Abstract
The bacterial enteropathogen Salmonella typhimurium employs a type III secretion system to inject bacterial toxins into the host cell cytosol. These toxins transiently activate Rho family GTP-binding protein-dependent signaling cascades to induce cytoskeletal rearrangements. One of these translocated Salmonella toxins, SopE, can activate Cdc42 in a Dbl-like fashion despite its lack of sequence similarity to Dbl-like proteins, the Rho-specific eukaryotic guanine nucleotide exchange factors. To elucidate the mechanism of SopE-mediated guanine nucleotide exchange, we have analyzed the structure of the complex between a catalytic fragment of SopE and Cdc42. SopE binds to and locks the switch I and switch II regions of Cdc42 in a conformation that promotes guanine nucleotide release. This conformation is strikingly similar to that of Rac1 in complex with the eukaryotic Dbl-like exchange factor Tiam1. However, the catalytic domain of SopE has an entirely different architecture from that of Tiam1 and interacts with the switch regions via different amino acids. Therefore, SopE represents the first example of a non-Dbl-like protein capable of inducing guanine nucleotide exchange in Rho family proteins.
Keywords: cytoskeleton/Dbl/GEF/GTP binding/Salmonella
Introduction
Ras-related GTP-binding proteins of the Rho family (e.g. RhoA/B, Cdc42 and Rac1/2) are key regulators of vital cellular functions including cytoskeletal rearrangements, cell shape, motility, cytokinesis, gene expression, cell aggregation and cell–cell adhesion (Hall, 1998). They exert their function by cycling between a biologically inactive GDP-bound and an active GTP-bound conformation (Bourne et al., 1990, 1991). As spontaneous guanine nucleotide dissociation is very slow, activation is dependent on guanine nucleotide exchange factors (GEFs), which enhance nucleotide exchange rates by inducing structural changes in the nucleotide-binding site (Vetter and Wittinghofer, 2001). All cellular Rho family-specific GEFs identified so far share a common module, the Dbl homology (DH) domain. This domain forms the catalytically active part of these molecules responsible for binding to the switch I and switch II regions of the Rho component (Cerione and Zheng, 1996; Cherfils and Chardin, 1999). These regions in GTP-binding proteins define major conformational differences between the active and inactive states (Milburn et al., 1990).
The bacterial enteropathogen Salmonella typhimurium is one of the leading causes of gastroenteritis in developed countries. During its evolution as a pathogen, S.typhimurium has acquired a variety of mechanisms to colonize, replicate and survive within its animal hosts (Groisman and Ochman, 1996). Essential for S.typhimurium virulence is a specialized protein secretion and delivery system termed type III that is encoded within the SPI-1 pathogenicity island of this bacterium (Galán and Curtiss, 1989; Watson et al., 1998; reviewed by Wallis and Galyov, 2000). This system mediates the transfer of several bacterial proteins that have the capacity to modulate signal transduction into the host cell cytosol. The translocated toxins trigger responses including cell death in macrophages, nuclear responses resulting in pro-inflammatory cytokine production, and actin cytoskeletal rearrangements leading to bacterial internalization by normally non-phagocytic cells such as epithelial cells (reviewed by Galán, 1999). In fact, S.typhimurium serves as an important model to study the cell biology of bacterial host cell invasion (Galán and Zhou, 2000). Recent work has demonstrated that S.typhimurium invasion of host cells is triggered mainly by three translocated effector proteins (Mirold et al., 2001; Zhou et al., 2001): SopE, SopE2 (see below) and SopB, a protein with inositol phosphatase activity (Hong and Miller, 1998; Norris et al., 1998).
Cdc42 is an essential component of the host cellular signaling cascades triggered by the translocated S.typhimurium effector proteins during invasion (Chen et al., 1996). The effector proteins SopE and SopE2 (70% amino acid sequence identity; Wood et al., 1996; Hardt et al., 1998b; Bakshi et al., 2000; Stender et al., 2000) bind directly to and activate host cellular Cdc42 by catalyzing guanine nucleotide exchange (Hardt et al., 1998a; Rudolph et al., 1999a; Stender et al., 2000). In fact, transient expression of SopE or SopE2 in cultured cells is sufficient to activate Cdc42, induce actin cytoskeletal reorganization and mediate internalization of non-invasive S.typhimurium mutants (Hardt et al., 1998b; Stender et al., 2000). Biochemical analyses have demonstrated that, unlike many other bacterial toxins, SopE and SopE2 activate host cellular Rho family proteins by transient interaction and not by chemical modification. Even though SopE and SopE2 do not share any recognizable sequence similarity to any known proteins from bacteria or eukaryotes, particularly the DH family proteins, they act as genuine GEFs (Rudolph et al., 1999a; Stender et al., 2000). Their ability to form nucleotide-free binary complexes with Rho proteins and to stimulate the rate of nucleotide exchange by several orders of magnitude are strikingly similar to those of genuine eukaryotic GEFs such as RCC1, a GEF for Ran (Klebe et al., 1995), or Cdc25, a GEF for Ras (Lenzen et al., 1998). Preliminary experiments indicate that its catalytic activity is much higher than that of genuine eukaryotic Rho-GEFs of the DH family (A.Wittinghofer, unpublished). For this reason, it was of great interest to study the structural basis of SopE-mediated guanine nucleotide exchange.
Here we have analyzed by X-ray crystallography at 2.3 Å resolution the interaction between the catalytic fragment of SopE and its host cellular target, Cdc42. We found that the structure of SopE is entirely different from that of Dbl-like RhoGEFs. Yet, the interaction of SopE locks the switch I and switch II regions of Cdc42 in the same conformation as that observed in the equivalent regions of Rac1 when bound to its celluar GEF Tiam1 (Worthylake et al., 2000), or in the Cdc42–Dbs complex (Rossman et al., 2002). These data provide the first insight into how proteins lacking any similarity to the Dbl-like protein family can act as efficient GEFs for Rho family GTP-binding proteins.
