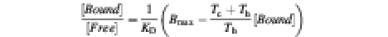Abstract
We report here the first three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor (IP3R). From cryo-electron microscopic images of purified receptors embedded in vitreous ice, a three-dimensional structure was determined by use of standard single particle reconstruction techniques. The structure is strikingly different from that of the ryanodine receptor at similar resolution despite molecular similarities between these two calcium release channels. The 24 Å resolution structure of the IP3R takes the shape of an uneven dumbbell, and is ∼170 Å tall. Its larger end is bulky, with four arms protruding laterally by ∼50 Å and, in comparison with the receptor topology, probably corresponds to the cytoplasmic domain of the receptor. The lateral dimension at the height of the protruding arms is ∼155 Å. The smaller end, whose lateral dimension is ∼100 Å, has structural features indicative of the membrane-spanning domain. A central opening in this domain, which is occluded on the cytoplasmic half, outlines a pathway for calcium flow in the open state of the channel.
Keywords: channel/cryo-electron microscopy/ IP3 receptor/single particle reconstruction
Introduction
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are ligand-gated calcium release channels for intracellular calcium stores in many eukaryotic cells (Berridge, 1993; Yoshida et al., 1997; Patel et al., 1999). They mediate a large array of calcium-regulated signal transduction events (Pozzan et al., 1994; Wilcox et al., 1998; Gailly and Colson-Van Shoor, 2001), from the secretion of granules in epithelial cells, to the control of gene expression, cell proliferation and cell death (Marks, 1997), to long-term depression in the nervous system (Inoue et al., 1998).
Three types of IP3Rs have been cloned (Furuichi et al., 1989; Mignery et al., 1989; Südhof et al., 1991; Blondel et al., 1993; Maranto, 1994), and are expressed in varying abundance in different tissues (Newton et al., 1994), including smooth muscle, cardiac myocytes (Moschella and Marks, 1993; Perez et al., 1997; Lipp et al., 2000), kidney (Blondel et al., 1993), endothelial cells (Go et al., 1995) and neurons. IP3Rs are tetramers of ∼300 kDa subunits. Hydrophobicity analysis has predicted 6–8 hydrophobic regions in each subunit, and >85% of the mass of the receptor to be located on the cytoplasmic side. Biochemical and molecular biology studies (Patel et al., 1999) have located the IP3-binding domain close to the N-terminus of the receptor subunit, and found that the long modulatory domain in the middle of the sequence contains the phosphorylation sites, splicing sites and the binding sites for various accessory proteins. Evidence has also accumulated to support the six transmembrane domain (TM) model for the receptor with a GXRXGGGXGD motif in the proposed pore loop, which contains two short hydrophobic sequences (Ramos-Franco et al., 1999; Williams et al., 2001).
Earlier negative stain electron microscopy (EM) studies of detergent-solubilized receptors purified from smooth muscle (Chadwick et al., 1990) and cerebellum (Maeda et al., 1990) revealed significant variation of the protein particles in terms of size and shape. EM images of partially ordered IP3Rs in the endoplasmic reticulum membranes of cerebellar Purkinje neurons (Katayama et al., 1996) provide an estimate of the lateral dimensions of the receptor on the cytoplasmic side of 12–16 nm. These studies failed to provide sufficient information to deduce the three-dimensional structure of the receptor.
A three-dimensional structure of the IP3R can provide a foundation for assimilating various structure–function studies into a coherent molecular image. Due to the limited quantity of receptor protein in most tissues and the large size of single receptor molecules, growth of three-dimensional crystals for X-ray diffraction or NMR studies of solubilized receptors is problematic for the structure determination of IP3R. Single particle reconstruction (SPR) from EM images of isolated receptors (Frank, 1996) can, however, be performed from picomole quantities of protein. To avoid the artifacts seen in negative stain images (Chadwick et al., 1990; Maeda et al., 1990; Frank, 1996), we chose to begin our SPR directly from cryo-EM images of the purified receptors. Thousands of images of detergent-solubilized receptors frozen in random orientations in vitreous ice were obtained by cryo-EM, and combined together by SPR to yield a 24 Å resolution structure. The structure is consistent with the predicted topology for the receptor, and predicts a possible pathway for calcium flow across the membrane.
