufficient to enable PTPα–NMDA receptor association in non-neuronal cells that normally express neither NMDA receptors nor PSD95. HEK293 and COS7 cells were transfected with cDNAs encoding NMDA NR1-1a, NR2A subunits (which are found in most NMDA receptors of adult animals; Dingledine et al., 1999; Cull-Candy et al., 2001) and/or PSD95. Co-immunoprecipitation experiments were performed on cell lysates using an antibody against PTPα. We found that expressed PSD95 was co-precipitated with PTPα from the lysate of cells lacking NMDA receptor subunit co-expression (Figure 2A). In contrast, PTPα antibodies could not precipitate overexpressed NMDA receptor subunit proteins without PSD95 co-expression (Figure 2A). Moreover, without NMDA NR2A subunit co-expression, PTPα failed to be co-precipitated with NMDA NR1-1a subunit, even if PSD95 was co-expressed (Figure 2A). Thus, PTPα may associate with a functional NMDA receptor by way of PSD95 association with the NR2A subunit.
Fig. 2. PTPα associates with NMDA receptors through the PDZ domain-containing protein, PSD95. (A) The lanes of the gel (left to right) were loaded with cell lysates (40 µg) and non-specific IgG or PTPα immunoprecipitates obtained from lysates of HEK293 cells transfected with cDNAs encoding NMDA receptor subunits and/or PSD95 as indicated under the blots by – (not transfected) or + (transfected). The filters were stripped sequentially and immunoblotted with antibodies against these proteins as indicated next to the arrows. (B) The lanes (left to right) in the left blot show PSD95 deletion mutants lacking the guanylate kinase (GK) domain (ΔGK), both the SH3 and GK domains [Δ(SH3 + GK)], and the PDZ3 (ΔPDZ3), PDZ2 (ΔPDZ2) and PDZ1 (ΔPDZ1) domains (Tezuka et al., 1999) expressed in HEK293 cells, and detected with an antibody against amino acids 1–54 of PSD95 (anti-PSD951–54). The right blot shows the PSD95 mutants, as indicated with arrows, detected in PTPα immunoprecipitates from these cells. C, cell lysates; IP, immunoprecipitation; IB, immunoblotting.
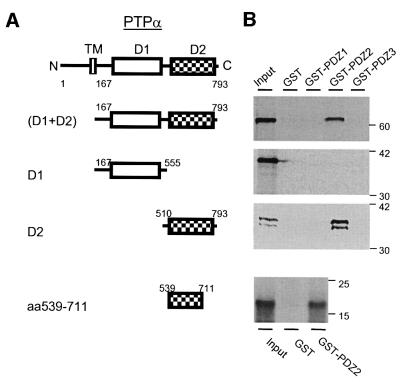
The data above led us to infer that PTPα may bind to PSD95, leading to its association with NMDA receptors. To verify this hypothesis, we directed our investigation towards determining how PTPα interacts with PSD95. cDNAs encoding PSD95 mutants, in which a segment corresponding to either PDZ1, 2, 3 or the SH3 and/or GK domain of PSD95 was deleted (Tezuka et al., 1999), were transfected into HEK293 or COS7 cells. The left blot in Figure 2B shows the expression of these mutants in HEK293 cells by immunoblotting with an antibody against PSD95 residues 1–54 (anti-PSD951–54). We found that all of the expressed PSD95 mutants except for one lacking residues 157–256 (corresponding to the PDZ2 domain of PSD95) could be co-immunoprecipitated by anti-PTPα antibodies (Figure 2B). These data suggest that PTPα association with PSD95 may be due to the binding of PTPα to the PDZ2 domain of PSD95. We substantiated this finding by performing in vitro GST fusion protein precipitation assays. Figure 3A shows the constructs of PTPα peptides used in these assays. Figure 3B shows that 35S-labelled peptides produced by in vitro transcription/translation corresponding to the entire cytoplasmic portion (D1 + D2) and the membrane-distal phosphatase domain including the C-terminal (D2), but not the membrane-proximal phosphatase domain (D1) of PTPα, could be precipitated by glutathione–agarose beads bound to GST fusion proteins containing the PSD95 PDZ2 domain. In contrast, neither GST alone nor the GST fusion proteins containing PSD95 PDZ1 or the PDZ3 domain could precipitate any of these 35S-labelled peptides (Figure 3B). Thus, the PTPα D2 domain appears to be involved in the interaction between PTPα and PSD95.
Fig. 3. The membrane-distal phosphatase domain (D2) of PTPα binds to the PSD95 PDZ2 domain in vitro. (A) Schematics of constructs encoding the entire intracellular portion (D1 + D2), D1 and D2 domains of PTPα, and a segment (amino acids 539–711) in the D2 domain. (B) Autoradiograph showing 35S-labelled PTPα peptides (Input) as indicated in (A), and PTPα peptides precipitated by GST fusion proteins conjugated with the PDZ1 (GST–PDZ1), PDZ2 (GST–PDZ2) or PDZ3 (GST–PDZ3) domain of PSD95. Numbers to the right of the blots indicate the position and size (kDa) of molecular mass markers.
Since there is no typical E(S/T)XV motif at the C-terminus of PTPα available for PSD95 PDZ2 domain binding, and recent studies have documented that the PDZ domain of PSD95 may also bind to the internal peptides of proteins lacking the typical E(S/T)XV terminal motif (Hillier et al., 1999; Tezuka et al., 1999; Sheng and Sala, 2001), we sought to identify further the sequence which may underlie the binding between the PTPα D2 and PSD95 PDZ2 domains. By using a D2 domain that was truncated from its N- and/or C-terminus, we found that a GST fusion protein containing the amino acids 539–711 of PTPα could still bind to the PDZ2 domain of PSD95 in vitro (Figure 3B). Thus, we conclude that PTPα binds to PSD95 via the D2–PDZ2 domain interaction, and thereby complexes with NMDA receptors.
In non-neuronal cells, it has been found that Src (Harder et al., 1998; Zheng et al., 2000) and Fyn (Bhandari et al., 1998) may complex with PTPα. To identify the role of PTPα in the regulation of NMDA receptor activity, we then investigated whether PTPα expression changes Src expression or the physical association of this kinase with NMDA receptors. cDNAs encoding NMDA receptor subunits and wild-type PSD95 were transfected into PTPα-deficient (PTPα–/–; Su et al., 1999) fibroblasts. Figure 4A shows the results of co-immunoprecipitation experiments using antibodies against NR2A/B subunits on lysates of these cells with or without the re-introduction of PTPα by transfection of PTPα cDNA into PTPα–/– fibroblasts. Compared with that observed in the fibroblasts without the re-introduction of PTPα, it was found that PTPα expression did not alter the ability of PSD95 or Src to associate with co-expressed NMDA receptors (Figure 4A). Western blot analysis using antibodies against Src (clone327), or C-terminally tyrosine-phosphorylated Src (Src-pTyr529), showed no significant difference in the total amount of Src in cells with PTPα expression versus those cells lacking PTPα, while a marked reduction in anti-Src-pTyr529 immunoreactivity, indicative of increased Src activity (Zheng et al., 1992; den Hertog et al., 1993; Ponniah et al., 1999; Su et al., 1999; Zheng et al., 2000), was found in the cells into which PTPα was re-introduced (Figure 4B and C).
