Abstract
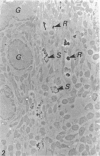
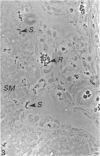
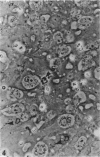
The spatiotemporal distribution of macrophages in the uterine wall of virgin mice and mice in the first half of pregnancy has been studied. Macrophages were identified using a combination of morphological criteria, the capacity to endocytose horseradish peroxidase and the expression of the Mac-1 antigen. In virgin mice and mice at preimplantation stages of pregnancy, macrophages were found throughout the endometrium, myometrium and mesometrial triangle. Following implantation, and in parallel with decidualisation, the density of macrophages appeared to decline in the decidua with advancing gestation. It is suggested that this change in density is due to a dilution of the macrophage population rather than a loss of individual cells. The numbers and distribution of decidual macrophages indicate that this group of cells does not play a major regulatory role in the success of pregnancy.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard O., Scheid M. P., Ripoche M. A., Bennett D. Immunological studies of mouse decidual cells. I. Membrane markers of decidual cells in the days after implantation. J Exp Med. 1978 Aug 1;148(2):580–591. doi: 10.1084/jem.148.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornillie F. J., Lauweryns J. M. Fluid and protein clearance in the rat endometrium. Part II: Ultrastructural evidence for the presence of alternative, non-lymphatic clearance mechanisms in the rat endometrium. Experientia. 1984 Nov 15;40(11):1264–1266. doi: 10.1007/BF01946666. [DOI] [PubMed] [Google Scholar]
- Daki N. M., Stewart I. J., Wild A. E. Receptors for IgG2b on cells of the mouse metrial gland. J Reprod Immunol. 1989 Dec;16(3):249–260. doi: 10.1016/0165-0378(89)90054-5. [DOI] [PubMed] [Google Scholar]
- De M., Wood G. W. Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol. 1990 Sep;126(3):417–424. doi: 10.1677/joe.0.1260417. [DOI] [PubMed] [Google Scholar]
- Elcock J. M., Searle R. F. Antigen-presenting capacity of mouse decidual tissue and placenta. Am J Reprod Immunol Microbiol. 1985 Mar;7(3):99–103. doi: 10.1111/j.1600-0897.1985.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Finn C. A., Martin L. Patterns of cell division in the mouse uterus during early pregnancy. J Endocrinol. 1967 Dec;39(4):593–597. doi: 10.1677/joe.0.0390593. [DOI] [PubMed] [Google Scholar]
- Finn C. A., McLaren A. A study of the early stages of implantation in mice. J Reprod Fertil. 1967 Apr;13(2):259–267. doi: 10.1530/jrf.0.0130259. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Head J. R., Lande I. J. Uterine lymphatics: passage of ink and lymphoid cells from the rat's uterine wall and lumen. Biol Reprod. 1983 May;28(4):941–955. doi: 10.1095/biolreprod28.4.941. [DOI] [PubMed] [Google Scholar]
- Head J. R., Seeling L. L., Jr Lymphatic vessels in the uterine endometrium of virgin rats. J Reprod Immunol. 1984 May;6(3):157–166. doi: 10.1016/0165-0378(84)90021-4. [DOI] [PubMed] [Google Scholar]
- Hunt J. S. Current topic: the role of macrophages in the uterine response to pregnancy. Placenta. 1990 Nov-Dec;11(6):467–475. doi: 10.1016/s0143-4004(05)80192-4. [DOI] [PubMed] [Google Scholar]
- Hunt J. S. Cytokine networks in the uteroplacental unit: macrophages as pivotal regulatory cells. J Reprod Immunol. 1989 Sep;16(1):1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Hunt J. S., Manning L. S., Wood G. W. Macrophages in murine uterus are immunosuppressive. Cell Immunol. 1984 May;85(2):499–510. doi: 10.1016/0008-8749(84)90262-4. [DOI] [PubMed] [Google Scholar]