Results
Purification and functional characterization of murine type 1 IP3R
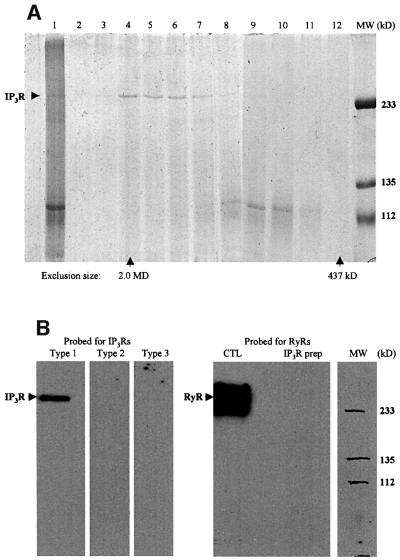
Cerebellum is the most abundant source for type 1 IP3R, making this tissue the best source for the purification of the receptors (Ferris et al., 1989; Maeda et al., 1990; Hingorani and Agnew, 1992). We purified the receptors from frozen mouse cerebellum, following the process with a standard [3H]IP3 binding assay (Thrower et al., 2000). After heparin and concanavalin A (Con A) affinity chromatography, the purified receptors were run through a size exclusion FPLC column (Figure 1A). The receptor peak eluted immediately after the 2.0 MDa dextran peak, consistent with the purified receptors being tetramers.
Fig. 1. Biochemical analysis of the purified IP3Rs. (A) SDS–PAGE of the loading sample and the FPLC fractions around the IP3R peak. The gel was stained with Coomassie Blue. In lane 1, 5.0 µg of the loading sample was applied. Lanes 2–12 correspond to the fractions from the elution volume 6.0–15 ml. A 20 µl aliquot of each fraction was used for each lane. The protein concentration in fractions corresponding to lanes 4–6 was ∼0.1 mg/ml. Fractions corresponding to these lanes were used in all other experiments. A control run of the high molecular weight standards showed that dextran 2000 came out at 7.8 ml and ferritin (437 kDa) at 14.5 ml, which correspond to lanes 4 and 12, respectively. (B) Immunoblotting of the purified IP3Rs with specific antibodies against the type 1, 2 and 3 IP3Rs and an antibody against RyRs (both types 1 and 2). The positive control for the type 2 IP3R antibody was microsomes made from mouse cardiac muscle, and that for the type 3 IP3R antibody was made from renal cells (data not shown). The positive control (CTL) for the RyR antibody was the microsomes made from mouse skeletal muscle.
To check whether it contained any type 2 or 3 IP3Rs or ryanodine receptors (RyRs), the receptor preparation was probed with antibodies specific for the various types of IP3Rs and RyRs (Figure 1B). Any such contamination was below the detection limit. In addition, the immunoblotting also demonstrated that the purified receptors suffered no detectable degradation during purification.
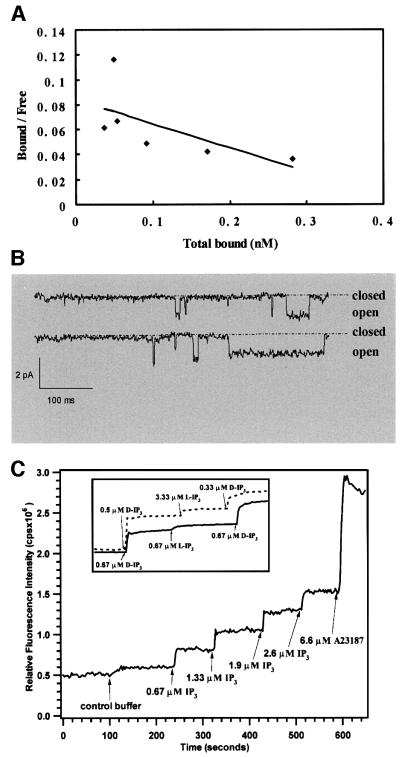
Functional assays showed that the purified receptors have properties characteristic of native IP3Rs. The IP3-binding affinity of the purified receptors was found to be ∼40 nM (Figure 2A). The apparent specific activity was 100–150 pmol/mg, consistent with previous studies employing this assay (Chadwick et al., 1990; Thrower et al., 2000). Single channel recordings (Figure 2B) showed that once reconstituted into lipid bilayers, the purified receptors presented the expected IP3-gated channel activity with ∼85 pS Ba2+ conductance, 4% maximum open probability and block by heparin. To verify their functional integrity further, the purified receptors were reconstituted into calcium-loaded lipid vesicles, and IP3-specific calcium efflux (Figure 2C) was measured by use of a Ca2+-sensitive dye. The results suggested that the majority of the receptors in vesicles could form functional calcium release channels. Moreover, as illustrated in the inset to Figure 2C, pharmacological identity of the receptors is satisfied by stereo-specific response to the d but not the l isoform of IP3.