Results and discussion
Structure determination and overview
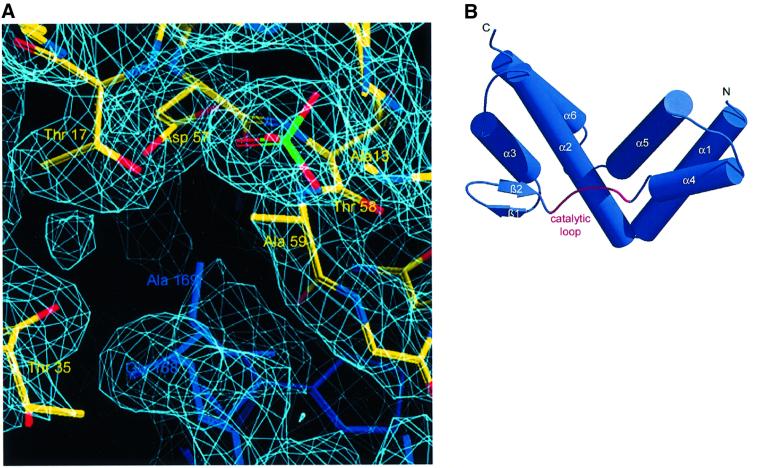
For crystallographic analysis, we used C-terminally truncated Cdc42 (amino acids 1–178) and the catalytic domain of SopE (SopEC, residues 78–240) (SopE from now onwards). It was shown previously that C-terminal truncation of Cdc42 does not interfere with SopE-mediated GEF activity (Rudolph et al., 1999a). The components of the complex were expressed in Escherichia coli and purified from cell lysates according to standard protocols. After mixing the constituents with a 2-fold molar excess of Cdc42, the SopE–Cdc42 complex was purified by size exclusion chromatography, crystallized, and the structure determined as described in Materials and methods. The current structural model of the asymmetric unit consists of two SopE–Cdc42 complexes, with most of the structure being well defined in its electron density. Altogether, six sulfate ions and 160 solvent molecules were added on the basis of Fo – Fc electron density maps. A summary of the crystallographic data analysis is given in Table I. Figure 1A shows a representative segment of the experimental electron density map.
Table I. Summary of crystallographic analysis.
| Native | CH3HgCl | K3UO2F5 | ||
|---|---|---|---|---|
| Data collectiona | ||||
| Resolution (Å) | 20–2.3 | 25–2.3 | 20–2.5 | |
| Highest shell (Å) | 2.4–2.3 | 2.4–2.3 | 2.6–2.5 | |
| Unique reflections | 40 507 (4737) | 76 154 (9239) | 59 109 (6611) | |
| Redundancy | 8.7 (7.6) | 2.6 (2.6) | 1.9 (1.9) | |
| I/σ | 15.2 (5.5) | 12.48 (5.18) | 7.49 (2.37) | |
| Rsym (%)b | 11.2 (32.8) | 5.6 (20.2) | 7.2 (29.4) | |
| RF (%)c | – | 28.9 | 13.8 | |
| Completeness (%) | 99.9 (100.0) | 97.5 (98.6) | 99.3 (99.8) | |
| MIRAS analysis | ||||
| Resolution (Å) | – | 20–2.6 | 20–2.6 | |
| Soaking | ||||
| Concentration/time | 0.1 mM/13 h | 5 mM/108 h | ||
| No. of sites | 4 | 3 | ||
| Rcullisd | 0.75 | 0.86 | ||
| Anomalous Rcullis | 0.89 | 0.99 | ||
| Phasing powere | 1.29 | 0.78 | ||
| Anomalous phasing power | 7.15 | 1.85 | ||
| Overall figure of merit | 0.52 | |||
| Refinement | ||||
| Resolution (Å) | 20–2.3 | |||
| Reflections | 40 297 | |||
| Rwork (%)f | 22.7 | |||
| Rfree (%)g | 25.8 | |||
| No. of atoms | ||||
| Protein | 5308 | |||
| Sulfate | 6 | |||
| Solvent | 160 | |||
| R.m.s. deviations | ||||
| Bonds (Å) | 0.006 | |||
| Angles (°) | 1.25 |
aNumbers in parentheses give values for the highest resolution bin.
bRsym = Σ|Ihi – Ih|/ΣIhi, where Ihi is the scaled intensity of the ith symmetry-related observation of reflection h and Ih is the mean value.
cRF = 2Σh|FPH – FP|/Σh|FPH + FP|, where FPH and FP are the derivative and native structure amplitudes.
dGeneralized Rcullis as implemented in CNS (Brünger et al., 1998).
eAs defined in CNS (Brünger et al., 1998).
fRwork = Σh|Fo – Fc|/ΣhFo, where Fo and Fc are the observed and calculated structure factor amplitudes for reflection h, respectively.
gValue of Rfree calculated for 10% randomly chosen reflections not included in the refinement.
Fig. 1. The GEF domain of SopE. (A) Segment of the experimental electron density map, contoured at 1.5σ showing a section of the complex interface. (B) Diagram of the structure of SopE, with helices as cylinders and the catalytic loop highlighted in red. (C) Sequences of the GEF domains of SopE (S.typhimurium, accession No. O52623; S.dublin, accession No. O06949; S.typhi, accession No. Q9RPM6) and SopE2 (accession No. Q9KIZ2), with the assignment of secondary structure elements according to the program DSSP (Kabsch and Sander, 1993). Selected residues in the interface (see Figure 2) are marked with filled circles. The catalytic loop involved in close contact with Cdc42 is highlighted. (D) Diagram of the structure of the DH domain of Tiam1, with helices as cylinders, as seen in the Rac1–Tiam1 complex (Worthylake et al., 2000), in the same orientation relative to the GTP-binding protein as SopE in (B). The functionally important lysine residue (see Figure 3) is included.
SopE
SopE is composed of six α-helices arranged in two three-helix bundles. The two bundles measure ∼40 Å in length and 30 Å in width and are arranged in a V-shaped fashion (Figure 1B). We observed only one small two-stranded β-sheet, which is followed by a peptide segment (residues 165–172) connecting the two sides of the ‘V’. This segment harbors a 166GAGA169 motif, which is proposed to represent the catalytic core of SopE (see Figure 1C). Analysis of the surface charge distribution with the program GRASP (Nicholls et al., 1991) did not show preferentially acidic or basic character.
A homology search with the program DALI (Holm and Sander, 1993) did not identify similarity with the Rho family-specific DH domains from the eukaryotic RhoGEFs Sos, Trio, βPIX or Tiam1 (Aghazadeh et al., 1998; Liu et al., 1998; Soisson et al., 1998; Worthylake et al., 2000). The DH domain is also an α-helical protein, but the location and topological arrangement of helices are quite different, as shown in Figure 1D. SopE also has no similarity to other nucleotide exchange factors for Ras-related GTP-binding proteins (Boriack-Sjodin et al., 1998; Cherfils et al., 1998; Mossessova et al., 1998; Renault et al., 1998). Structural relationships were found with proteins containing three-helix bundle ‘motifs’, with ribosome recycling factor (Selmer et al., 1999; Kim et al., 2000) and the helical segment of human guanylate-binding protein showing the highest scores of 5.8 and 5.7, respectively (Prakash et al., 2000). These similarities do not reflect common functional aspects, but rather confirm the three-helix bundle as a structural element of a large number of proteins.
