Abstract
The normal functional anatomy of the aqueous humour outflow pathways in the domestic pig is poorly documented in the literature despite its being readily available and of a similar size to the human eye. Anterior segment tissue from 12 pig eyes was appropriately fixed and investigated by light microscopy, and scanning and transmission electron microscopy. The configuration of the iridocorneal angle tissues is similar to other nonprimate mammals in several respects, i.e. it possesses a deep ciliary cleft crossed by stout pectinate ligaments and delicate uveal cords, poorly developed ciliary musculature, and an angular aqueous plexus. However, there were some noteworthy features which may make it a suitable model for specific types of glaucoma related research. These features include a shallow scleral sulcus which contains a wedge-shaped mass of corneoscleral tissue comparable in size to the human trabecular meshwork. This tissue was more trabecular than 'reticular' in arrangement, the latter being the more common in nonprimate mammalian species. The relevance of the present findings to the use and limitations of the porcine eye as a model of the human aqueous outflow pathways is discussed.
Full text
PDF


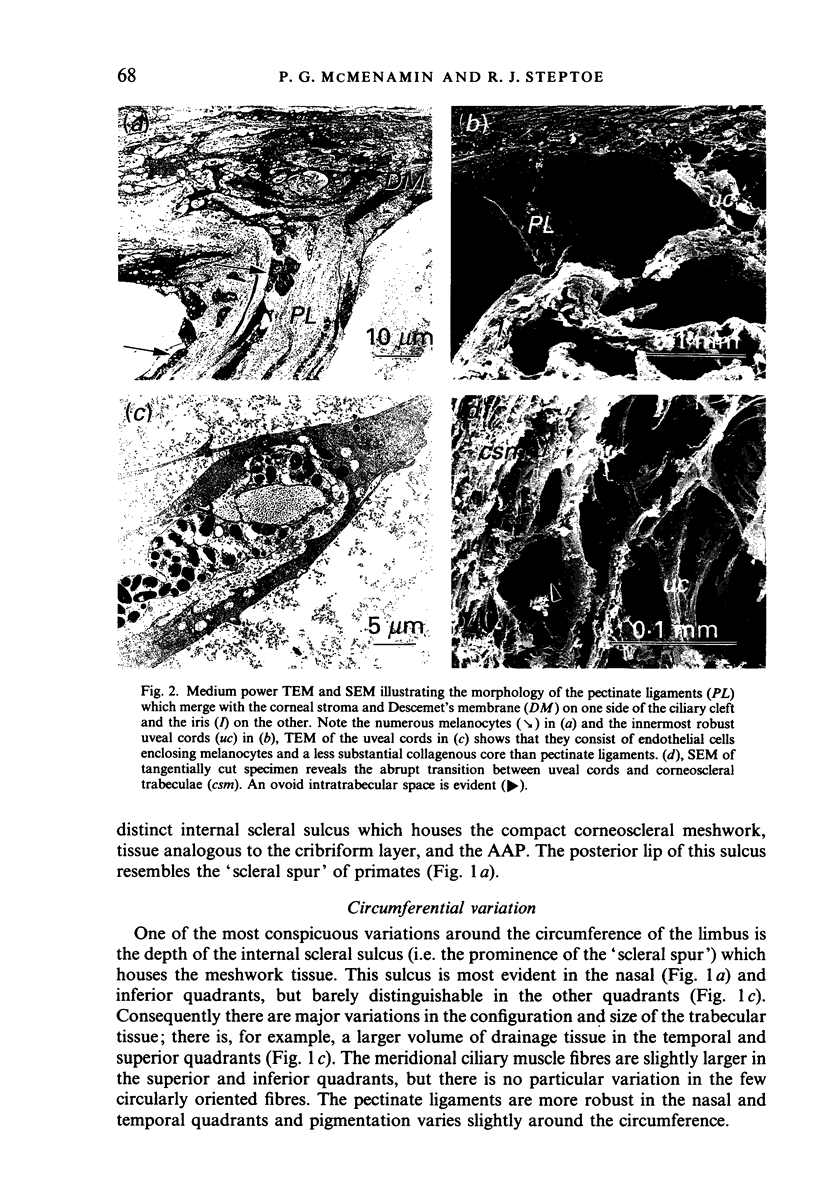
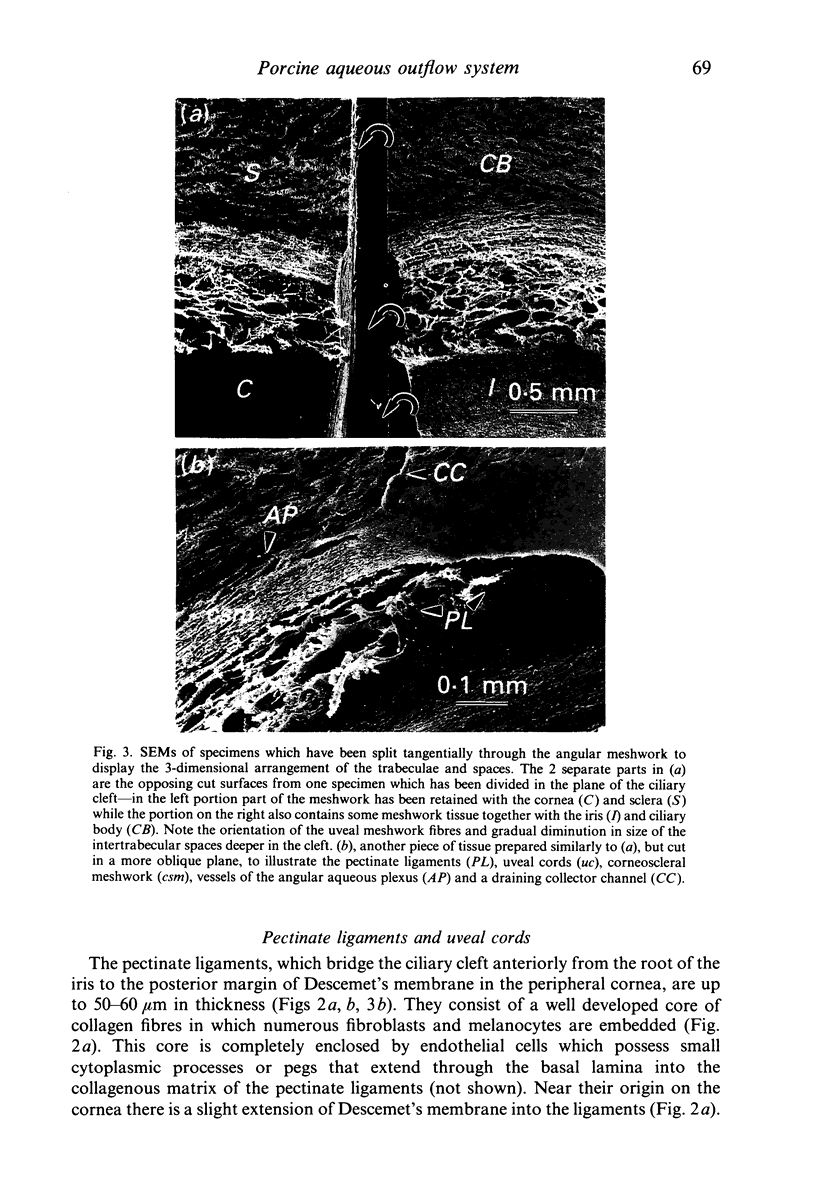






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Wang J., Epstein D. L. Metabolism of calf trabecular (reticular) meshwork. Invest Ophthalmol Vis Sci. 1980 Jan;19(1):13–20. [PubMed] [Google Scholar]
- Bedford P. G., Grierson I. Aqueous drainage in the dog. Res Vet Sci. 1986 Sep;41(2):172–186. [PubMed] [Google Scholar]
- Grierson I., Lee W. R. Changes in the monkey outflow apparatus at graded levels of intraocular pressure: a qualitative analysis by light microscopy and scanning electron microscopy. Exp Eye Res. 1974 Jul;19(1):21–33. doi: 10.1016/0014-4835(74)90068-2. [DOI] [PubMed] [Google Scholar]
- Grierson I., Nagasubramanian S., Edwards J., Millar L. C., Ennis K. The effects of various levels of intraocular pressure on the rabbit's outflow system. Exp Eye Res. 1986 Apr;42(4):383–397. doi: 10.1016/0014-4835(86)90032-1. [DOI] [PubMed] [Google Scholar]