Fig. 2. Functional assay of the purified IP3Rs. (A) [3H]IP3 binding from a competition assay. Shown here is the Scatchard plot, which was fitted with an equation for the ratio of bound to free [3H]IP3:
which yielded a KD of 40 nM. Here, Tc is the total cold IP3 and Th the total [3H]IP3. The term, ((Tc + Th)/Th)[Bound] expresses the total bound IP3. (B) Single channel recordings from reconstituted IP3R in lipid bilayers. The channel activity was recorded in the presence of 2.0 µM IP3 in the cis-side. The single channel conductance (Ba2+) was 83 pS, and the maximum open probability ∼4%. (C) Calcium efflux from IP3R vesicles as monitored with Indo-1. Stepwise addition of IP3 resulted in Ca2+ release from vesicles up to a 50% maximum. The maximum Ca2+ available for efflux is shown by addition of the ionophore, calcimycin A23187. The inset represents a comparison between cerebellar microsomes (dashed line) and reconstituted IP3R vesicles (solid line) when challenged with either d- or l-IP3. The slight rise with l-IP3 addition is consistent with the presence of contaminating Ca2+.
Cryo-EM images and three-dimensional reconstruction
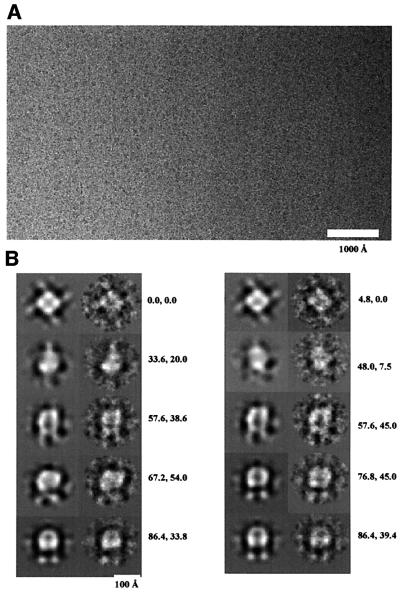
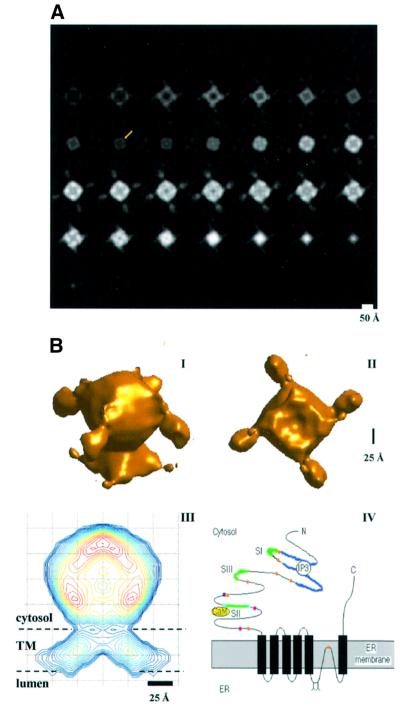
Isolated IP3R particles embedded in vitreous ice were imaged while being kept at liquid nitrogen temperature. Figure 3A shows some of the particle images. The receptor particles are regular in size (∼150 Å), and isolated from each other. About 4500 particle images were selected interactively from digitized micrographs. The image processing was performed with the software package EMAN (Ludtke et al., 1999). A subset of 400 particle images was first used to generate a starting model. This was then refined against the remaining particle images by iterative cycles of reprojection from the model, multireference alignment, classification and three-dimensional reconstruction. After 10 iterations, a stably converged model was achieved. Reprojections from the converged model match very well with the class averages at the same orientations (Figure 3B). As a test for robustness of the three-dimensional reconstruction, four different starting models were used for the refinement; in each case, convergence to the same three-dimensional structure was achieved.