The complex with Cdc42
The overall structure of Cdc42 is similar to that found in crystal structures of other Rho family proteins (e.g. Hirshberg et al., 1997; Wei et al., 1997). Conformational changes are restricted to the switch regions of Cdc42 that interact directly with SopE (discussed below). The switch I and switch II regions of Ras-related GTP-binding proteins are involved in nucleotide binding and define the major conformational differences between GTP- and GDP-bound forms (Milburn et al., 1990) (see below). Presumably as a consequence of the crystallization conditions (see Materials and methods), sulfate ions are found in various favorable binding sites, the functionally most important of which is the phosphate-binding (P) loop (Saraste et al., 1990). This sulfate ion is located in the position which would be occupied by the β-phosphate moiety when nucleotide is present. Like the β-phosphate, it is stabilized by interactions with main chain amide groups derived from the P-loop motif (GxxxxKS/T) and the side chain amino group of Lys16. A similar situation is found in the complex between Rac1 and the RhoGEF Tiam1 (Worthylake et al., 2000) (see below), in the Ran–RCC1 complex (Renault et al., 2001) or in guanosine-bound Ras (Scheffzek et al., 1994).
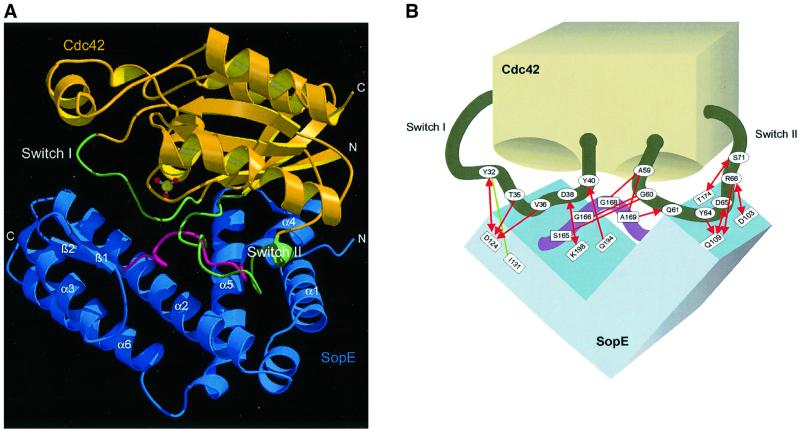
The complex interface
The complex interface is formed primarily by helices α2, α4 and α5 from SopE, including flanking regions that communicate mainly with the switch regions of Cdc42 (Figure 2A and B). The interface buries ∼2800 Å of accessible surface area, which is similar to that of the Rac1–Tiam1 (Worthylake et al., 2000) and Dbs–Cdc42 (Rossman et al., 2002) complexes and somewhat higher than those of other Rho regulatory complexes (Rittinger et al., 1997; Hoffman et al., 2000; Scheffzek et al., 2000). As in other G-protein–GEF complexes (Kawashima et al., 1996; Wang et al., 1997; Boriack-Sjodin et al., 1998; Goldberg, 1998), the base-binding pocket is not occluded by the GEF component and is accessible from the solvent. SopE does not interact with P-loop residues, which is consistent with the observation that Cdc42G12V is an efficient substrate (Rudolph et al., 1999a).
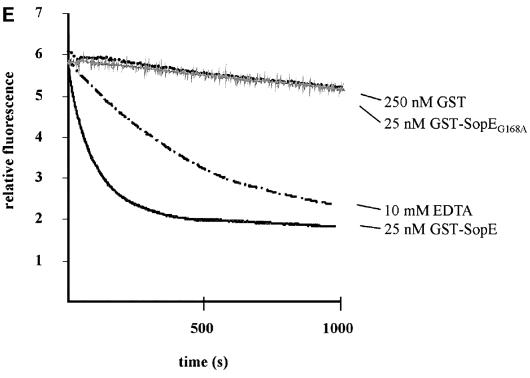
Fig. 2. The Cdc42–SopE complex. (A) Ribbon representation of the complex with SopE in blue and Cdc42 in yellow. The switch regions are shown in green. The catalytic loop inserted between the switch regions is highlighted in red. (B) Schematic representation of the Cdc42–SopE interaction with important interactions indicated. Hydrophobic contacts are in green, polar interactions in red. Arrowheads mark side chain and straight ends indicate main chain contributions. (C) Close up view of the interface region with the switch regions of GDP-bound Cdc42 (in light gray) included. Side chains of residues considered important for the interaction are shown. Dashed segments indicate disordered regions, modeled stereochemically. The gray ball near Ala59 is the Mg2+ ion from the Cdc42–GDP structure. (D) Stereo view of the nucleotide-binding region, showing the specific rearrangements of the switch regions associated with SopE–Cdc42 interaction, as discussed in the text. (E) Multiple turnover kinetics of nucleotide exchange on Cdc42·mantGDP mediated by GST–SopEG168A. Release of mantGDP was followed by fluorescence spectrometry in buffer S (50 mM NaCl, 50 mM Tris–HCl pH 7.6, 5 mM MgCl2) in the presence of 1 mM GDP. The assays were performed with 1 µM Cdc42·mantGDP and 250 nM GST, 10 mM EDTA, 25 nM GST–SopEG168A (gray line) or 25 nM GST–SopE (excitation wavelength 366 nm, emission wavelength 440 nm, step size 1 nm, bandpass 4 nm).
In order to explore the catalytic mechanism of SopE-mediated guanine nucleotide exchange, we have superimposed the structure of the nucleotide-free SopE–Cdc42 complex (Figure 2A) with the structure of GDP-loaded Cdc42 (Rudolph et al., 1999b). The core of the G-domains is virtually identical in both structures, but major conformational changes are observed in the switch I/II regions of Cdc42 (Figure 2C). The most notable feature of SopE contributing to these conformational changes is the catalytic 166GAGA169-loop of SopE, which is inserted between the switch regions of Cdc42 and appears to exert a push-and-pull-type movement whereby switch I is pushed aside and switch II is pulled towards the loop (Figure 2C). Insertion of the 166GAGA169-loop of SopE induces two consecutive peptide flips in the C-terminal half of switch I, rotating Phe37cdc42 by ∼90° and Tyr40cdc42 by almost 180° (Figure 2D). The resulting conformation is stabilized by a salt bridge between Asp38cdc42 and Lys198sopE along with a hydrogen bond between the hydroxyl group of Tyr40cdc42 and the main chain carbonyl group of Gln194sopE. In addition, the hydrophobic core burying the aromatic side chains of Phe37cdc42 and Tyr40cdc42 appears to contribute to stabilization (Figure 2). The major contribution of the catalytic 166GAGA169-loop to the proposed mechanism of SopE-mediated nucleotide release is supported by site-directed mutagenesis of Gly168sopE to alanine. The mutant protein, under the conditions employed, does not stimulate nucleotide exchange on Cdc42 as measured by a fluorescence assay where Cdc42-bound fluorescent mant-nucleotide is exchanged against a large excess of unlabeled nucleotide (Figure 2E).