- Hernandez M. R., Weinstein B. I., Wenk E. J., Gordon G. G., Dunn M. W., Southren A. L. The effect of dexamethasone on the in vitro incorporation of precursors of extracellular matrix components in the outflow pathway region of the rabbit eye. Invest Ophthalmol Vis Sci. 1983 Jun;24(6):704–709. [PubMed] [Google Scholar]
- Johnson D. H., Tschumper R. C. Human trabecular meshwork organ culture. A new method. Invest Ophthalmol Vis Sci. 1987 Jun;28(6):945–953. [PubMed] [Google Scholar]
- McMenamin P. G., Lee W. R., Aitken D. A. Age-related changes in the human outflow apparatus. Ophthalmology. 1986 Feb;93(2):194–209. doi: 10.1016/s0161-6420(86)33762-x. [DOI] [PubMed] [Google Scholar]
- McMenamin P. G., Lee W. R. Effects of prolonged intracameral perfusion with mock aqueous humour on the morphology of the primate outflow apparatus. Exp Eye Res. 1986 Jul;43(1):129–141. doi: 10.1016/s0014-4835(86)80051-3. [DOI] [PubMed] [Google Scholar]
- McMenamin P. G., al-Shakarchi M. J. The effect of various levels of intraocular pressure on the rat aqueous outflow system. J Anat. 1989 Feb;162:67–82. [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y., Taniguchi Y. Distributions of 35S-sulfate and 3H-glucosamine in the angular region of the hamster: light and electron microscopic autoradiography. Invest Ophthalmol Vis Sci. 1983 Jun;24(6):697–703. [PubMed] [Google Scholar]
- Remé C., d'Epinay S. L. Periods of development of the normal human chamber angle. Doc Ophthalmol. 1981 Jul 15;51(3):241–268. doi: 10.1007/BF00143888. [DOI] [PubMed] [Google Scholar]
- Richardson T. M., Marks M. S., Ausprunk D. H., Miller M. A morphologic and morphometric analysis of the aqueous outflow system of the developing cat eye. Exp Eye Res. 1985 Jul;41(1):31–51. doi: 10.1016/0014-4835(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Rohen J. W., Kaufman P. L., Eichhorn M., Goeckner P. A., Bito L. Z. Functional morphology of accommodation in the raccoon. Exp Eye Res. 1989 Apr;48(4):523–527. doi: 10.1016/0014-4835(89)90035-3. [DOI] [PubMed] [Google Scholar]