Fig. 3. Cryo-EM images of the IP3Rs and image processing. (A) A field of IP3R particles, here visible as dark spots of ∼150 Å diameter. The image was taken at 1.2 µm defocus, 42 000× with a Tecnai 12 microscope operated at 120 kV. The electron dose for each exposure was ∼10 e/Å2. (B) Projections of the converged model before CTF correction (left in each pair) compared with the corresponding class averages of the raw data (right in the pair). The orientation angles shown were sampled from the asymmetrical triangle. The two orientation angles (β and γ) for each pair are shown next to them. The good match shows that the model is consistent with the data set.
Correction for the contrast transfer function in the model was performed through Wiener filtering (Hawkes, 1980; Grigorieff, 1998). Sections across the resultant density map in planes normal to its 4-fold symmetry axis (C4 axis) are shown in Figure 4A. Based on the typical protein density (0.81 Da/Å3), a threshold density value was determined to account for the 1.2 MDa mass for each IP3R particle. The surface rendering of this model in two different orientations is shown in Figure 4B. The lateral dimensions of the structure vary along the C4 axis, one end smaller than the other, rendering the appearance of an uneven dumbbell. In Figure 4A, the first five slices from the bottom of the structure show an opening enclosed in the center, which corresponds to the deep indentation on the bottom of the small end as shown in Figure 4B (panel II).
Fig. 4. The three-dimensional structure of IP3R and its predicted orientation in the membrane. (A) Horizontal slices across the IP3R model normal to the 4-fold symmetry axis and running from the small end to the bulky end. Each slice is 5.0 Å thick. The height of the receptor structure is ∼170 Å. In one of the slices of the waist region, a yellow arrow points to one of the pillars, which form the connections between the transmembrane domain and the cytoplasmic domain. (B) Panels I and II give the surface presentation of the three-dimensional structure of IP3R from two viewing angles: from the upper lateral side (I) and from the bottom along its C4 axis (II). Assuming the average protein density of 1.35 g/cm3, a threshold value for the converged model after CTF correction was determined to give the estimated molecular mass of 1.21 MDa and was defined as 1.0δ. This threshold defined the surface shown. The small and large ends of the particle are joined at the narrowest part of the structure, the waist. The large end has four arms protruding laterally by ∼50 Å. The bottom of the small end (II) shows an indentation (∼25 Å deep) in the center. In panels III and IV, a sagittal slice across the model, along the C4 axis and between two neighboring protruding arms, is compared with the predicted topology of the IP3R subunit in the membrane. The contour lines are set at relative densities of 1, 1.5, 2.0, 2.5, 3–18 and 18.8δ, and are colored across the spectrum from blue (1.0δ) to red (18.8δ). The large end is assumed to be the cytoplasmic domain and the small end the membrane-spanning domain. Such an arrangement is consistent with the predicted topology of the receptor (panel IV). The six transmembrane domain model is assumed, and the pore loop (P-loop) contains two short hydrophobic sequences. The ligand-binding domain (purple) is presented as being occupied by an IP3 molecule. Also shown are the calcium-binding sites (orange circles), the three splicing sites (SI, SII and SIII, green lines), the calmodulin (CaM) anchoring site, the phosphorylation sites (cyan circles) and the two N-glycosylation sites (Y).
Discussion
In this study, we present, for the first time, the three-dimensional structure of the type 1 IP3R. The structure was obtained from images of purified receptors, which were also shown to be functional by IP3 binding, single channel recording and IP3-specific Ca efflux assays. The IP3R structure is significantly different from the 22 Å resolution structure of the RyR (Serysheva et al., 1995, 1999; Orlova et al., 1996), the other type of intracellular calcium release channel. At the current resolution (24 Å), the structure not only reveals the molecular morphology of the receptor, but also defines the spatial arrangement of its subdomains. Previously, only two-dimensional projection images of the receptor were available by negative stain and freeze etching EM (Chadwick et al., 1990; Maeda et al., 1990; Katayama, 1996), giving limited information about the receptor shape and size. By using cryo-EM, we have overcome the limitations of these former studies. The receptor images we obtained were regular in size and well preserved in shape, allowing a reliable three-dimensional structure to be generated from SPR.