The displacement of switch I seems to be stabilized by interactions of Asp124sopE with the hydroxyl group of Tyr32cdc42, as well as with the main chain amide groups of Thr35cdc42 and Val36cdc42. The side chains of these residues also participate in the interface. Presumably as a consequence of the observed interactions, the N-terminal portion of switch I becomes disordered (residues 26–31) and extruded from the nucleotide-binding pocket (Figure 2B and C). Switch II is displaced towards the catalytic loop and its C-terminal end is involved in interactions with the area around helices α2 and α4 of SopE, forming an extensive interface (Figure 2B and C). Hydrophobic contributions come from Tyr64cdc42, and a number of polar contacts involving Tyr64cdc42, Asp65cdc42, Arg66cdc42 and Ser71cdc42 apparently stabilize the interface in this area (Figure 2B and C).
The most extensive contacts are formed between the catalytic loop of SopE and the N-terminal part of switch II, involving residues Ala59cdc42, Gly60cdc42 and Gln61cdc42. They form a network of polar main chain and van der Waals interactions with Ser165sopE, Gly166sopE, Gly168sopE and Ala169sopE. The most dramatic effect on the conformation of switch II is a peptide flip involving Ala59cdc42, whereby its methyl group points into and thus blocks the Mg2+-binding site (Figure 2C). Strikingly, a similar conformation of Ala59cdc42 has been observed in GDP-bound RhoA in the absence of Mg2+ (Shimizu et al., 2000) and also in other G-protein–GEF complexes (Boriack-Sjodin et al., 1998; Worthylake et al., 2000). The conformation of Ala59cdc42 is stabilized by a main chain–main chain interaction with Ala169sopE.
Implications for nucleotide release
We have shown earlier that SopE increases the guanine nucleotide exchange on Rho proteins 105-fold above the intrinsic reaction rate (Rudolph et al., 1999a), which is similar to the catalytic rates of GEFs for Ras-related proteins (Klebe et al., 1995; Lenzen et al., 1998; Hutchinson and Eccleston, 2000). The structure of the nucleotide-free SopE–Cdc42 complex provides important insights to explain the accelerated guanine nucleotide release stimulated by the bacterial exchange factor.
Reorientation of switch I causes extrusion of residues potentially important for binding of the nucleotide, especially of the guanine base. The rearrangements in switch I include the disordering of its N-terminal segment, as inferred from ill-defined electron density in this region (Figure 2C). This leads to extrusion of Phe28cdc42 from the nucleotide-binding pocket, where it usually provides a major contribution to the binding of GDP/GTP. Similarly to Ras, the mutation of this amino acid to leucine leads to 140-fold increased nucleotide dissociation rates (Reinstein et al., 1991; Lin et al., 1997), highlighting the importance of this hydrophobic interaction for nucleotide affinity.
In the nucleotide-free Ras–Sos complex, the switch I region of Ras is flipped out of its position close to the nucleotide by a long helical extension on Sos, and it has been argued that this is the key feature driving guanine nucleotide release (Boriack-Sjodin et al., 1998). Here we also find that most of the switch I region is pushed aside due to the action of the catalytic loop of SopE, arguing that reorientation of residues such as Glu31cdc42 in addition to Phe28cdc42 might contribute to accelerated nucleotide release. In the Rac1–GppNHp, but not in the Cdc42–GDP complex, Glu31rac1 is coordinated to the 3′-oxygen of the ribose and this residue is conserved in Rho family proteins. Therefore, we speculate that the specific reorientation of Phe28cdc42 and Glu31cdc42 and/or the general rearrangement of the effector loop, which runs alongside the nucleotide and partially shields it from the solvent, might both contribute to the accelerated nucleotide release.
In switch II, a peptide flip positions the methyl group of Ala59cdc42 within 2 Å of the Mg2+-binding site (Figure 2C and D). Since Mg2+ is important for high-affinity binding of GDP/GTP from Ras-like GTP-binding proteins (Vetter and Wittinghofer, 2001) and excess Mg2+ decreases dissociation of GDP from Cdc42 by 140-fold (Zhang et al., 2000), the SopE-induced displacement of Mg2+ might be an important step in guanine nucleotide release. The conformation of switch II in SopE-bound Cdc42 is strikingly similar to that of switch II regions in Mg2+-free GDP-bound RhoA (Shimizu et al., 2000) and Tiam1-bound Rac1 (Worthylake et al., 2000), with Ala59rac1 pointing into the Mg2+-binding site. It is possible that part of the switch II region is stabilized in a Mg2+-free conformation and that the GEF activity of SopE is due at least partially to its effect on removal of the metal ion. The role of the methyl group of Ala59 in the GEF reaction has been investigated for the Ras–Sos system by site-directed mutagenesis (Hall et al., 2001). These studies showed that this residue might not be important for nucleotide release in the Ras–Sos reaction. In order to assess the role of the almost invariant Ala59 for the RhoGEF mechanism in more detail, however, site-directed mutagenesis combined with fast kinetic analysis would be necessary. Reorientation of Thr35 in switch I, in turn, is unlikely to have an effect on nucleotide affinity since it is coordinated to Mg2+ only in the triphosphate form, and its mutation to alanine in Ras reduces Mg2+ affinity in that complex only 4-fold (Spoerner et al., 2001).
Studies on Ras have shown that the β-phosphate contributes significantly to binding affinity of the nucleotide, since GMP and guanosine bind with five orders of magnitude weaker affinity than GTP or GDP (John et al., 1990). Since removal of Mg2+ or Phe28cdc42 would only partially account for the 105-fold stimulation of nucleotide release from Cdc42 by SopE, it is possible that SopE, like other GEFs, interferes with binding of the phosphate moiety of the nucleotide. Structures of Ras, Arf and EF-Tu complexed with their cognate GEFs indeed show GEF side chains pointing into phosphate-binding regions (Kawashima et al., 1996; Wang et al., 1997; Boriack-Sjodin et al., 1998; Goldberg, 1998). However, no such residues were found in our structure or in other GEF–G-protein complexes where a sulfate ion is bound in the P-loop (Worthylake et al., 2000; Renault et al., 2001). On the basis of the Ran–RCC1 complex (Renault et al., 2001), it has been suggested that the sulfate-bound structure in the crystal represents an intermediate along the kinetic pathway of nucleotide exchange, and that rearrangement of the nucleotide-binding site after release of the anion may produce conformational changes that lead to the nucleotide-free structures observed in Ras–Sos, Arf–Sec7 and EF-Tu–EF-Ts (Kawashima et al., 1996; Boriack-Sjodin et al., 1998; Goldberg, 1998).
The SopE-induced conformational rearrangements of switch I and switch II shown here disrupt several favorable interactions necessary for tight guanine nucleotide binding and induce nucleotide release. Since the nucleotide-binding pocket is almost freely accessible from the solvent, unrestricted rebinding of nucleotide and formation of a low-affinity trimeric complex would be possible. In the course of the complete GEF reaction, concomitant restructuring of the switch regions would not be compatible with the SopE binding and would thus lead to dissociation of SopE and formation of a high-affinity nucleotide complex.