- Rohen J. W., Lütjen-Drecoll E. Age changes of the trabecular meshwork in human and monkey eyes. A light and electron microscopic study. Altern Entwickl Aging Dev. 1971;1:1–36. [PubMed] [Google Scholar]
- Samuelson D. A., Gelatt K. N. Aqueous outflow in the beagle. I. Postnatal morphologic development of the iridocorneal angle: pectinate ligament and uveal trabecular meshwork. Curr Eye Res. 1984 Jun;3(6):783–794. doi: 10.3109/02713688409000790. [DOI] [PubMed] [Google Scholar]
- Samuelson D. A., Gelatt K. N. Aqueous outflow in the beagle. II. Postnatal morphologic development of the iridocorneal angle: corneoscleral trabecular meshwork and angular aqueous plexus. Curr Eye Res. 1984 Jun;3(6):795–807. doi: 10.3109/02713688409000791. [DOI] [PubMed] [Google Scholar]
- Samuelson D., Smith P., Brooks D. Morphologic features of the aqueous humor drainage pathways in horses. Am J Vet Res. 1989 May;50(5):720–727. [PubMed] [Google Scholar]
- Scott J. E., Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965 Oct 1;5(3):221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C. The functional morphology of the outflow systems of ocular and cerebrospinal fluids. Exp Eye Res. 1977;25 (Suppl):65–116. doi: 10.1016/s0014-4835(77)80010-9. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C., Tripathi B. J. The mechanism of aqueous outflow in lower mammals. Exp Eye Res. 1972 Jul;14(1):73–79. doi: 10.1016/0014-4835(72)90146-7. [DOI] [PubMed] [Google Scholar]
- Tripathi R. C. Ultrastructure of the exit pathway of the aqueous in lower mammals. (A preliminary report on the "angular aqueous plexus"). Exp Eye Res. 1971 Nov;12(3):311–314. doi: 10.1016/0014-4835(71)90155-2. [DOI] [PubMed] [Google Scholar]
- Tsukahara S. The existence of smooth muscle adjacent to the Schlemm's canal of the normal albino rat eye. Acta Ophthalmol (Copenh) 1978 Oct;56(5):735–741. doi: 10.1111/j.1755-3768.1978.tb06637.x. [DOI] [PubMed] [Google Scholar]