The IP3R channel takes the shape of an uneven dumbbell with one end significantly larger than the other (Figure 4B, panels I and II). The purified receptor has a very low open probability, only 4% when maximally activated (Thrower et al., 2002). Considering the fact that the receptors were imaged in the absence of agonists, we assume that the structure reflects the IP3R in the closed state. Given the known topology of the receptor, the small end probably presents the transmembrane domain (TM; (panels III and IV in Figure 4B), and the large end the cytoplasmic domain (CD). The two domains join at a low density region (called the waist hereafter; Figure 4A). Such assignment gives a 14% density ratio of the TM to the whole structure; this is consistent with the ∼11% mass ratio of the TM to the whole receptor estimated from the primary sequence and the predicted topology. The TM has an opening to the bottom, i.e. the luminal side, which tapers to ∼20 Å in diameter at its half height along its C4 axis, and is completely occluded at its cytoplasmic half. The occlusion of the opening in the TM represents the block of a putative central pathway for ion flux. Even though the gate and selectivity filter of the channel are not visible at the current resolution, consideration of the occlusion of the ion flux by the closed gate and the allowable size of the central opening in the selectivity filter suggests their locations in the cytoplasmic half of the TM. This contrasts with the proposed pore region architecture modeled after the KcsA potassium channel by Williams et al. (2001). Crossing the membrane from the luminal side, the TM density is tilted ∼35–45° towards the C4 axis (Figure 4B, panel III), making the lateral dimension of the TM at the luminal surface almost 2-fold that on the cytoplasmic surface. The dimensions of the TM, both laterally and vertically, are similar to those of the membrane-integral portions of the voltage-gated sodium and potassium channels (Sato et al., 1998; Sokolova et al., 2001).
The density in the waist region (Figure 4A) is low except at four high-density pillars. These pillars correspond to the major connections between the CD and the TM of the tetrameric receptor. The crevices between the pillars (the yellow arrow in Figure 4A pointing to one pillar) might allow ions to flow, reminiscent of the lateral flow pathways suggested by the four lateral ‘windows’ between the T1 and membrane-integral portion of the Shaker potassium channel (Kobertz et al., 2000; Sokolova et al., 2001). Furthermore, the channel gate rests between the waist and the central opening in the luminal half of the TM, an ideal position to control ion flow.
The CD resembles a bulb with four small arms protruding laterally by ∼50 Å (Figure 4B, panels I and II). The four arms make the projection views of the receptor along the C4 axis similar to one of the views observed in an earlier negative stain EM study (Chadwick et al., 1990). The dimensions of this domain are consistent with estimates from a quick-freeze deep-etch EM study of bovine cerebellar IP3Rs (Katayama et al., 1996). Currently, there is no function associated with the four protruding arms, although they could be hypothesized as regulatory domains or specific ligand-binding sites.
The various domains of the IP3R structure fit well with the predicted topology of the IP3R subunits (Figure 4B, panel IV; Michikawa et al., 1994; Ramos-Franco et al., 1999). The six TM model puts the N- and C-termini of the receptor on the cytoplasmic side, and a long loop (106 amino acid residues, including two short hydrophobic sequences and two N-glycosylation sites) between the fifth and sixth transmembrane segments on the luminal side. A short sequence of this long loop was proposed to be the pore loop (Ramos-Franco et al., 1999; Williams, 2001). This arrangement of the total mass for each subunit of a receptor conforms well to the electron density distribution of the IP3R structure.
The shape of the TM in the IP3R structure leads us to believe that the structure for the channel portion of the IP3R is distinct from that of the RyR (Serysheva et al., 1995, 1999; Orlova et al., 1996). Despite the conserved GXRXGGGXGD motif, the sequence of the pore loop of the IP3R is markedly different from that of the RyR. In three-dimensional reconstructions, the channel portion of the RyR in the closed state has no apparent opening on the luminal side, although its lateral dimension at the luminal side (∼120 Å) is close to that of the IP3R (Orlova et al., 1996; Samsó and Wagenknecht, 1998). The transmembrane density of the RyR structure tapers slightly towards the luminal side, while the opposite is true for that of the IP3R. The different topologies proposed for the RyR (four TMs) and the IP3R (six TMs) may be the basis for the structural differences. Spherical reconstruction (Jiang et al., 2001) has the advantage of resolving the TMs of membrane proteins in a lipid environment, and may be applicable in the future for resolving the discrepancy between these two closely related calcium release channels.
The present depiction of the IP3R structure will be valuable to the field of calcium signaling as a template for future structure–function studies of this important channel. It forms the basis for improving the structure to a better resolution, and sets the stage for investigating the structural alterations following the binding of specific ligands and various accessory proteins by tracing the resultant global conformational changes of the receptor complexes. For example, chromogranins bind to the luminal face of the receptor, dramatically increasing its open probability in the presence of IP3 (Thrower et al., 2002), and may allow us to observe the receptor channel in its open state. The structure of the type 1 IP3R probably represents a general model for all three types of IP3Rs since they have 60–70% homology in amino acid sequence (Südhof et al., 1991), and share the same topology according to hydrophobicity analysis.