Fig. 4. Removal of PTPα does not affect the association of Src with NMDA receptors, but reduces Src activity. (A) The left blots show immunoprecipitates obtained using an anti-NR2A/B antibody (rabbit IgG) on the lysates of PTPα–/– fibroblasts co-transfected with cDNAs encoding PSD95 and NR2A (left lane), or PTPα, PSD95 and NR2A (right lane). The symbols beneath the blots show transfection status as –, not transfected; +, transfected. The blots on the right show the immunoprecipitates obtained using non-specific IgG (rabbit). The filters were probed sequentially with antibodies against NR2A (rabbit IgG), PSD95 (mouse IgG) and Src (mouse IgG) as indicated. (B) Western blot analysis of cell lysates used for the immunoprecipitation shown in (A) was conducted with the indicated antibodies (arrows). (C) The bar graph shows the mean ratios (± SE, six experiments) of the band intensity of Src (open bar, detected with the antibody clone327) and C-terminally tyrosine-phosphorylated Src (filled bar, detected with the antibody anti-Src-pTyr529) in cells with PTPα expression (labelled as PTPα+) versus those in cells without PTPα expression (labelled as PTPα–). The dashed line indicates the level of protein detected in cells without PTPα expression. IP: immunoprecipitation. *P < 0.05 (Wilcoxon test).
PTPα activity is necessary for initiating and maintaining the regulation of recombinant NMDA receptors by endogenous Src family PTKs in fibroblasts
To determine the role of PTPα in the regulation of NMDA receptor functions, we recorded whole-cell currents mediated by the NMDA NR1-1a/NR2A receptor expressed in PTPα–/– fibroblasts with or without the re-introduction of the phosphatase (Figure 5). In all of the patch clamp recording experiments conducted in fibroblasts, cDNA encoding wild-type PSD95 was co-transfected. Whole-cell currents were evoked with l-aspartate or NMDA (250 µM) applied through a double-barrel pipette system. The averaged peak and steady-state amplitudes of whole-cell currents recorded in PTPα–/– cells were 476 ± 69 and 404 ± 52 pA, respectively (n = 28, mean ± SEM). The decay of whole-cell currents during the agonist application was fitted using two exponential components with time constants 185 ± 40 ms (τfast) and 1217 ± 147 ms (τslow), respectively. Compared with the currents recorded in PTPα–/– cells, the re-introduction of PTPα into PTPα–/– cells significantly increased the amplitude of currents mediated by the recombinant NMDA receptors (peak, 839 ± 148 pA; steady state, 663 ± 113 pA; n = 38; also see Figure 5A), but did not significantly change the decay time (τfast, 129 ± 14 ms; τslow, 1094 ± 146 ms). To clarify whether the effects of the re-introduction of PTPα into PTPα–/– cells on recombinant NMDA receptors resulted from the direct modulation of NMDA receptors by PTPα, we transfected cDNAs encoding NMDA NR1-1a, NR2A subunits and wild-type PSD95 into fibroblasts lacking PTKs Src, Fyn and Yes (SYF cells; Klinghoffer et al., 1999) with or without PTPα cDNA co-transfection, and recorded currents evoked by l-aspartate or NMDA. The amplitudes of the peak and steady-state whole-cell currents mediated by NMDA receptors expressed in SYF cells with (n = 39) and without (n = 20) overexpression of PTPα were 674 ± 66 and 458 ± 55 pA, and 758 ± 95 and 425 ± 83 pA, respectively. No statistically significant difference could be found. Thus, it is implied that the enhancement of NMDA receptor-mediated responses in cells in which PTPα was re-introduced may be a result of PTPα-dependent activation of Src family PTKs.
Fig. 5. Deletion of the PTPα gene abolishes the Src kinase regulation of recombinant NMDA receptors. (A) The peak amplitudes (mean ± SE) of whole-cell responses mediated by recombinant NMDA NR1-1a/NR2A receptors expressed in PTPα–/– fibroblasts with (filled bar, +) and without (open bar, –) re-introduction of PTPα. #, P < 0.05, Mann–Whitney test. (B) An example of whole-cell currents and current–voltage (I/V) relationships mediated by recombinant NMDA NR1-1a/NR2A receptors in PTPα-expressing cells (labelled as PTPα+) before and during the application of PP2 (10 µM, Calbiochem, San Diego, CA). (C) A summary of PP2 or PP3 effects on recombinant NMDA receptors expressed in PTPα–/– cells with PTPα re-introduced. (D) The effects of PP2 on recombinant NMDA receptors expressed in SYF cells or SYF cells co-transfected with cDNA encoding pp60c-Src (labelled as SYF + c-Src). (E) A summary of PP2 effects on the NMDA receptors expressed in PTPα–/– cells without re-introduction of PTPα (labelled as PTPα–) or with re-introducion of catalytically inactive PTPα [labelled as PTPα (C433A)] (Harder et al., 1998). In (C), (D) and (E), the filled and open bars, respectively, indicate the peak and steady-state amplitudes (mean ± SE) normalized to their control responses before application of drugs as indicated. Dashed lines in (C), (D) and (E) indicate the control level of l-aspartate-evoked whole-cell currents before the application of drugs as indicated. Values in parentheses indicate the number of cells tested. *P < 0.05; **P < 0.01 (Wilcoxon test).
Then, we examined the effects of the Src family PTK inhibitor, PP2 (0.5–10 µM), on NMDA NR1-1a/NR2A receptors co-expressed in cells in which PTPα was re-introduced. An example presenting the effect of PP2 applied to cells with the re-introduction of PTPα is shown in Figure 5B. We found that PP2 application significantly reduced NMDA NR1-1a/NR2A receptor-mediated whole-cell currents (Figure 5B and C) without changing the reversal potential of currents recorded (Figure 5B). These data indicate that the PP2-induced reduction of current amplitudes recorded in cells in which PTPα was re-introduced results from a decrease in NMDA receptor-mediated whole-cell conductance. The PP2 effect was concentration dependent (Figure 5C), while PP3 (an inactive PP2 isomer) had no effect (Figure 5C). To confirm that the PP2 effects detected in these experiments were produced by the inhibition of Src family PTKs, we examined the effects of PP2 on NMDA NR1-1a/NR2A receptors expressed in SYF cells. We found that PP2 application had no effect on the recombinant NMDA receptors expressed in SYF cells unless Src was re-introduced (Figure 5D). This indicates that the recombinant NMDA NR1-1a/NR2A receptors in cells expressing PTPα may be constitutively regulated by endogenous Src, Fyn and/or Yes.
Surprisingly, PP2 (10 µM) application did not produce any inhibition of recombinant NMDA receptors expressed in PTPα–/– cells (Figure 5E). To determine whether the lack of PP2-induced inhibition was related to the absence of PTPα phosphatase activity, we investigated the effects of PP2 on NMDA receptors expressed in PTPα–/– cells co-transfected with cDNA encoding catalytically inactive PTPα (PTPα with a cysteine to alanine mutation at residue 433 in the D1 phosphatase domain) (Harder et al., 1998). PP2 had no effect on NMDA receptors expressed in these cells (Figure 5E), suggesting that PTPα activity may be necessary for initiating and maintaining the NMDA receptor regulation by Src family PTKs.
To confirm the role of PTPα activity in the regulation of NMDA receptors by endogenous PTKs, we examined the effects of another PTK inhibitor, lavendustin A (10 µM) (Wang and Salter, 1994; Hemmings, 1997). We found that similarly to PP2, lavendustin A application did not induce any significant reduction of recombinant NMDA receptor-mediated currents recorded from cells without PTPα expression or expressing catalytically inactive PTPα (the peak and steady-state amplitudes during lavendustin A application were 100 ± 3 and 98 ± 4% of control, n = 8, or 92 ± 6 and 92 ± 4% of control, n = 7). In contrast, lavendustin A significantly reduced NMDA receptor-mediated responses (the peak and steady-state amplitudes were 72 ± 4 and 73 ± 5% of control, n = 7, P < 0.05, Wilcoxon test) without changing the reversal potential of currents in cells in which PTPα was re-introduced (data not shown). Lavendustin B, an inactive isomer of lavendustin A, had no such effects (the peak and steady-state amplitudes during lavendustin B application were 97 ± 4 and 87 ± 3% of control, n = 6). These data provide strong support for the concept that PTPα may govern the activity of endogenous PTKs in the regulation of NMDA receptors.