Comparison with the eukaryotic Rac1–Tiam1 complex and evolutionary relationship
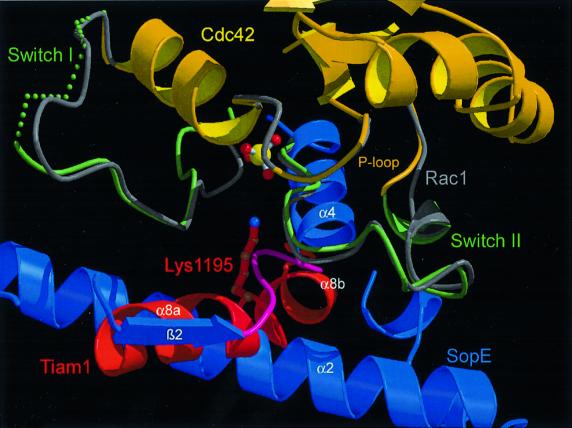
In order to compare the catalytic mechanism of SopE-mediated guanine nucleotide exchange with that of eukaryotic RhoGEFs, we have superimposed the structure of the Cdc42–SopE complex with that of the Rac1–Tiam1 complex (Worthylake et al., 2000). Alignment of both complexes on the G-domain cores revealed that the switch regions of the Rho proteins have a remarkably similar local arrangement (Figure 3) such as the flip of Phe28cdc42/rac1 and Ala59cdc42/rac1 as well as displacement of Phe37cdc42/rac1. Only the local arrangement around Tyr40cdc42/rac1 differs significantly between the two structures (Figure 3). In the Rac1–Tiam1 complex, the side chain of Tyr40rac1 is rotated by 180° relative to its orientation in the Cdc42–SopE complex. Nevertheless, SopE and the eukaryotic GEF Tiam1 induce very similar overall rearrangements in the switch I and switch II regions of the Rho component to accelerate nucleotide release.
Fig. 3. Similar GEF mechanisms by SopE and Tiam1 using distinct structural elements. The Cdc42–SopE and Rac1–Tiam1 complexes have been aligned on the G-domain shown in gray. The P loop and switch I/II regions are shown for the individual molecules. Helices α2 and α4 from SopE are in blue, and helix α8 from Tiam1 in red. The view shows the similar location of the catalytic loop from SopE and the important Lys1195 from Tiam1.
In sharp contrast, however, the GEF components of both protein complexes are completely different (Figure 1B and D). While both GEFs have a high α-helical content, they have different tertiary folds and both use different amino acids to interact with their Rho target (Figure 3). Even the catalytic cores of both GEFs differ markedly: to distort switch I and II, SopE employs main chain contacts of the 166GAGA169-loop, which is composed entirely of small side chains, while Tiam1 uses helix α8 and particularly the large side chain of Lys1195 (Figure 3). Mutations of Lys1195 in Tiam1 or of the analogous amino acid in the DH domain of Trio have been reported to decrease the GEF activity by at least 80%, supporting its importance for the catalytic mechanism (Liu et al., 1998; Worthylake et al., 2000). The only similarity between both GEFs is that Asp124sopE and Glu1047tiam engage in analogous contacts with the side chain of Tyr32cdc42/rac1 and the main chain amides of Thr35cdc42/rac1 and Val36cdc42/rac1 (see Figure 5 in Worthylake et al., 2000). Based on mutational analyses of DH proteins, substitution of the homologous glutamate by alanine in the exchange factor Trio reduces its activity to 50% of the wild-type rate (Liu et al., 1998). Comparing the complexes leads us to conclude that SopE is not just mimicking Rho family-specific eukaryotic GEFs of the DH family, but that it represents an entirely novel class of RhoGEFs. Why SopE appears to be a more efficient guanine nucleotide exchange catalyst is presently unclear and has been investigated in more detail, using as a conceptual framework the structural information from the SopE and DH complexes.
Bacterial pathogens have evolved a variety of strategies to manipulate signaling inside animal cells. Many bacterial toxins modulate Rho signaling pathways by covalently modifying Rho proteins (Aktories et al., 2000). These modifications either activate or inactivate Rho proteins. The translocated toxins SopE, SopE2 and SptP from S.typhimurium and ExoS from Pseudomonas aeruginosa belong to a new group of toxins interfering with Rho signaling by non-covalent interaction with host cell GTP-binding proteins of the Rho subfamily. These toxins allow S.typhimurium to modulate reversibly the activity of Rho proteins. Therefore, they can be viewed as functional analogs of regulators of their host cells. Here we have demonstrated that SopE very closely copies the functional mechanism of GEFs from eukaryotic cells, but uses a different fold and residues for the task.
The structural and functional properties of SopE are in sharp contrast to those of SptP and other similar toxins from Yersinia spp. and Pseudomonas spp. These proteins, which act as transient inactivators of Rho family members by increasing the rate of GTP hydrolysis, use the same catalytic motif (the ‘arginine finger’; Scheffzek et al., 1998) as eukaryotic GTPase-activating proteins (GAPs) and therefore constitute examples of molecular mimicry (Stebbins and Galán, 2000; Würtele et al., 2001).
Considering the long standing co-evolution between Salmonella spp. and their mammalian hosts, it is intriguing to speculate that hosts have little chance to evolve variants of Rho resistant to modulation. Since SopE (and SptP) binds to virtually identical elements of the Rho target as the eukaryotic GEFs (and GAPs), any mutation interfering with the SopE interaction would almost certainly also negatively affect interaction with the host cellular GEFs (and GAPs). In addition, SopE recognizes several different members of the Rho family. Therefore, multiple mutations in different genes would be necessary to evolve host resistance.
Materials and methods
Protein expression and purification
Salmonella typhimurium SopE (residues 78–240) and human Cdc42 (residues 1–178) were expressed in E.coli BL21 as recombinant GST fusion proteins. Cells grown to an OD600 of 0.8 in LB medium with 100 µg/ml ampicillin were induced at 30°C with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and cultivated for 7–10 h. After homogenizing the cells with a fluidizer (Microfluidics, Newton, MA), the suspension was centrifuged at 30 000 g for 30 min. GST fusion proteins of SopE and Cdc42 were purified on glutathione–Sepharose (Pharmacia). After application, the column was washed extensively with buffer A [50 mM Tris–HCl pH 7.5, 100 mM NaCl, 5 mM dithiothreitol (DTT)], followed by buffer B (50 mM Tris–HCl pH 7.5, 500 mM NaCl, 5 mM DTT) and again with buffer A. After cleavage with thrombin overnight on the column, the proteins were eluted with buffer A and thrombin was removed by chromatography on benzamidine–Sepharose 6B (Pharmacia). Further purification by gel filtration chromatography (Superdex 75, Pharmacia) resulted in the SopE construct (residues 78–240), which contains two additional amino acids (Gly76–Ser77), and the Cdc42 construct that also included glycine and serine at the N-terminus. We obtained ∼1 mg of protein per gram of cells in both cases.