Materials and methods
Purification of type 1 IP3Rs from mouse cerebellum
The preparation followed published procedures with many minor modifications (Ferris et al., 1989; Hingorani and Agnew, 1992; Thrower et al., 2000). Special care was taken to minimize the degradation of the receptors during the purification by making the procedure as short as possible and using protease inhibitors at every stage. Briefly, 30 frozen mice cerebella (on average ∼2.3 g; Pel Freez Biologicals, Rogers, AK) were used for each preparation. The cerebella were put into 30 ml of cold buffer A (50 mM Tris–HCl pH 8.0, 1.0 mM EGTA and 1.0 mM β-mercaptoethanol supplemented with protease inhibitors). The tissue was homogenized manually by 15 strokes in a 40 ml Knotes glass homogenizer. The homogenate was then centrifuged at 100 000 g (SW28 rotor in a Beckman L8-70M ultracentrifuge) for 30 min. The pellet was homogenized again in buffer A, yielding the microsome preparation, and the final volume was adjusted to 70 ml with buffer A. The microsomes used for calcium flux experiments (Figure 2C) were prepared in EGTA-free buffer A plus 1.0 mM Ca2+.
For detergent extraction, CHAPS was added at 1.2% to the microsome preparation. The extract mixture was incubated for 25 min with intermittent inversions, and subsequently was centrifuged at 45 000 g (SS34 rotor in a Sorvall RC 5 plus centrifuge) for 10 min. The supernatant contained the solubilized receptors, and was combined and incubated with 10 ml of heparin–agarose beads (Sigma) for 15 min with end-over-end rotation. Thereafter, the beads were collected, washed with 50 ml of buffer B (buffer A + 0.25 M NaCl + 1.0% CHAPS), and then eluted with 10 ml of buffer C (buffer A + 0.6 M NaCl + 0.5% CHAPS). The eluate was collected and incubated with 1.0 ml of Con A–Sepharose beads (Sigma) for 1.5 h. Finally, the beads were collected, washed with 10 ml of buffer D (buffer A + 0.5% CHAPS + 1.0 mM Ca2+ + 1.0 mM Mg2+) and eluted with 8 ml of buffer E (buffer A + 0.5% CHAPS + 1.0 M methyl-α-d-mannopyranoside + 4.0 mM EGTA).
To remove small size impurities, the preparation was concentrated to 2.0 mg/ml in a Vivaspin G-100 concentrator (Vivascience, Binbrook, Lincoln, UK), and then injected into a Superose 6 HR10/30 FPLC gel filtration column in an ÄKTA system (Amersham Pharmacia Biotech Inc., Piscataway, NJ), and eluted at a flow rate of 0.3 ml/min with buffer G (0.4% CHAPS, 5 mM Tris–HCl pH 8.0, 50 mM NaCl, 50 mM KCl, 1.0 mM EGTA and protease inhibitors). The IP3R peak eluted as the first peak at 8.1 ml (Figure 1A).
Reconstitution of IP3Rs into lipid vesicles
Small unilamellar vesicles (SUVs) of egg phosphatidylcholine (PC; Avanti Polar Lipids, Alabaster, AL) were prepared in dialysis buffer (10 mM Tris–HCl pH 8.0, 50 mM NaCl, 50 mM KCl, 1.0 mM EGTA, 10 µM protease inhibitors). Purified receptors were concentrated to 0.4–0.5 mg/ml, and washed once with 2.0 ml of buffer G. The concentrated receptors were then mixed with a suspension of SUVs (1.0 mg/ml lipids) in equal volume. The solubilization of egg PC SUVs by CHAPS was characterized as described in Rigaud et al. (1995). The mixture was stirred for 30 min, and then loaded into a piece of pre-cleaned membrane tubing (10 mm wide, molecular weight cut-off 12 000–14 000, Spectrum Laboratories, Inc., Rancho Dominguez, CA), and dialyzed against 2000 vols of dialysis buffer for 24 h with two buffer changes in the middle. The vesicles were collected. Nycodenz (Sigma) was added thereafter to 15% in the vesicle suspension. The mixture was loaded into a centrifuge tube and covered with a small volume (∼50 µl) of the dialysis buffer. Centrifugation at 200 000 g for 2 h (SW55S rotor in a Sorvall M150GX, Kendro Laboratory Products, Newtown, CT) concentrated the vesicles to the top buffer layer, leaving the non-incorporated IP3Rs in the bottom. The vesicles were collected and used for bilayer recording. For calcium flux assay, the vesicles were prepared in the same way except that the dialysis buffer contained 1.0 mM Ca2+ and no EGTA.