Blockade of the PTPα catalytic (D1) domain inhibits NMDA receptor activity and turns off the regulation of NMDA receptors by Src family PTKs in hippocampal neurones
PTPα, like the majority of receptor-like PTPs, contains two cytoplasmic phosphatase domains, with the D1 domain displaying most of the enzyme’s catalytic activity (Lim et al., 1998; Blanchetot and den Hertog, 2000). We found that application of a monoclonal antibody (clone21, 2.5 µg/ml, mouse IgM; Transduction Lab, Lexington, KY), raised against the D1 domain of PTPα, into fibroblasts expressing PTPα significantly inhibited the activity of NMDA receptors expressed in these cells (Figure 6A–C). In contrast, application of the antibody into PTPα–/– fibroblasts, or application of non-specific mouse IgM (an isotype control of the antibody, clone21) into PTPα-expressing fibroblasts had no effect on NMDA receptor activity (Figure 6C). Thus, these data demonstrate that the effect of the anti-PTPα D1 antibody on NMDA receptors in PTPα-expressing cells is produced by specific blockade of PTPα.
Fig. 6. The blockade of the PTPα D1 domain inhibits NMDA receptor-mediated whole-cell currents and abolishes the effect of PP2 on NMDA receptors in hippocampal neurones. (A) An example of NMDA NR1-1a/NR2A receptor-mediated whole-cell currents recorded from a fibroblast with re-introduced PTPα using a recording pipette filled with intracellular solution containing the antibody, clone21 (2.5 µg/ml). (B) An example showing the current–voltage relationships (I/V) recorded at 1 and 25 min after breakthrough from one fibroblast into which PTPα was re-introduced. (C) The amplitudes [normalized to the first trace (It/I1) after breakthrough, mean ± SE] of NMDA receptor-mediated currents recorded in the fibroblasts. Clone21/PTPα-, clone21 was applied into PTPα–/– fibroblasts (open triangles); Clone21/PTPα+, clone21 was applied into PTPα–/– cells re-introduced with PTPα (filled circles); IgM, non-specific mouse IgM was applied onto the cells with re-introduced PTPα (open circles). (D and E) An example of NMDA receptor-mediated whole-cell currents recorded from hippocampal neurones with applied clone21. (F) An example showing the current– voltage relationships (I/V) recorded at 1 and 20 min after breakthrough from one hippocampal neurone. (G) An example of NMDA receptor-mediated whole-cell currents recorded from hippocampal neurones with applied non-specific mouse IgM. (H) Normalized amplitudes (mean ± SE) of NMDA receptor-mediated currents recorded in hippocampal neurones. (I) Averaged traces of NMDA receptor-mediated whole-cell currents recorded from one hippocampal neurone before (Control) and during application of PP2 (10 µM). (J) Pre-loading clone21 into neurones abolished the effect of PP2, while the PP2 effect was not changed by pre-loading neurones with non-specific IgM. The dashed line indicates the control level of peak responses mediated by NMDA receptors before PP2 or PP3 application. Values in parentheses indicate the number of cells tested.*P < 0.05; **P < 0.01 (paired t-test or Wilcoxon test).
In order to identify the function of PTPα in central neurones, we investigated the effects of clone21 on NMDA receptor activity in cultured hippocampal neurones. We found that application of clone21 (2.5 µg/ml) into neurones through recording pipettes reduced NMDA receptor-mediated whole-cell currents (Figure 6D and E). The average current amplitude recorded at 20 min after breakthrough was reduced to 52 ± 5% of the first response (Figure 6H) with no change in the reversal potential (Figure 6F). Application of an equal concentration of non-specific mouse IgM into neurones did not reduce NMDA receptor-mediated currents (Figure 6G and H). Furthermore, we found that the blockade of the D1 phosphatase domain of PTPα by pre-loading clone21 into neurones abolished the effect of PP2 on NMDA receptor activity (Figure 6J), while PP2 significantly inhibited NMDA currents in neurones intracellularly pre-loaded with non-specific IgM (Figure 6J). PP2 application inhibited NMDA receptor-mediated whole-cell responses recorded from hippocampal neurones without clone21 pre-application (Figure 6I and J), while application of PP3 produced no effect (Figure 6J). These findings further suggest that endogenous PTPα may be an up-regulator of NMDA receptors and govern the regulation of NMDA receptors by Src family PTKs in central neurones.
PTPα is a novel up-regulator of synaptic NMDA receptors and plays an important role in the induction of synaptic long-term potentiation (LTP) in CA1 hippocampal neurones
In light of the findings that PTPα may bind directly to PSD95 (Figures 2 and 3), and that the blockade of the main catalytic domain of PTPα may down-regulate NMDA receptor-mediated channel activity in neurones (Figure 6), it would be logical to expect that PTPα may be very important in the regulation of excitatory synaptic transmission, namely the up- or down-regulation of PTPα activity may enhance or reduce synaptic responses mediated by glutamate. Previous studies have demonstrated that the cytoplasmic portion, (D1 + D2), of PTPα represents the functional domain of the enzyme that regulates the phosphatase activity, protein targeting and interaction (Zheng et al., 1992, 2000; den Hertog et al., 1993; Neel and Tonks, 1997; Harder et al., 1998; den Hertog, 1999). To obtain direct evidence showing the function of PTPα in central neurones, we examined the effects of application of GST fusion protein containing (D1 + D2) into neurones on glutamate-mediated synaptic responses.
We recorded miniature excitatory post-synaptic currents (mEPSCs) in cultured hippocampal neurones. Consistent with previous findings (Bekkers and Stevens, 1989; Yu et al., 1997; Yu and Salter, 1998), most of the recorded mEPSCs in hippocampal neurones consisted of two components: one with a fast decay, which could be blocked by bath application of the non-NMDA receptor antagonist DNQX (5 µM; data not shown), the other one with a slow decay, which could be blocked by bath application of the NMDA receptor antagonist APV (50 µM; Figure 7A). GST–(D1 + D2) was applied into neurones through recording electrodes. We found that the application of (D1 + D2) into hippocampal neurones may potentiate the NMDA, but not non-NMDA (Figure 7A and C), receptor-mediated mEPSC component in a concentration-dependent manner (Figure 7B). No significant changes in glutamate-mediated mEPSCs could be noted when 0.05 µM (D1 + D2) was applied into neurones (Figure 7B). With increases in the concentration of the applied protein, the NMDA receptor-mediated mEPSC component was enhanced (Figure 7B). At 15 min after application of 1.8 µM (D1 + D2) into neurones, the NMDA receptor-mediated mEPSC component increased to 148 ± 5% of initial values (Figure 7A and B), while no significant changes in the non-NMDA receptor-mediated mEPSC component could be found (Figure 7A and C). Application of GST alone (1.8 µM) into neurones did not induce any significant increase in mEPSCs (Figure 7B).