The Cdc42–SopE complex was formed by incubating the two purified proteins at 4°C in buffer [20 mM Tris–HCl pH 7.5, 150 mM NaCl, 5 mM MgCl2, 200 mM (NH4)2SO4, 0.1 mM ZnCl2 and 5 U of alkaline phosphatase/mg Cdc42] using an ∼2-fold molar excess of Cdc42. Alkaline phosphatase and ZnCl2 were used in order to remove the nucleotide. The complex solution was purified through size exclusion chromatography using a Superdex 75 gel filtration column (Pharmacia, 16/60) and a running buffer with 20 mM Tris–HCl pH 7.5, 30 mM NaCl, 1 mM (NH4)2SO4, 2 mM EDTA and 5 mM DTT. Fractions containing the complex were pooled and concentrated to 44 mg/ml.
Crystallization
Crystals were grown at room temperature by vapor diffusion from hanging drops formed by equal volumes (1 + 1 µl) of protein (44 mg/ml) and crystallization buffer [1.9 M ammonium sulfate, 0.1 M sodium citrate pH 5.6, 2% polyethylene glycol (PEG)], suspended over a reservoir of 500 µl. Small crystalline needles (average size 100 × 10 × 10 µm3) appeared after 1–2 days. They were improved by addition of 0.05 M betaine and using microseeding protocols, and reached a typical size of 250 × 40 × 20 µm3. Crystals belong to the trigonal space group P3121 with cell dimensions a = b = 87.5 Å, c = 200.5 Å, α = β = 90°, γ = 120°, and contain two Cdc42–SopE complexes in the asymmetric unit with a solvent content of 58%.
Data collection
Initial data sets of tiny needles, diffracting to a resolution of ∼2.5 Å, were collected at the microfocus beamline (ID13) of the European Synchrotron Radiation Facility (ESRF, Grenoble, France). Improved crystals were used for routine data collection on beamline ID14-1 at ESRF.
Crystals were prepared for flash cooling by transfer to a stabilization buffer (2.0 M ammonium sulfate, 0.1 M sodium citrate pH 5.6, 2% PEG, 0.05 M betaine) containing 15% (v/v) glycerol as cryoprotectant. Data sets of typically 2.3 Å in resolution were collected from cryofrozen crystals at a temperature of 100 K using the rotation method (crystal to detector distance, 150 cm; oscillation angle, 1°; wavelength, 0.934 Å; detector MAR 165 mm CCD). Data were processed and scaled with the XDS package (Kabsch, 1993) (see Table I).
Structure determination and refinement
The structure was determined by multiple isomorphous replacement anomalous scattering (MIRAS), combined with molecular replacement. Heavy atom soaks were performed in stabilization buffer (see above) containing 0.1–5 mM heavy metals of seven tested compounds. CH3HgCl- and K3UO2F5-soaked crystals produced derivatives of sufficient quality to be used for phasing. All MIRAS analysis (see Table I) was carried out with the program CNS (Brünger et al., 1998). Initial heavy atom positions in the mercury derivative were determined using difference Patterson methods and used to obtain the heavy atom coordinates in the uranyl derivative by difference Fourier methods. Phases were calculated, and improved using ‘solvent flipping’ as implemented in CNS. The resulting electron density map was of good quality and was used to position the Cdc42 model (Rudolph et al., 1999b) in the unit cell, followed by tracing the SopE portion of the asymmetric unit. After several cycles of interactive model building with O (Jones et al., 1991) and refinement with CNS, the model was completed finally to two Cdc42–SopE complexes, 160 water molecules and six sulfate ions. Inspection of the model with PROCHECK (Laskowski et al., 1993) did not reveal unfavorable Φ,Ψ combinations. Visualization of structural models was done with the programs MOLSCRIPT (Kraulis, 1991) and RASTER3D (Merritt and Bacon, 1997).
Fluorescence spectrometry
The Cdc42·mantGDP complex was prepared as published recently (Friebel et al., 2001). Fluorescence measurements were performed as multiple turnover kinetics in an Aminco-Bowman Series 2 Luminescence spectrometer. The excitation wavelength was 366 nm, and emission was recorded at 440 nm. Release of mantGDP was followed in buffer S (40 mM HEPES pH 7.4, 100 mM NaCl, 5 mM MgCl2) in the presence of 1 mM GDP. The assays contained 1 µM Cdc42·mantGDP and 250 nM GST, 10 mM EDTA, 25 nM GST–SopEG168A or 25 nM GST–SopE.
Coordinates
Coordinates have been deposited with the Protein Data Bank under the ID code 1gzs.
Acknowledgments
Acknowledgements
We thank Susi Gunawan for help with protein purification, the staff at the beamlines ID13 (A.Perrakis) and ID14-1 (H.Barhaldi) of the European Synchrotron Radiation Facility (ESRF), Grenoble, France, for technical support, Ingrid Vetter, Axel Scheidig, Martin Würtele, members of the Saraste and of the Conti group at EMBL for discussions, Matti Saraste for support, and Rita Schebaum for secretarial assistance. This work was supported by the Deutsche Forschungsgemeinschaft with research grants to K.S., A.W. and W.D.H.
