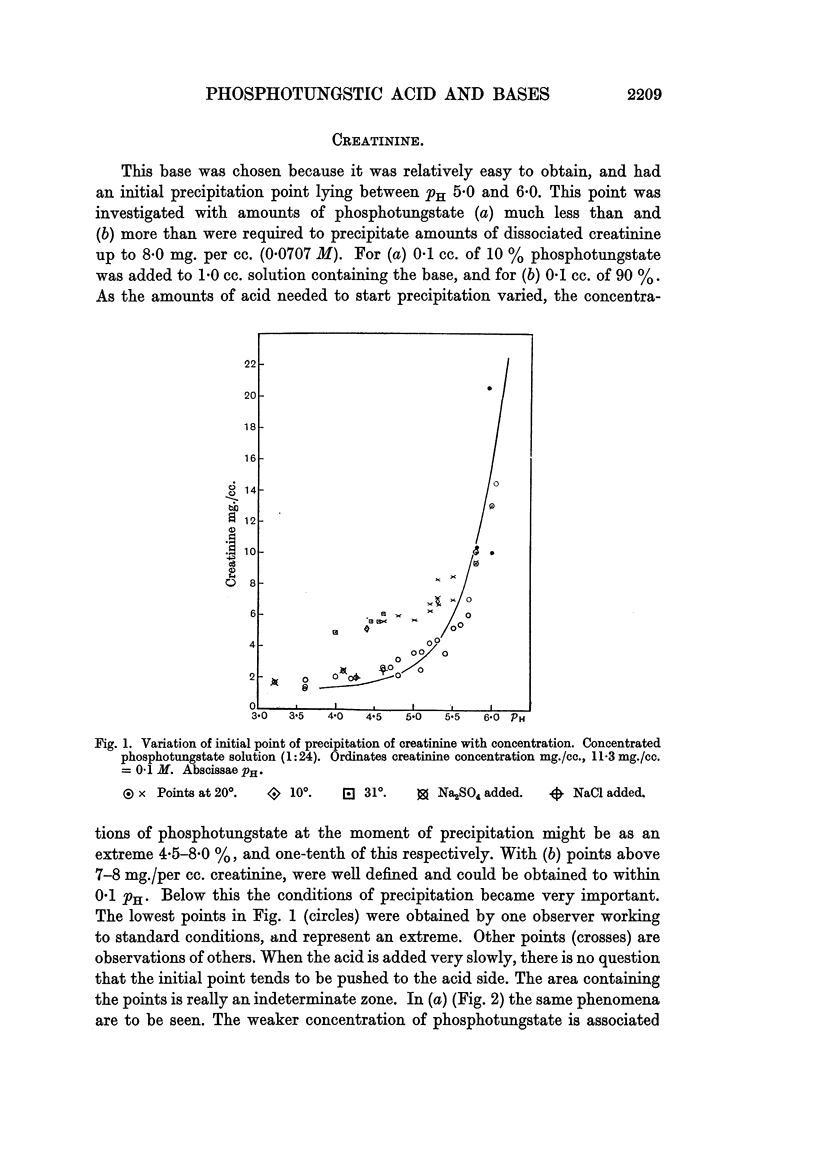
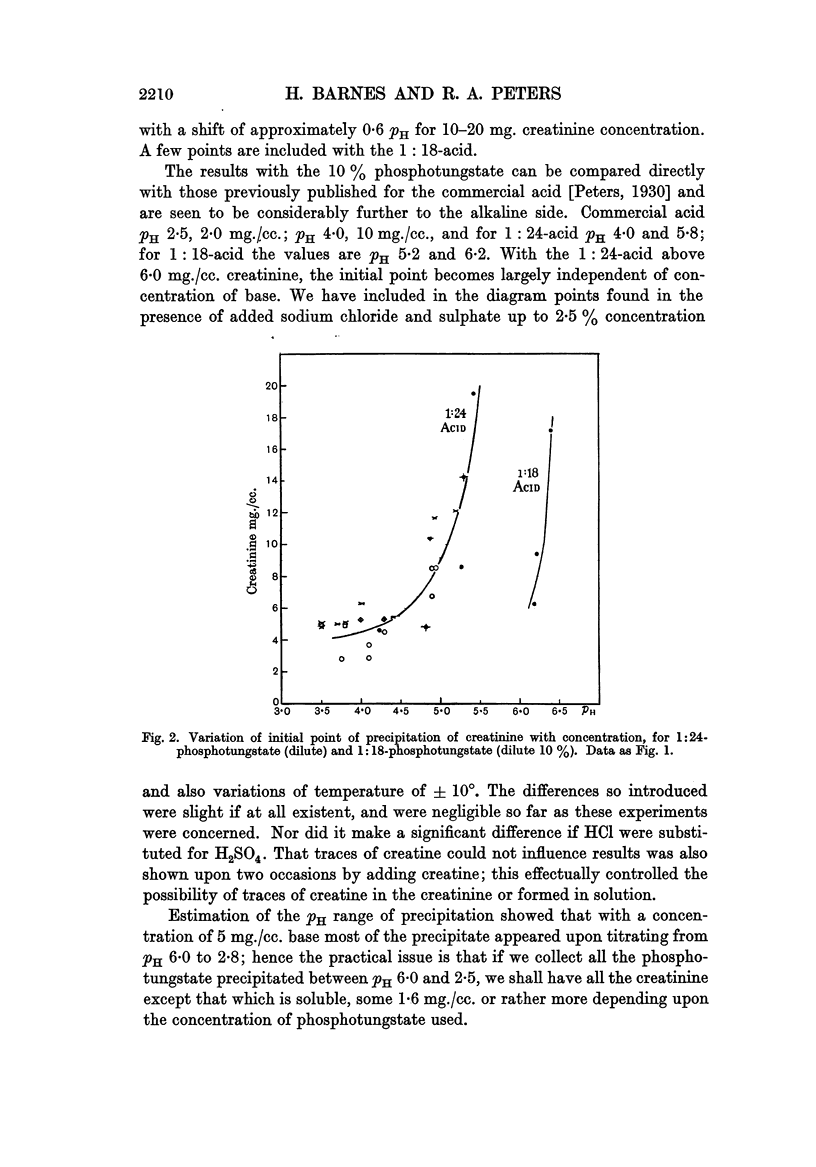
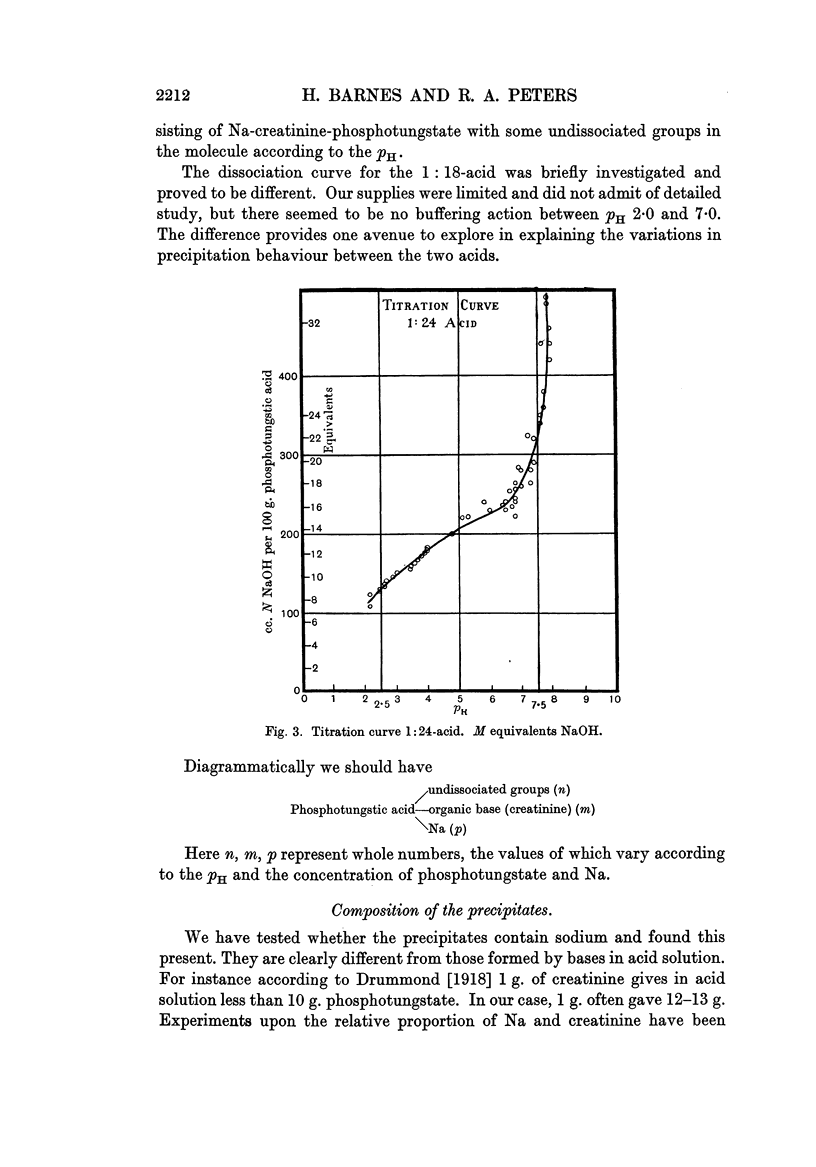
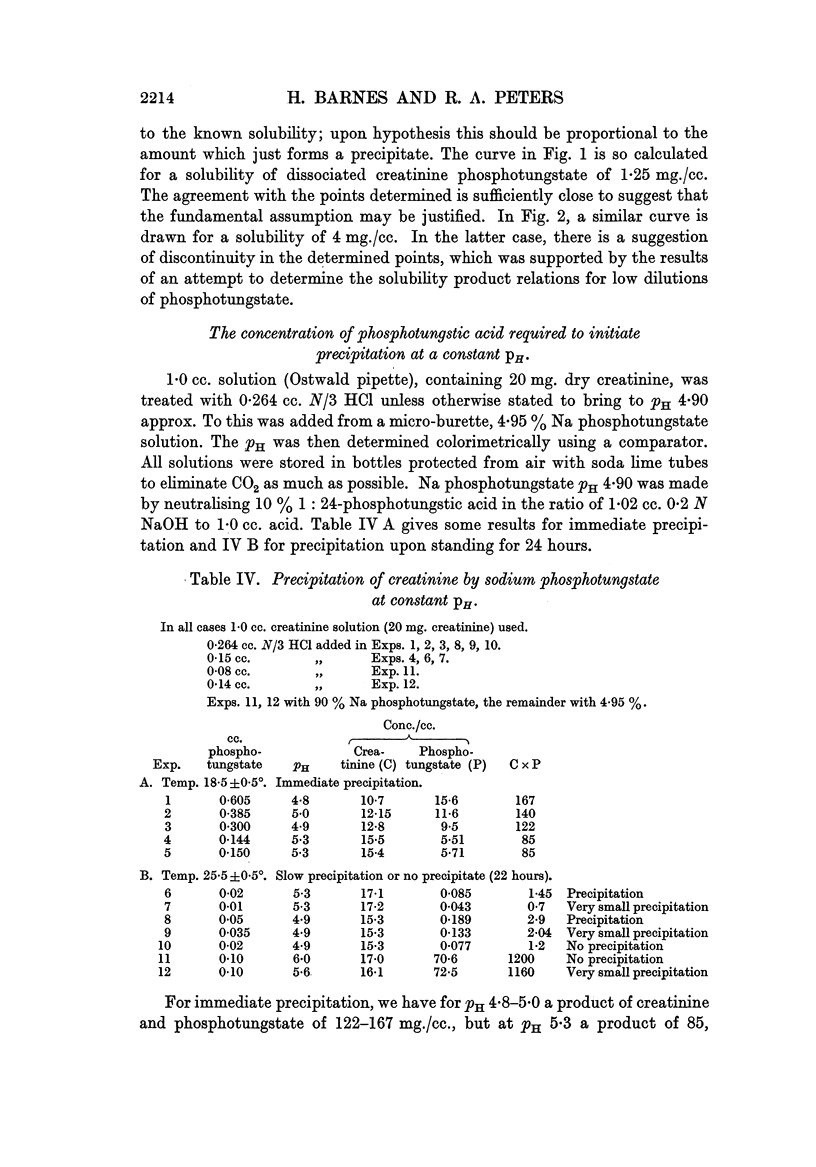
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birch T. W., Harris L. J. A redetermination of the titration dissociation constants of arginine and histidine with a demonstration of the zwitterion constitution of these molecules. Biochem J. 1930;24(2):564–575. doi: 10.1042/bj0240564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannan R. K., Shore A. The creatine-creatinine equilibrium. The apparent dissociation constants of creatine and creatinine. Biochem J. 1928;22(4):920–929. doi: 10.1042/bj0220920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond J. C. Observations on the Phosphotungstates of certain Bases and Amino-acids. Biochem J. 1918;12(1-2):5–24. doi: 10.1042/bj0120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnersley H. W., Peters R. A. Antineuritic Yeast Concentrates. I. Biochem J. 1925;19(5):820–826. doi: 10.1042/bj0190820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. A. The effect of hydrogen ion concentration upon the precipitation of certain basic substances by phosphotungstic acid. Biochem J. 1930;24(6):1852–1855. doi: 10.1042/bj0241852. [DOI] [PMC free article] [PubMed] [Google Scholar]