Fig. 7. The role of PTPα in the regulation of glutamate-mediated post-synaptic responses. (A) An example of averaged mEPSCs recorded when GST fusion protein containing the entire intracellular portion of PTPα [(D1 + D2), 1.8 µM] was applied into neurones. (B) Normalized charges (mean ± SE) of NMDA components recorded in neurones with GST applied alone (1.8 µM, n = 7), or with (D1 + D2) in concentrations of 0.05 (n = 6), 0.18 (n = 6) or 1.8 µM (n = 7). (C) The effects of PTPα domains [(D1 + D2) (1.8 µM) or D1 (1.8 µM)] on NMDA (filled bars) and non-NMDA (open bars) receptor-mediated mEPSC components at 10–15 min after breakthrough. (D1 + D2), application of (D1 + D2) into neurones; (D1 + D2)/PP2, (D1 + D2) was applied into neurones treated with PP2 (10 µM); (D1 + D2)/no ATP, (D1 + D2) was applied into neurones recorded with pipettes filled with intracellular solution containing no ATP; D1, application of D1 only into neurones. (D) An example of averaged mEPSCs recorded in cultured hippocampal neurones with clone21 applied. (E) Normalized charges of NMDA components recorded from neurones with application of clone21 (2.5 µg/ml, filled circles, n = 7), non-specific mouse IgM (2.5 µg/ml, open circles, n = 8) or both clone21 (2.5 µg/ml) and (D1 + D2) (0.05 µM, open squares, n = 9) through recording pipettes. (F) Normalized charges of non-NMDA receptor components recorded in these neurones during the period of 10–15 min after breakthrough. Clone21/(D1 + D2), both clone21 and (D1 + D2) were applied into neurones through recording pipettes. (G) Averaged current traces representing three consecutive EPSCs before and 20 min after tetanic stimulation from one experiment in which IgM and clone21 were applied, respectively, into two adjacent neurones as indicated. (H) EPSPs evoked by tetanic stimulation (100 Hz for 500 ms, bar) recorded in the current clamp mode from one experiment in which IgM and clone21 were applied into two adjacent neurones as indicated. (I) Plots of averaged EPSC amplitudes (mean ± SEM) for each minute plotted from neurones pre-loaded with non-specific IgM (open circles, n = 8) or with antibody clone21 (closed circles, n = 8). The arrow indicates the application of tetanic stimulation. (J) Averaged current traces representing three consecutive EPSCs at 1 and 30 min after breakthrough recorded from one experiment in which GST only and GST–(D1 + D2) were applied, respectively, into two adjacent neurones as indicated. (K) Plots of averaged EPSC amplitudes for each minute plotted from neurones pre-loaded with GST–(D1 + D2) (closed circles, n = 7) or with GST alone (open circles, n = 7). The EPSC amplitudes were normalized to the amplitude of EPSCs during the first minute (=100%, control) after breakthrough. Values in parentheses indicate the number of cells tested *P < 0.05; **P < 0.01 (Wilcoxon test in C and E, t-test in I and K).
To determine mechanisms underlying the effects of (D1 + D2), we investigated whether the up-regulation of NMDA receptors induced by the application of (D1 + D2) was produced through Src family PTK activity. Consistent with our previous finding that NMDA, but not non-NMDA, receptor-mediated mEPSCs are altered by activation or inhibition of endogenous Src family PTKs (Yu et al., 1997), we found that bath application of the Src family PTK inhibitor, PP2 (10 µM), did not significantly change non-NMDA receptor-mediated mEPSCs (83 ± 13% of control, n = 3), but reduced the NMDA receptor-mediated mEPSC component to 64 ± 4% of control (n = 3). Importantly, application of (D1 + D2) into neurones treated with PP2 could not produce any significant increase in NMDA receptor-mediated synaptic responses (Figure 7C). Moreover, we also found that the effect of (D1 + D2) could be prevented by removal of ATP from the intracellular solution (Figure 7C). Thus, application of PTPα (D1 + D2) protein into neurones may potentiate NMDA receptor activity via the activation of Src family PTKs. Furthermore, we found that application of GST fusion protein containing only the D1 domain did not produce the same effect as that induced by (D1 + D2) (Figure 7C). Thus, it is implied that the D2 domain interaction may be necessary for the regulation of NMDA receptors by PTPα.
Conversely, intracellular application of the anti-PTPα D1 antibody, clone21 (2.5 µg/ml), through recording pipettes significantly reduced the NMDA receptor-mediated component of mEPSCs (Figure 7D and E). At 15 min after the antibody application, the NMDA mEPSC component was reduced to 64 ± 3% of that recorded at 2 min after breakthrough (Figure 7D and E), while no significant change could be found in the non-NMDA receptor-mediated mEPSC component (Figure 7F). The effect of the antibody on NMDA mEPSCs could be blocked by co-application of 0.05 µM (D1 + D2) (Figure 7E), while the application of 0.05 µM (D1 + D2) alone into neurones did not significantly alter NMDA receptor-mediated synaptic responses (Figure 7B). As a control, non-specific mouse IgM applied into neurones had no effect (Figure 7E).
The role of endogenous PTPα in the regulation of synaptic plasticity was also investigated. Tetanic stimulation-induced long-lasting potentiation of EPSCs of CA1 pyramidal neurones in hippocampal slices of adult rats was recorded. The EPSCs were evoked by stimulating the Schaffer collateral pathway. The effects of antibody clone21 and non-specific IgM applied into post-synaptic neurones were examined in two adjacent neurones recorded simultaneously with the double whole-cell patch recording configuration. One neurone was patched with a recording pipette filled with intracellular solution supplemented with antibody clone21 (2.5 µg/ml), and the other was supplemented with an equal amount of non-specific IgM. No difference in the basal EPSCs recorded from neurones applied with clone21 and non-specific IgM was found until 20 min after breakthrough (Figure 7I). The tetanic stimulation delivered at 20 min after breakthrough produced an enhancement of EPSCs in neurones with intracellular application of non-specific IgM that was similar to that observed in neurones without the IgM application (intracellular solution only, data not shown); the peak amplitude of EPSCs at 30 min after tetanic stimulation was 163 ± 10.2% (n = 8) of baseline (Figure 7G and I). Pre-application of antibody clone21 (2.5 µg/ml) into post-synaptic neurones significantly reduced the amplitude of the long-lasting increase in EPSCs (Figure 7G and I), but did not significantly affect the membrane depolarization during tetanic stimulation (Figure 7H) or the short-term potentiation of EPSCs immediately after the tetanic stimulation (Figure 7I) when compared with those recorded from neurones applied with non-specific IgM. Conversely, intracellular application of GST fusion protein containing (D1 + D2) (1.8 µM) through recording pipettes significantly increased the EPSC amplitude (Figure 7J and K). At 30 min, the EPSC amplitudes were increased to 147 ± 8% (n =7) of that recorded at the first minute after breakthrough (Figure 7J and K). In contrast, application of GST alone (1.8 µM) did not have such an effect (Figure 7J and K). Thus, we have demonstrated that PTPα may be an important factor involved in the induction of activity-dependent enhancement of synaptic function in the CNS.