References
- Aghazadeh B., Zhu,K., Kubiseski,T.J., Liu,G.A., Pawson,T., Zheng,Y. and Rosen,M.K. (1998) Structure and mutagenesis of the Dbl homology domain. Nat. Struct. Biol., 5, 1098–1107. [DOI] [PubMed] [Google Scholar]
- Aktories K., Schmidt,G. and Just,I. (2000) Rho GTPases as targets of bacterial protein toxins. Biol. Chem., 381, 421–426. [DOI] [PubMed] [Google Scholar]
- Bakshi C.S., Singh,V.P., Wood,M.W., Jones,P.W., Wallis,T.S. and Galyov,E.E. (2000) Identification of SopE2, a Salmonella secreted protein which is highly homologous to SopE and involved in bacterial invasion of epithelial cells. J. Bacteriol., 182, 2341–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boriack-Sjodin P.A., Margarit,S.M., Bar-Sagi,D. and Kuriyan,J. (1998) The structural basis of the activation of Ras by Sos. Nature, 394, 337–343. [DOI] [PubMed] [Google Scholar]
- Bourne H.R., Sanders,D.A. and McCormick,F. (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature, 348, 125–132. [DOI] [PubMed] [Google Scholar]
- Bourne H.R., Sanders,D.A. and McCormick,F. (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature, 349, 117–127. [DOI] [PubMed] [Google Scholar]
- Brünger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr., D54, 905–921. [DOI] [PubMed] [Google Scholar]
- Cerione R.A. and Zheng,Y. (1996) The Dbl family of oncogenes. Curr. Opin. Cell Biol., 8, 216–222. [DOI] [PubMed] [Google Scholar]
- Chen L.M., Hobbie,S. and Galán,J.E. (1996) Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science, 274, 2115–2118. [DOI] [PubMed] [Google Scholar]
- Cherfils J. and Chardin,P. (1999) GEFs: structural basis for their activation of small GTP-binding proteins. Trends Biochem. Sci., 24, 306–311. [DOI] [PubMed] [Google Scholar]
- Cherfils J., Menetrey,J., Mathieu,M., Le Bras,G., Robineau,S., Beraud-Dufour,S., Antonny,B. and Chardin,P. (1998) Structure of the Sec7 domain of the Arf exchange factor ARNO. Nature, 392, 101–105. [DOI] [PubMed] [Google Scholar]
- Friebel A., Ilchmann,H., Aepfelbacher,M., Ehrbar,K., Machleidt,W. and Hardt,W.D. (2001) SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem., 276, 34035–34040. [DOI] [PubMed] [Google Scholar]
- Galán J.E. (1999) Interaction of Salmonella with host cells through the centisome 63 type III secretion system. Curr. Opin. Microbiol., 2, 46–50. [DOI] [PubMed] [Google Scholar]
- Galán J.E. and Curtiss,R.,3rd (1989) Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl Acad. Sci. USA, 86, 6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J.E. and Zhou,D. (2000) Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl Acad. Sci. USA, 97, 8754–8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. (1998) Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP–myristoyl switching. Cell, 95, 237–248. [DOI] [PubMed] [Google Scholar]
- Groisman E.A. and Ochman,H. (1996) Pathogenicity islands: bacterial evolution in quantum leaps. Cell, 87, 791–794. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hall B.E., Yang,S.S., Boriack-Sjodin,P.A., Kuriyan,J. and Bar-Sagi,D. (2001) Structure-based mutagenesis reveals distinct functions for Ras switch 1 and switch 2 in Sos-catalyzed guanine nucleotide exchange. J. Biol. Chem., 276, 27629–27637. [DOI] [PubMed] [Google Scholar]
- Hardt W.D., Chen,L.M., Schuebel,K.E., Bustelo,X.R. and Galán,J.E. (1998a) S.typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell, 93, 815–826. [DOI] [PubMed] [Google Scholar]
- Hardt W.D., Urlaub,H. and Galán,J.E. (1998b) A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc. Natl Acad. Sci. USA, 95, 2574–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshberg M., Stockley,R.W., Dodson,G. and Webb,M.R. (1997) The crystal structure of human rac1, a member of the rho-family complexed with a GTP analogue. Nat. Struct. Biol., 4, 147–152. [DOI] [PubMed] [Google Scholar]
- Hoffman G.R., Nassar,N. and Cerione,R.A. (2000) Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell, 100, 345–356. [DOI] [PubMed] [Google Scholar]
- Holm L. and Sander,C. (1993) Protein structure comparison by alignment of distance matrices. J. Mol. Biol., 233, 123–138. [DOI] [PubMed] [Google Scholar]
- Hong K.H. and Miller,V.L. (1998) Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J. Bacteriol., 180, 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson J.P. and Eccleston,J.F. (2000) Mechanism of nucleotide release from Rho by the GDP dissociation stimulator protein. Biochemistry, 39, 11348–11359. [DOI] [PubMed] [Google Scholar]
- John J., Sohmen,R., Feuerstein,J., Linke,R., Wittinghofer,A. and Goody,R.S. (1990) Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry, 29, 6058–6065. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Zou,J.-Y., Cowan,S.W. and Kjeldgaard,M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr., A47, 110–119. [DOI] [PubMed] [Google Scholar]
- Kabsch W. (1993) Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr., 26, 795–800. [Google Scholar]
- Kabsch W. and Sander,C. (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers, 22, 2577–2637. [DOI] [PubMed] [Google Scholar]
- Kawashima T., Berthet-Colominas,C., Wulff,M., Cusack,S. and Leberman,R. (1996) The structure of the Escherichia coli EF-Tu·EF-Ts complex at 2.5 Å resolution. Nature, 379, 511–518. [DOI] [PubMed] [Google Scholar]
- Kim K.K., Min,K. and Suh,S.W. (2000) Crystal structure of the ribosome recycling factor from Escherichia coli. EMBO J., 19, 2362–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe C., Bischoff,F.R., Ponstingl,H. and Wittinghofer,A. (1995) Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry, 34, 639–647. [DOI] [PubMed] [Google Scholar]
- Kraulis P.J. (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr., 24, 946–950. [Google Scholar]
- Laskowski R.A., MacArthur,M.W., Moss,D.S. and Thornton,J.M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr., 26, 283–291. [Google Scholar]
- Lenzen C., Cool,R.H., Prinz,H., Kuhlmann,J. and Wittinghofer,A. (1998) Kinetic analysis by fluorescence of the interaction between Ras and the catalytic domain of the guanine nucleotide exchange factor Cdc25Mm. Biochemistry, 37, 7420–7430. [DOI] [PubMed] [Google Scholar]
- Lin R., Bagrodia,S., Cerione,R. and Manor,D. (1997) A novel Cdc42Hs mutant induces cellular transformation. Curr. Biol., 7, 794–797. [DOI] [PubMed] [Google Scholar]
- Liu X. et al. (1998) NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell, 95, 269–277. [DOI] [PubMed] [Google Scholar]
- Merritt E.A. and Bacon,D.J. (1997) Raster3D: photorealistic molecular graphics. Methods Enzymol., 277, 505–524. [DOI] [PubMed] [Google Scholar]
- Milburn M.V., Tong,L., deVos,A.M., Brunger,A., Yamaizumi,Z., Nishimura,S. and Kim,S.H. (1990) Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science, 247, 939–945. [DOI] [PubMed] [Google Scholar]