Characterization of IP3Rs
SDS–PAGE/immunoblotting. Gel analysis of the receptors was performed in a standard way (Bollag et al., 1996). For IP3Rs and RyRs, a 7% resolution gel with a 3% stacking gel was used. For western blots, the protein was transferred from the gel to a sheet of Millipore Immobilon-P transfer membrane (Bedford, MA) in a mini-Trans-Blot cell (Bio-Rad, Hercules, CA). The membrane was then blocked overnight with 5% non-fat dry milk in buffer TBS-T (150 mM NaCl, 10 mM Tris–HCl pH 7.4 and 0.1% Tween-20). For type 1 IP3R, the primary antibody was a laboratory-made, rabbit anti-mouse monoclonal directed against the C-terminal 20 amino acid residues of the receptor, and was incubated with the membrane for 1 h in TBS-T + 0.5% milk. The membrane was then washed and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Amersham Bio Sciences, Piscataway, NJ) in the same buffer. The final detection of HRP was performed with the Pierce ECL plus kit (Rockford, IL). Polycolonal rabbit anti-mouse antibody against type 2 IP3R was purchased from Chemicon International (Temecula, CA). Type 3 IP3Rs were probed with a mouse monoclonal antibody (Transduction Laboratories, Lexington, UK). The mouse monoclonal antibody for RyRs (types 1 and 2) was obtained from Affinity BioReagents (Golden, CO). An HRP-conjugated horse anti-mouse IgG (Amersham) was used for detection of the mouse antibodies.
[3H]IP3 binding assay. The [3H]IP3 binding assay was performed according to the standard polyethylene glycol (PEG) precipitation procedure (Thrower et al., 2000). Briefly, the solubilized receptors or the reconstituted IP3R vesicles were incubated with [3H]IP3 for 15 min in the presence or absence of 1.0 µM IP3. Then 0.5% γ-globulin (Sigma) and 30% PEG 8000 were added in sequence to precipitate the IP3Rs with the bound IP3. The mixture was centrifuged at 14 000 g for 5 min. The pellet was rinsed once, resuspended and counted in a scintillation counter. For the competition test, different concentrations of cold IP3 were used.
Bilayer recordings. A Teflon chamber partitioned by a thin Teflon film with a small hole (∼100 µm) in the middle was used to make the recordings. To form a bilayer, the hole in the Teflon film was pre-painted with a decane solution of 1,2-diphytanoyl-sn-glycero-3-PC/1,2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS) in 6:1 weight ratio. Once the solvent dried, the two sides were filled with buffer solutions. The cis-side buffer contained 250 mM HEPES–Tris pH 7.35 plus 0.5 mM EGTA, and the trans-side had 250 mM HEPES–Tris pH 5.5 plus 53 mM Ba2+. The hole was painted again with a decane solution of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE)/DOPS in 6:1 weight ratio. Once a bilayer was formed, reconstituted IP3R vesicles were added into the cis chamber and mixed with stirring. Normally, within 5 min, insertion of the receptor into the bilayer would occur. The channel activity was recorded at 0 mV with 2 µM IP3 added to the cis-side.
Calcium efflux from IP3R vesicles. Ca2+-loaded vesicles or microsomes were prepared as stated above. Membrane-impermeant Ca2+-sensitive fluorescent dye, Indo-1 (Molecular probes, Eugene, OR), was prepared in calcium-free water, as were IP3 isoforms used in these experiments. Time-based fluorescence measurement was performed in a SPEX FluoroMax-3 spectrofluorometer (Jobin Yvon Inc., Edison, NJ) with a temperature control unit (Wavelength Electronics Inc., Bozeman, MT) to keep the cuvette at 10°C, which minimizes non-specific calcium leak. For each run, 40 µl of Ca2+-loaded vesicles were quickly run through a 1.0 ml G-50 spin column equilibrated in the assay buffer (40 mM HEPES, 100 mM NaCl, 5 mM KCl pH 7.6, 8 g/100 ml Chelex-100) to remove all Ca2+ outside the vesicles. The vesicles were then added into the assay buffer containing 3.3 µM Indo-1 to start the experiment. At specific moments, agonists or antagonists were added into the cuvette, and time-based changes in the Indo-1 fluorescence intensity were recorded.