- Krcek J. P., Dickson A. D., Biddle F. G. Mononuclear cell accumulations in the trophoblastic giant cell circulation in inbred strains of mice. J Anat. 1983 Mar;136(Pt 2):283–292. [PMC free article] [PubMed] [Google Scholar]
- LOBEL B. L., DEANE H. W. Enzymic activity associated with postpartum involution of the uterus and with its regression after hormone withdrawal in the rat. Endocrinology. 1962 Apr;70:567–578. doi: 10.1210/endo-70-4-567. [DOI] [PubMed] [Google Scholar]
- Larkin L. H. Electron microscopy of granule release in metrial gland cells of the pregnant rat. Anat Rec. 1972 Jan;172(1):109–126. doi: 10.1002/ar.1091720110. [DOI] [PubMed] [Google Scholar]
- Matthews C. J., Searle R. F. The role of prostaglandins in the immunosuppressive effects of supernatants from adherent cells of murine decidual tissue. J Reprod Immunol. 1987 Oct;12(2):109–124. doi: 10.1016/0165-0378(87)90039-8. [DOI] [PubMed] [Google Scholar]
- Parr E. L., Young L. H., Parr M. B., Young J. D. Granulated metrial gland cells of pregnant mouse uterus are natural killer-like cells that contain perforin and serine esterases. J Immunol. 1990 Oct 1;145(7):2365–2372. [PubMed] [Google Scholar]
- Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- Peel S., Stewart I. J., Bulmer D. Experimental evidence for the bone marrow origin of granulated metrial gland cells of the mouse uterus. Cell Tissue Res. 1983;233(3):647–656. doi: 10.1007/BF00212232. [DOI] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest. 1989 Jul;61(1):27–36. [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988 Jun 1;140(11):3947–3955. [PubMed] [Google Scholar]
- Scodras J. M., Parhar R. S., Kennedy T. G., Lala P. K. Prostaglandin-mediated inactivation of natural killer cells in the murine decidua. Cell Immunol. 1990 May;127(2):352–367. doi: 10.1016/0008-8749(90)90138-h. [DOI] [PubMed] [Google Scholar]
- Searle R. F., Bell S. C., Billington W. D. Ia antigen-bearing decidual cells and macrophages in cultures of mouse decidual tissue. Placenta. 1983 Apr-Jun;4(2):139–148. doi: 10.1016/s0143-4004(83)80027-7. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stewart I. A morphological study of granulated metrial gland cells and trophoblast cells in the labyrinthine placenta of the mouse. J Anat. 1984 Dec;139(Pt 4):627–638. [PMC free article] [PubMed] [Google Scholar]
- Stewart I., Mukhtar D. D. The morphology of cultured cells from the mouse endometrium and decidua. J Anat. 1988 Feb;156:197–206. [PMC free article] [PubMed] [Google Scholar]
- Stewart I., Peel S. Changes in the cell population of the pregnant rodent uterus in relation to the differentiation of granulated metrial gland cells. J Anat. 1982 Aug;135(Pt 1):111–118. [PMC free article] [PubMed] [Google Scholar]
- Stewart I., Peel S. The structure and differentiation of granulated metrial gland cells of the pregnant mouse uterus. Cell Tissue Res. 1977 Nov 23;184(4):517–527. doi: 10.1007/BF00220975. [DOI] [PubMed] [Google Scholar]
- Tawfik O. W., Hunt J. S., Wood G. W. Partial characterization of uterine cells responsible for suppression of murine maternal anti-fetal immune responses. J Reprod Immunol. 1986 Nov;9(3):213–224. doi: 10.1016/0165-0378(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P. Selective inhibition of nonspecific eosinophil staining or identification of eosinophilic granulocytes by paired counterstaining in immunofluorescence studies. J Histochem Cytochem. 1981 May;29(5):595–400. doi: 10.1177/29.5.6166662. [DOI] [PubMed] [Google Scholar]