Discussion
Previous studies have demonstrated that NMDA receptors are associated with endogenous Src family PTKs (Yu et al., 1997; Tezuka et al., 1999), which regulate NMDA receptor functions (Yu et al., 1997; Lu et al., 1999; Huang et al., 2001; Vissel et al., 2001). In more detailed mechanistic studies, it has been found that Src may form a complex with NMDA receptors via the binding of its SH2 domain to a region N-terminal to the PSD95 PDZ1 domain (Hajdur et al., 2001), while Fyn participates via the binding of its SH2 domain to the PSD95 PDZ3 domain (Tezuka et al., 1999). Both PSD95 PDZ1 and PDZ2 domains may bind to the ESDV motif at the C-terminus of NMDA receptor NR2 subunits (Kornau et al., 1995; Sheng and Sala, 2001). Our present study demonstrated that the Src kinase activator, PTPα, may also bind to PSD95. Recently, it was documented that the PDZ domain of PSD95 can bind to the internal peptides of proteins with a β-finger structure, but lacking the typical E(S/T)XV terminal motif (Hillier et al., 1999; Sheng and Sala, 2001). Since there is thus far no crystallized PTPα D2 domain available to aid in determining the structure of the PTPα D2 domain, whether such a β-finger structure exists in the PTPα D2 domain is unknown. Nonetheless, our study revealed that: (i) the amount of PTPα co-immunoprecipitated with NMDA receptors after the depletion of PSD95 from rat brain lysates was significantly reduced; (ii) in heterologous cells, PTPα may co-precipitate all of the expressed PSD95 mutants except for one lacking the PDZ2 domain; and (iii) the peptide corresponding to the entire cytoplasmic portion (D1 + D2), the D2 phosphatase domain or the internal part (amino acids 539–711) of the PTPα D2 domain, but not the D1 phosphatase domain of PTPα, could be precipitated by a GST fusion protein containing the PSD95 PDZ2 domain in vitro. According to these findings, we conclude that PTPα may bind to PSD95 through a direct interaction between the internal peptide amino acids 539–711 of the PTPα D2 domain and the PSD95 PDZ2 domain. By combining our present findings with those reported by other groups demonstrating the interactions of Src family kinases and PSD95 (Kornau et al., 1995; Tezuka et al., 1999; Sheng and Sala, 2001), we propose a model showing that Src family PTKs, their activator (PTPα) and substrate (NMDA receptors) are closely linked together by the scaffold protein, PSD95 (Figure 8).
Fig. 8. A schematic of the NMDA receptor-associated PTK–PTP signalling complex. NMDAR, NMDA receptor; N, N-terminus; C, C-terminus; Uniq, unique domain; Extr., extracellular; Intr., intracellular.
In this model, the D2 domain of PTPα plays a key role in the interaction of the phosphatase with the synaptic scaffold protein, PSD95. The D2 domain in most receptor-like PTPs is found to be involved in protein–protein interactions (Bilwes et al., 1996; Felberg and Johnson, 1998; Majeti et al., 1998; Wallace et al., 1998; Jiang et al., 1999). Through the D2 domain binding, receptor-like PTPs may form homo- or heterodimers, and thereby regulate the enzyme activity (Bilwes et al., 1996; Felberg and Johnson, 1998; Majeti et al., 1998; Wallace et al., 1998; Jiang et al., 1999). Furthermore, a recent study has demonstrated that the D2 domain interaction may regulate the clustering and location of receptor-like PTPs, such as LAR (Serra-Pages et al., 1998). We found that application of the D1 domain only did not produce the same effect as that induced by (D1 + D2) (Figure 7C). Thus, it has been implied that the D2 domain interaction may be necessary for the regulation of synaptic functions by receptor-like PTPs.
Previous studies, which discovered that NMDA receptor activity may be reduced by application of PTK inhibitors (Wang and Salter, 1994; Wang et al., 1996; Yu et al., 1997), indicate that NMDA receptors may be constitutively regulated by endogenous Src family kinases. However, until now, it has remained unclear whether certain structures are required for this regulation. The ‘kinase–kinase activator–kinase substrate complex’ shown in Figure 8 may represent such a structure required for the initiation and maintenance of the constitutive regulation of NMDA receptors by endogenous Src family PTKs.
Our present study shows that knockout of PTPα activity abolished the effects of PTK inhibitors PP2 and lavendustin A on NMDA receptors. This finding strongly suggests that the constitutive regulation of NMDA receptors by PTKs, particularly Src family PTKs, may depend upon the activity of PTPα. So far, there is no evidence to support the possibility that PTPα may modulate NMDA receptor activity directly. Therefore, PTPα may in reality act as an endogenous driver for initiation and maintenance of the constitutive regulation of NMDA receptors by endogenous Src family PTKs. This concept was confirmed further by the observation that blockade of the main catalytic domain of PTPα occludes the effect of PP2 on NMDA receptors in cultured hippocampal neurones.
The regulation of NMDA receptor activity by protein kinases and phosphatases has been studied extensively (for reviews see Dingledine et al., 1999; Kennedy, 2000; Ali and Salter, 2001; Greengard, 2001). According to these studies, protein kinases and phosphatases appear to act in opposition in the regulation of NMDA receptor activity, i.e. kinases up-regulate, while phosphatases down-regulate NMDA receptor activity. However, our present study revealed that: (i) PTPα-deficient cells exhibited down-regulated whole-cell responses mediated by recombinant NMDA receptors expressed in fibroblasts; (ii) application of PTPα protein into hippocampal neurones significantly increased synaptic NMDA receptor activity; and (iii) blockade of the main catalytic domain of endogenous PTPα inhibited NMDA receptor activity and LTP induction. Thus, we have demonstrated the first direct evidence indicating that a phosphatase may also up-regulate ligand-gated ion channel functions and excitatory synaptic transmission in the CNS.
LTP is believed to be an important cellular mechanism related to learning and memory processes. In studies of mechanisms of LTP induction, serine/threonine phosphatases have been found to be actively involved in the suppression of the induction and/or maintenance of LTP (for reviews see Kennedy, 2000; Winder and Sweatt, 2001). Functions of PTPs in the regulation of LTP induction have also been investigated recently (Uetani et al., 2000; Pelkey et al., 2002). Uetani et al. (2000) found an enhanced LTP in CA1 and CA3 neurones in PTPδ-deficient animals, suggesting that PTPδ may be a tonic down-regulator of LTP induction. Pelkey et al. (2002) reported that STEP, a cytoplasmic PTP, is a down-regulator of NMDA receptors and acts as a tonic brake on LTP induction. In contrast to all of the findings mentioned above, we found that the blockade of endogenous PTPα inhibited LTP induction in CA1 neurones, and that application of the PTPα functional domain (D1 + D2) into neurones induced an enhancement of synaptic responses. These data suggest that PTPα may be a critical factor in the signalling pathway(s) responsible for LTP induction. To date, PTPα is the first phosphatase found to be actively involved in the induction of LTP. Thus, further characterization of mechanisms underlying the function of PTPα in LTP induction will be essential for the understanding of activity-dependent neuroplasticity in the CNS.
Materials and methods
Immunoprecipitation and western blotting
Brain tissue from adult rats was homogenized in ice-cold homogenizing buffer (320 mM sucrose, 10 mM Tris–HCl pH 7.4, 1 mM NaHCO3 pH 7.4, 1 mM MgCl2) supplemented with 1 mM sodium orthovanadate and 1% protease inhibitor cocktail after two washes with ice-cold phosphate-buffered saline (PBS), and subsequently spun at 5000 g for 15 min. The supernatant was collected and spun at 40 000 g (Beckman ultracentrifuge, Palo Alto, CA) for 60 min. Pellets were then dissolved in a lysis buffer (10 mM Tris–HCl pH 9, 150 mM NaCl, 0.5% Triton X-100, 1% sodium deoxycholate, 0.5% SDS, 2 mM EDTA, 1 mM sodium orthovanadate and 1% protease inhibitor cocktail) for subsequent experiments. For collection of cultured cells, attached cells were mechanically dissociated from the dish after two washes with ice-cold PBS, and then spun at 4000 g for 5 min. Cell pellets in the lysis buffer were kept on ice for 1 h, and then sonicated and centrifuged at 15 000 g for 20 min. The supernatant was collected for subsequent experiments. In immunoprecipitation experiments, solubilized proteins (1 mg) were incubated with antibodies (see below) at 4°C and gently shaken overnight. Antibodies used for immunoprecipitation were: anti-NR1 (1 µg, mouse IgG; PharMingen, San Diego, CA), anti-NR2A/B (0.5 µg, rabbit IgG; Chemicon, Temecula, CA), anti-PTPα (1 µg, rabbit IgG; F.Jirik, University of British Columbia; Harder et al., 1998), clone21 (1.3 µg, mouse IgM; Transduction labs, Lexington, KY), clone327 (0.5 µg, mouse IgG; Oncogene, Cambridge, MA) and anti-PSD95 (4 µl, mouse IgG; ABR, Golden, CO). The immune complexes were collected with 10 µl of protein G–Sepharose beads for 2 h at 4°C. Immunoprecipitates were then washed four times with ice-cold PBS, resuspended in 2× Laemmli sample buffer and boiled for 5 min. These samples were subjected to SDS–PAGE and transferred to a nitrocellulose membrane. The blotting analysis was performed by repeated stripping and successive probing with antibodies: anti-NR1, anti-NR2A/B, anti-PTPα, clone327, anti-Src-pTyr529 (rabbit IgG; Biosource, Camarillo, CA), anti-PSD95 and anti-PSD951–54 (rabbit IgG; T.Yamamoto, Tokyo University). All chemicals used were purchased from Sigma, except where indicated.