- Mirold S., Ehrbar,K., Weissmüller,A., Prager,R., Tschäpe,H., Rüssmann,H. and Hardt,W.D. (2001) Salmonella host cell invasion emerged by acquisition of a mosaic of separate genetic elements, including Salmonella pathogenicity island 1 (SPI1), SPI5 and sopE2. J. Bacteriol., 183, 2348–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova E., Gulbis,J.M. and Goldberg,J. (1998) Structure of the guanine nucleotide exchange factor Sec7 domain of human arno and analysis of the interaction with ARF GTPase. Cell, 92, 415–423. [DOI] [PubMed] [Google Scholar]
- Nicholls A., Sharp,K.A. and Honig,B. (1991) Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins: Struct. Funct. Genet., 11, 281–296. [DOI] [PubMed] [Google Scholar]
- Norris F.A., WilsonM.P., Wallis,T.S., Galyov,E.E. and Majerus,P.W. (1998) SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl Acad. Sci. USA, 95, 14057–14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash B., Praefcke,G.J., Renault,L., Wittinghofer,A. and Herrmann,C. (2000) Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature, 403, 567–571. [DOI] [PubMed] [Google Scholar]
- Reinstein J., Schlichting,I., Frech,M., Goody,R.S. and Wittinghofer,A. (1991) p21 with a phenylalanine 28→leucine mutation reacts normally with the GTPase activating protein GAP but nevertheless has transforming properties. J. Biol. Chem., 266, 17700–17706. [PubMed] [Google Scholar]
- Renault L., Nassar,N., Vetter,I., Becker,J., Klebe,C., Roth,M. and Wittinghofer,A. (1998) The 1.7 Å crystal structure of the regulator of chromosome condensation (RCC1) reveals a seven-bladed propeller. Nature, 392, 97–101. [DOI] [PubMed] [Google Scholar]
- Renault L., Kuhlmann,J., Henkel,A. and Wittinghofer,A. (2001) Structural basis for guanine nucleotide exchange on Ran by the regulator of chromosome condensation (RCC1). Cell, 105, 245–255. [DOI] [PubMed] [Google Scholar]
- Rittinger K., Walker,P.A., Eccleston,J.F., Smerdon,S.J. and Gamblin,S.J. (1997) Structure at 1.65 Å of RhoA and its GTPase-activating protein in complex with a transition-state analogue. Nature, 389, 758–762. [DOI] [PubMed] [Google Scholar]
- Rossman K.L., Worthylake,D.K., Snyder,J.T., Siderovski,D.P., Campbell,S.L. and Sondek,J. (2002) A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange. EMBO J., 21, 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M.G., Weise,C., Mirold,S., Hillenbrand,B., Bader,B., Wittinghofer,A. and Hardt,W.D. (1999a) Biochemical analysis of SopE from Salmonella typhimurium, a highly efficient guanosine nucleotide exchange factor for RhoGTPases. J. Biol. Chem., 274, 30501–30509. [DOI] [PubMed] [Google Scholar]
- Rudolph M.G., Wittinghofer,A. and Vetter,I.R. (1999b) Nucleotide binding to the G12V-mutant of Cdc42 investigated by X-ray diffraction and fluorescence spectroscopy: two different nucleotide states in one crystal. Protein Sci., 8, 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald,P.R. and Wittinghofer,A. (1990) The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci., 15, 430–434. [DOI] [PubMed] [Google Scholar]
- Scheffzek K., Kabsch,W., Schlichting,I., Pai,E.F., Lautwein,A., Frech,M., Wittinghofer,A. and Goody,R.S. (1994) Crystallization and preliminary X-ray structure investigation of thermally unstable p21H-ras guanosine complexes. Acta Crystallogr., D50, 521–526. [DOI] [PubMed] [Google Scholar]
- Scheffzek K., Ahmadian,M.R. and Wittinghofer,A. (1998) GTPase activating proteins: helping hands to complement an active site. Trends Biochem. Sci., 23, 257–262. [DOI] [PubMed] [Google Scholar]
- Scheffzek K., Stephan,I., Jensen,O.N., Illenberger,D. and Gierschik,P. (2000) The Rac–RhoGDI complex and the structural basis for the regulation of Rho proteins by RhoGDI. Nat. Struct. Biol., 7, 122–126. [DOI] [PubMed] [Google Scholar]
- Selmer M., Al-Karadaghi,S., Hirokawa,G., Kaji,A. and Liljas,A. (1999) Crystal structure of Thermotoga maritima ribosome recycling factor: a tRNA mimic. Science, 286, 2349–2352. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Ihara,K., Maesaki,R., Kuroda,S., Kaibuchi,K. and Hakoshima,T. (2000) An open conformation of switch I revealed by the crystal structure of a Mg2+-free form of RHOA complexed with GDP. Implications for the GDP/GTP exchange mechanism. J. Biol. Chem., 275, 18311–18317. [DOI] [PubMed] [Google Scholar]
- Soisson S.M., Nimnual,A.S., Uy,M., Bar-Sagi,D. and Kuriyan,J. (1998) Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell, 95, 259–268. [DOI] [PubMed] [Google Scholar]
- Spoerner M., Herrmann,C., Vetter,I.R., Kalbitzer,H.R. and Wittinghofer,A. (2001) Dynamic properties of the Ras switch I region and its importance for binding to effectors. Proc. Natl Acad. Sci. USA, 98, 4944–4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins C.E. and Galán,J.E. (2000) Modulation of host signaling by a bacterial mimic: structure of the Salmonella effector SptP bound to Rac1. Mol. Cell, 6, 1449–1460. [DOI] [PubMed] [Google Scholar]
- Stender S., Friebel,A., Linder,S., Rohde,M., Mirold,S. and Hardt,W.D. (2000) Identification of SopE2 from Salmonella typhimurium, a conserved guanine nucleotide exchange factor for Cdc42 of the host cell. Mol. Microbiol., 36, 1206–1221. [DOI] [PubMed] [Google Scholar]
- Vetter I.R. and Wittinghofer,A. (2001) The guanine nucleotide-binding switch in three dimensions. Science, 294, 1299–1304. [DOI] [PubMed] [Google Scholar]
- Wallis T.S. and Galyov,E.E. (2000) Molecular basis of Salmonella-induced enteritis. Mol. Microbiol., 36, 997–1005. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang,Y., Meyering-Voss,M., Sprinzl,M. and Sigler,P.B. (1997) Crystal structure of the EF-Tu·EF-Ts complex from Thermus thermophilus. Nat. Struct. Biol., 4, 650–656. [DOI] [PubMed] [Google Scholar]
- Watson P.R., Galyov,E.E., Paulin,S.M., Jones,P.W. and Wallis,T.S. (1998) Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect. Immun., 66, 1432–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Zhang,Y., Derewenda,U., Liu,X., Minor,W., Nakamoto,R.K., Somlyo,A.V., Somlyo,A.P. and Derewenda,Z.S. (1997) Crystal structure of RhoA-GDP and its functional implications. Nat. Struct. Biol., 4, 699–703. [DOI] [PubMed] [Google Scholar]
- Wood M.W., Rosqvist,R., Mullan,P.B., Edwards,M.H. and Galyov,E.E. (1996) SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol. Microbiol., 22, 327–338. [DOI] [PubMed] [Google Scholar]
- Worthylake D.K., Rossman,K.L. and Sondek,J. (2000) Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature, 408, 682–688. [DOI] [PubMed] [Google Scholar]
- Würtele M., Wolf,E., Pederson,K.J., Buchwald,G., Ahmadian,M.R., Barbieri,J.T. and Wittinghofer,A. (2001) How the Pseudomonas aeruginosa ExoS toxin downregulates Rac. Nat. Struct. Biol., 8, 23–26. [DOI] [PubMed] [Google Scholar]
- Zhang B., Zhang,Y., Wang,Z. and Zheng,Y. (2000) The role of Mg2+ cofactor in the guanine nucleotide exchange and GTP hydrolysis reactions of Rho family GTP-binding proteins. J. Biol. Chem., 275, 25299–25307. [DOI] [PubMed] [Google Scholar]
- Zhou D, Chen,L.M., Hernandez,L., Shears,S.B. and Galán,J.E. (2001) A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrange ments and bacterial internalization. Mol. Microbiol., 39, 248–259. [DOI] [PubMed] [Google Scholar]