Cell culture and transfection
For biochemical experiments, HEK293 and COS7 cells, and PTPα–/– fibroblasts (Su et al., 1999) (a gift of Dr J.Sap, New York University) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco-BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (Gibco-BRL). These cells were transfected with expression vectors (pcDNA3 or pBCMGneo) containing cDNAs encoding NR1-1a (8 µg), NR2A (32 µg), PTPα (1 µg), and wild-type (1 µg, a gift of Dr D.Bredt) or mutant (1 µg; a gift of Dr T.Tezuka and T.Yamamoto) (Tezuka et al., 1999) PSD95 in which amino acids 532–724 [corresponding to the guanylate kinase domain (GK), ΔGK], amino acids 411–724 [including both the GK and SH3 domains, Δ(GK + SH3)], amino acids 255–433 (corresponding to the PDZ3 domain, ΔPDZ3), amino acids 157–256 (corresponding to PDZ2, ΔPDZ2) and amino acids 54–158 (corresponding to PDZ1, ΔPDZ1) of PSD95 were deleted. The calcium phosphate method (Gibco-BRL, Gaithersburg, MD) was used for transfection following the protocol recommended by the manufacturer. Cells transfected with both NR1-1a and NR2A cDNAs were cultured in medium supplemented with the NMDA receptor antagonist APV (500 µM) for 48–72 h before lysis.
For electrophysiological recordings, cDNAs encoding the NMDA receptor subunits NR1-1a (0.3 µg) and NR2A (1.2 µg), PSD95 (0.15 µg), green fluorescent protein (GFP; 0.15 µg), wild-type PTPα (0.15 µg), the catalytically inactive mutant of PTPα [PTPα with a cysteine to alanine mutation at residue 433 (Harder et al., 1998), 0.15 µg], pp60c-Src (0.3 µg) or the corresponding empty vectors were transfected into PTPα–/– or SYF fibroblasts (Klinghoffer et al., 1999) (ATCC, Manassas, VA) cultured in 35 mm culture dishes using the lipofectamine method (Gibco-BRL). After 5–12 h, transfected cells were maintained in DMEM supplemented with 10% fetal bovine serum and APV (500 µM) for 48 h before recording.
Neurones used for electrophysiological recordings were prepared from cultures of fetal hippocampal tissues of Wistar rats (E18) as previously described (Yu et al., 1997). Hippocampal tissue was then mechanically dissociated by trituration and plated onto 35 mm, collagen-coated culture dishes at a density of <1 × 106 cells/ml. Hippocampal neurones were used for electrophysiological recordings 12–17 days after plating.
In vitro GST pull-down assay
DNA sequences encoding the entire cytoplasmic portion of PTPα (D1 + D2, amino acids 167–793), the membrane-proximal phosphatase domain (D1, amino acids 167–555), the membrane-distal phosphatase domain (D2, amino acids 510–793) and amino acids 539–711 of PTPα (Harder et al., 1998) were synthesized using PCR. Plasmid pBCMGSneo containing the full-length cDNA of PTPα (a gift from Dr Jirik; Harder et al., 1998) was used as the template.
Primer sequences made for the PCR for D1 + D2, D1, D2 and amino acids 539–711 of PTPα were 5′-TTTAAGCTCAGGCCACCTGTGAGGC-3′ and 5′-TTTGATATCTTACTTGAAGTTGGCATAATC-3′; 5′-TTTAAGCTCAGGCCACCTGTGAGGC-3′ and 5′-TTTGATATCTTAGGTCCCTGGGATTTTGTTG-3′; 5′-TTTAAGCTTGACAAGATGCGGACTGG-3′ and 5′-TTTGATATCTTACTTGAAGTTGGCATAATC-3′; and 5′-TTTAAGCTTGACAAGATGCGGACTGG-3′ and 5′-TTTGATATCTTACTGCTGCTTCTGCACGGC-3′, respectively. All the PTPα fragments were confirmed by DNA sequencing and constructed in the HindIII–EcoRV sites in vector pcDNA3 (Invitrogen, Carlsbad, CA) for in vitro transcription and translation.
pGEX2T- PSD95 PDZ1 (amino acids 55–156), PDZ2 (amino acids 158–256) and PDZ3 (amino acids 257–432) plasmids were gifts from Dr T.Yamamoto (Tezuka et al., 1999). The PSD95–GST fusion proteins were produced in Escherichia coli (BL-21 strain; Invitrogen) by isopropyl-β-d-thiogalactopyranoside (IPTG; 0.1 µM) induction and purified with glutathione–Sepharose beads. The bead-bound fusion proteins (30 µg) conjugated with the PDZ1, 2 and 3 domains of PSD95 were each mixed with 2 µl of in vitro synthesized [35S]methionine-labelled PTPα peptides D1 + D2, D1, D2 or amino acids 539–711 (TNT-coupled reticulocyte lysate systems; Promega, Madison, WI) in binding buffer (250 mM NaCl, 50 mM HEPES pH 7.9, 0.5 mM EDTA, 0.1% NP-40, 1 mM dithiothreitol) for 10 min at 4°C. The beads were then washed four times with binding buffer, and bound proteins eluted with elution buffer (20 mM Tris–HCl pH 7.9, 100 mM KCl, 20 mM glutathione) before separation by SDS–PAGE and detection by autoradiography.
Whole-cell recordings in cultured cells
For whole-cell recordings in hippocampal neurones, the cultures were bathed in a standard extracellular solution containing 140 mM NaCl, 5.4 mM KCl, 33 mM glucose, 1.3 mM CaCl2, 25 mM HEPES, 0.001 mM tetrodotoxin (TTX) and 0.003 mM glycine pH 7.35; osmolarity 310–320 mOsm. The standard extracellular solution containing 0.03 mM glycine was used for recordings in fibroblasts. Recording pipettes were made from thin-walled borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) pulled to a diameter of 1–2 µm at the tip with a resistance of 4–7 MΩ, and filled with a standard intracellular solution composed of 140 mM CsCl, 1 mM BAPTA, 10 mM HEPES, 2 mM MgCl2 and 4 mM K-ATP pH 7.25; osmolarity 300–310 mOsm. All the agents used for testing PTPα or Src family PTKs were freshly prepared from high concentration stock solutions and diluted (1:200 or 1:1000) with the standard intra- or extracellular solution for recording experiments.
Whole-cell currents were evoked by application of NMDA receptor agonists l-aspartate or NMDA (250 µM). The standard and NMDA receptor agonist-containing extracellular solutions were applied to cells via a double-barrel perfusion pipette made from two square glass capillary tubes attached to a stepper motor (SF-77B perfusion fast-step system; Warner Instrument, Hamden, CT) which was controlled by a computer. The time constant of solution exchange was ∼5 ms. Recordings were conducted under the voltage clamp condition at a holding potential of –60 mV except where indicated. Whole-cell currents were recorded using Axopatch 200B amplifiers (Axon Instruments, Foster City, CA). Data were filtered at 2 kHz and digitized on-line using Digidata 1200 DAC units (Axon Instruments). On-line data acquisition and off-line analysis were performed using pClamp8 software (Axon Instruments). Current–voltage (I/V) relationships were calculated by the subtraction of currents during the voltage ramp from –60 to +60 mV without l-aspartate or NMDA application from currents during the analogous ramp over the steady state of l-aspartate- or NMDA-evoked responses.
For mEPSC recordings in hippocampal neurones, the cultures were bathed in an extracellular solution containing 100 mM Na2SO4, 9 mM Cs2SO4, 33 mM glucose, 1.3 mM CaCl2, 25 mM HEPES, 0.001 mM TTX, 0.003 mM glycine, 0.01 mM bicuculline and 0.01 mM strychnine pH 7.35; osmolarity 310–320 mOsm. Recording pipettes were filled with the standard intracellular solution (see above). mEPSCs were detected semi-automatically off-line and analysed after alignment of the rising edge using Synaptic Toolbench software (Yu et al., 1997; Yu and Salter, 1998). The threshold for detection was approximately –4 pA and was optimized for each cell so as to collect >95% of mEPSCs. Traces with noise artefacts or with more than one mEPSC per 200 ms recording period were not included in the analysis. Detected mEPSCs were averaged during the periods following breakthrough: 0–2 min, 2–5 min, 5–10 min and 10–15 min. Averages were required to contain at least 25 traces. For each neurone tested, charges (Q) mediated by non-NMDA and NMDA receptors were calculated respectively by integrating currents mediated by these receptors, and normalized to that measured during the initial 2 min period (Q2).
Whole-cell recordings in brain slices
Hippocampal slices (300 µm) from 28- to 30-day-old Sprague–Dawley rats were prepared as previously described (Lu et al., 1998). In brief, the slice in the recording chamber was superfused continuously with artificial cerebrospinal fluid (ACSF, 2 ml/min) saturated with 95% O2/5% CO2 at 30 ± 1°C. The ACSF contained 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgCl2, 2 mM CaCl2, 26 mM NaHCO3, 10 mM dextrose and 0.01 mM bicuculline. CA1 pyramidal neurones were visualized with infrared illumination and differential interference contrast. Two adjacent neurones (separated by a distance of <20 µm) were selected for double whole-cell patch clamp recordings. Recording electrodes (3–5 MΩ) were filled with intracellular solution containing 142.5 mM Cs-gluconate, 7.5 mM CsCl, 10 mM HEPES, 0.2 mM EGTA, 2 mM Mg-ATP, 0.3 mM GTP, and 5 mM QX-314 pH 7.4; osmolarity 290–300 mOsm. For double-patch clamp recordings, one neurone was patched with an electrode filled with the intracellular solution and additionally with the antibody clone21 or GST–(D1 + D2), and the other electrode with intracellular solution and additionally with an equal amount of non-specific mouse IgM or GST alone. EPSCs were recorded at a holding potential of –70 mV with Axopatch 200B amplifiers. Currents were filtered at 2 kHz with a low-pass filter, and data were digitized at 10 kHz and stored on-line using the pclamp8 system (Axon Instruments). The input and series resistance were monitored using voltage steps (1 mV, 200 ms) at 5 min intervals throughout the duration of the experiment. Series resistance ranged from 11 to 16 MΩ, and input resistance was 266–288 MΩ. For each neurone, if the input resistance changed >20% relative to a 10 min period prior to tetanic stimulation, the neurone was rejected from the statistical analysis. The resting membrane potential of recorded neurones was –64 to –68 mV. Neither the antibody nor GST fusion protein application affected resting membrane potential or input resistance. The Schaffer collateral pathway, ∼50 µm from CA1 pyramidal cell bodies, was stimulated with a bipolar tungsten electrode that delivered test stimuli at a frequency of 0.1 Hz. The tetanic stimulation consisted of two 100 Hz stimuli of 1 s duration with an inter-train interval of 10 s in current clamp mode. The averaged amplitude of EPSCs for the 10 min period before tetanic stimulation was defined as baseline (i.e. 100%).
Acknowledgments
Acknowledgements
We thank Drs M.W.Salter, Y.-T.Wang, H.Van Tol and L.-Y.Wang for critical comments on the manuscript. We are also grateful to Dr J.Sap for the gifts of PTPα–/– cells and anti-PTPα antibody, as well as critical comments on the manuscript, Drs T.Yamamoto and T.Tezuka for the gifts of the PSD95 constructs and anti-PSD951–54 antibody, as well as critical comments on the manuscript, Dr F.R.Jirik for the gifts of the antibodies and plasmids for PTPα, and for comments on the manuscript, and Dr D.Bredt for the gift of PSD95 plasmid. This work was supported by grants from Canadian Institutes of Health Research (CIHR) and Ontario Neurotrauma Foundation to X.-M.Y., who is a New Investigator of CIHR.
References
- Ali D.W. and Salter,M.W. (2001) NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr. Opin. Neurobiol., 11, 336–342. [DOI] [PubMed] [Google Scholar]
- Bekkers J.M. and Stevens,C.F. (1989) NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature, 341, 230–233. [DOI] [PubMed] [Google Scholar]
- Bhandari V., Lim,K.L. and Pallen,C.J. (1998) Physical and functional interactions between receptor-like protein-tyrosine phosphatase α and p59fyn. J. Biol. Chem., 273, 8691–8698. [DOI] [PubMed] [Google Scholar]
- Bilwes A.M., den Hertog,J., Hunter,T. and Noel,J.P. (1996) Structural basis for inhibition of receptor protein-tyrosine phosphatase-α by dimerization. Nature, 382, 555–559. [DOI] [PubMed] [Google Scholar]
- Blanchetot C. and den Hertog,J. (2000) Multiple interactions between receptor protein-tyrosine phosphatase (RPTP) α and membrane-distal protein-tyrosine phosphatase domains of various RPTPs. J. Biol. Chem., 275, 12446–12452. [DOI] [PubMed] [Google Scholar]
- Brown M.T. and Cooper,J.A. (1996) Regulation, substrates and functions of src. Biochim. Biophys. Acta, 1287, 121–149. [DOI] [PubMed] [Google Scholar]
- Cooper J.A., Gould,K.L., Cartwright,C.A. and Hunter,T. (1986) Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science, 231, 1431–1434. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S., Brickley,S. and Farrant,M. (2001) NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol., 11, 327–335. [DOI] [PubMed] [Google Scholar]
- den Hertog J. (1999) Protein-tyrosine phosphatases in development. Mech. Dev., 85, 3–14. [DOI] [PubMed] [Google Scholar]
- den Hertog J., Pals,C.E., Peppelenbosch,M.P., Tertoolen,L.G., de Laat, S.W. and Kruijer,W. (1993) Receptor protein tyrosine phosphatase α activates pp60c-src and is involved in neuronal differentiation. EMBO J., 12, 3789–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R., Borges,K., Bowie,D. and Traynelis,S.F. (1999) The glutamate receptor ion channels. Pharmacol. Rev., 51, 7–61. [PubMed] [Google Scholar]
- Felberg J. and Johnson,P. (1998) Characterization of recombinant CD45 cytoplasmic domain proteins. Evidence for intramolecular and intermolecular interactions. J. Biol. Chem., 273, 17839–17845. [DOI] [PubMed] [Google Scholar]
- Greengard P. (2001) The neurobiology of slow synaptic transmission. Science, 294, 1024–1030. [DOI] [PubMed] [Google Scholar]
- Grosshans D.R., Clayton,D.A., Coultrap,S.J. and Browning,M.D. (2001) LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nature Neurosci., 5, 27–33. [DOI] [PubMed] [Google Scholar]
- Hajdur L.V., Pelkey,K.A., Huang,Y.Q. and Salter,M.W. (2001) Phosphotyrosine-independent interaction between SH2 domain of tyrosine kinase Src and PSD-95. Soc. Neurosci. Abstr., 27, 352.6 (Abstract). [Google Scholar]
- Harder K.W., Moller,N.P., Peacock,J.W. and Jirik,F.R. (1998) Protein-tyrosine phosphatase α regulates Src family kinases and alters cell–substratum adhesion. J. Biol. Chem., 273, 31890–31900. [DOI] [PubMed] [Google Scholar]
- Hemmings H.C.J. (1997) Protein kinase and phosphatase inhibitors. In Hemmings,H.C.J. (ed.), Regulatory Protein Modification: Technique and Protocols. Humana Press, Totowa, NJ, pp. 121–218.
- Hillier B.J., Christopherson,K.S., Prehoda,K.E., Bredt,D.S. and Lim,W.A. (1999) Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS–syntrophin complex. Science, 284, 812–815. [PubMed] [Google Scholar]
- Huang Y.Q. et al. (2001) CAKβ/Pyk2 kinase is a signalling link for induction of long-term potentiation in CA1 hippocampus. Neuron, 29, 485–496. [DOI] [PubMed] [Google Scholar]
- Jiang G., den Hertog,J., Su,J., Noel,J., Sap,J. and Hunter,T. (1999) Dimerization inhibits the activity of receptor-like protein-tyrosine phosphatase-α. Nature, 401, 606–610. [DOI] [PubMed] [Google Scholar]
- Kennedy M.B. (2000) Signal-processing machines at the postsynaptic density. Science, 290, 750–754. [DOI] [PubMed] [Google Scholar]
- Klinghoffer R.A., Sachsenmaier,C., Cooper,J.A. and Soriano,P. (1999) Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J., 18, 2459–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau H.C., Schenker,L.T., Kennedy,M.B. and Seeburg,P.H. (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science, 269, 1737–1740. [DOI] [PubMed] [Google Scholar]
- Lim K.L., Kolatkar,P.R., Ng,K.P., Ng,C.H. and Pallen,C.J. (1998) Interconversion of the kinetic identities of the tandem catalytic domains of receptor-like protein-tyrosine phosphatase PTPα by two point mutations is synergistic and substrate-dependent. J. Biol. Chem., 273, 28986–28993. [DOI] [PubMed] [Google Scholar]
- Lu W.Y., Xiong,Z.G., Lei,S., Orser,B.A., Dudek,E., Browning,M.D. and MacDonald,J.F. (1999) G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nature Neurosci., 2, 331–338. [DOI] [PubMed] [Google Scholar]
- Lu Y.M., Roder,J.C., Davidow,J. and Salter,M.W. (1998) Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science, 279, 1363–1367. [DOI] [PubMed] [Google Scholar]
- Majeti R., Bilwes,A.M., Noel,J.P., Hunter,T. and Weiss,A. (1998) Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science, 279, 88–91. [DOI] [PubMed] [Google Scholar]
- Manzerra P., Behrens,M.M., Canzoniero,L.M., Wang,X.Q., Heidinger,V., Ichinose,T., Yu,S.P. and Choi,D.W. (2001) Zinc induces a Src family kinase-mediated up-regulation of NMDA receptor activity and excitotoxicity. Proc. Natl Acad. Sci. USA, 98, 11055–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S., Okada,M., MacAuley,A., Cooper,J.A. and Nakagawa,H. (1991) Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature, 351, 69–72. [DOI] [PubMed] [Google Scholar]
- Neel B.G. and Tonks,N.K. (1997) Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol., 9, 193–204. [DOI] [PubMed] [Google Scholar]
- Pelkey K.A., Askalan,R., Paul,S., Kalia,L.V., Nguyen,T.H., Pitcher,G.M., Salter,M.W. and Lombroso,P.J. (2002) Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron, 34, 127–138. [DOI] [PubMed] [Google Scholar]
- Ponniah S., Wang,D.Z., Lim,K.L. and Pallen,C.J. (1999) Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol., 9, 535–538. [DOI] [PubMed] [Google Scholar]
- Sap J., D’Eustachio,P., Givol,D. and Schlessinger,J. (1990) Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc. Natl Acad. Sci. USA, 87, 6112–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Pages C., Medley,Q.G., Tang,M., Hart,A. and Streuli,M. (1998) Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins. J. Biol. Chem., 273, 15611–15620. [DOI] [PubMed] [Google Scholar]
- Sheng M. and Sala,C. (2001) Pdz domains and the organization of supramolecular complexes. Annu. Rev. Neurosci., 24, 1–29. [DOI] [PubMed] [Google Scholar]
- Su J., Muranjan,M. and Sap,J. (1999) Receptor protein tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol., 9, 505–511. [DOI] [PubMed] [Google Scholar]
- Tezuka T., Umemori,H., Akiyama,T., Nakanishi,S. and Yamamoto,T. (1999) PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-d-aspartate receptor subunit NR2A. Proc. Natl Acad. Sci. USA, 96, 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.M. and Brugge,J.S. (1997) Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol., 13, 513–609. [DOI] [PubMed] [Google Scholar]
- Uetani N., Kato,K., Ogura,H., Mizuno,K., Kawano,K., Mikoshiba,K., Yakura,H., Asano,M. and Iwakura,Y. (2000) Impaired learning with enhanced hippocampal long-term potentiation in PTPδ-deficient mice. EMBO J., 19, 2775–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vactor D. (1998) Protein tyrosine phosphatases in the developing nervous system. Curr. Opin. Cell Biol., 10, 174–181. [DOI] [PubMed] [Google Scholar]
- Vissel B., Krupp,J.J., Heinemann,S.F. and Westbrook,G.L. (2001) A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nature Neurosci., 4, 587–596. [DOI] [PubMed] [Google Scholar]
- Wallace M.J., Fladd,C., Batt,J. and Rotin,D. (1998) The second catalytic domain of protein tyrosine phosphatase δ (PTPδ) binds to and inhibits the first catalytic domain of PTPσ. Mol. Cell. Biol., 18, 2608–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.T. and Salter,M.W. (1994) Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature, 369, 233–235. [DOI] [PubMed] [Google Scholar]
- Wang Y.T., Yu,X.-M. and Salter,M.W. (1996) Ca(2+)-independent reduction of N-methyl-d-aspartate channel activity by protein tyrosine phosphatase. Proc. Natl Acad. Sci. USA, 93, 1721–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder D.G. and Sweatt,J.D. (2001) Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nature Rev. Neurosci., 2, 461–474. [DOI] [PubMed] [Google Scholar]
- Yu X.-M. and Salter,M.W. (1998) Gain control of NMDA-receptor currents by intracellular sodium. Nature, 396, 469–474. [DOI] [PubMed] [Google Scholar]
- Yu X.-M., Askalan,R., Keil,G.J.I. and Salter,M.W. (1997) NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science, 275, 674–678. [DOI] [PubMed] [Google Scholar]
- Zheng X.M., Wang,Y. and Pallen,C.J. (1992) Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature, 359, 336–339. [DOI] [PubMed] [Google Scholar]
- Zheng X.M., Resnick,R.J. and Shalloway,D. (2000) A phosphotyrosine displacement mechanism for activation of Src by PTPα. EMBO J., 19, 964–978. [DOI] [PMC free article] [PubMed] [Google Scholar]