Cryo-EM imaging and three-dimensional reconstruction
Cryo-electron microscopy. Freezing of the cryo-EM samples was performed as described by Jiang et al. (2001) except that the Quantifoil grids (Quantifoil Micro Tools GmbH, Jena, Germany) with 1.2–2.0 µm holes were used. Onto the perforated carbon film of these grids was laid a thin (∼10 nm) carbon film. Solubilized, purified IP3Rs were concentrated to ∼1.5 mg/ml in buffer G, and 3 µl were loaded onto the carbon film of a grid, blotted in a 4°C atmosphere and then frozen in liquid ethane. Grids were transferred under liquid nitrogen to a Gatan 626 cryo-holder (Gatan Inc., Pleasanton, CA), and observed under a Tecnai 12 electron microscope with a LaB6 filament operated at 120 kV. Images were collected at 40 000×, 1.2 µm defocus by use of the low-dose mode. Micrographs were screened using an optical diffractometer. Good negatives were scanned at 7.0 µm/pixel in a Zeiss SCAI scanner (Z/I imaging, Huntsville, AL), and subsequently were averaged to yield 5 Å/pixel at the object level.
Single particle reconstruction. IP3R particles were selected interactively using the software package of EMAN (Ludtke et al., 1999) or Web (Frank et al., 1995). Before any processing, phase flipping was performed for each particle image using CTFIT in EMAN. The images were also band-pass filtered. A subgroup of 400 particles were center-aligned against their total sum, and then classified iteratively by multivariate statistical analysis and aligned by multireference alignment to generate stable class averages. Two class averages with contrastingly different symmetry properties (Ludtke, 1999; EMAN documentation at http://ncmi.bcm.tmc. edu/∼stevel/EMAN/doc/index.html) were used to construct an initial model (model A) of 4-fold symmetry in the MATLAB software environment (Mathworks, Natick, MA). This model was refined iteratively against another group of 4100 particles to give the 24 Å resolution structure. The refinement followed the standard procedure. The model was projected first in 190 different orientations in the asymmetrical triangle to generate a reprojection set. Each raw image was then compared with the reprojection set by multireference alignment to find the best orientation angles. The raw images with the same orientation were grouped together as a class. For each class, a classification was employed to find outliers (10–20%); a new class average was then generated from the majority of the class members. The class averages from all orientation angles were combined to generate a new three-dimensional model by direct Fourier space reconstruction (Ludtke et al., 1999). The new model was then used as the starting model for the next iteration. The final model converged very well after 8–10 iterations. The effective resolution was estimated by the 0.5 criterion for the Fourier shell correlation (FSC; van Heel, 1987; Böttcher, 1997; Zhou et al., 2001) between two models reconstructed from two halves of the data set (Frank, 1996).
To test the robustness of the model, the refinement was repeated with four different initial models. The first one (model B) was obtained by filling the center of model A with the maximum density. The second model (model C) was derived by introducing a helical twist into model B, along the C4 axis by 1° per 5 Å slice. The structure of Figure 4 after a thresholding operation using a three times higher threshold than that used for Figure 4B became the third starting model (model D). Finally, model D was twisted helically along its C4 axis, again by 1° per slice, to give model E. These four initial models were refined against the same data set and were found to converge to the same structure. Also from the same data set, classification and alignment analysis using the alternative software package IMAGIC (van Heel et al., 1996) yielded similar results. With these tests, we believe that the structure in Figure 4A is an unbiased representation of the data set.
Acknowledgments
Acknowledgements
We are grateful to Ms Brenda DeGray for technical assistance and to Ms Hulda Michel for picking particles. The results in this work are based on a dissertation (Q.-X.J.) submitted to fulfill in part the requirements for the degree of Doctor of Philosophy at Yale University. This work was supported by grants from the National Institutes of Health to B.E.E (GM63496) and F.J.S. (NS21501).
References
- Berridge M.J. (1993) Inositol trisphosphate and calcium signaling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda,J., Janssen,H., Seino,S. and Bell,G.I. (1993) Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract and other tissues. J. Biol. Chem., 268, 11356–11363. [PubMed] [Google Scholar]
- Bollag D.M., Rozycki,M.D. and Edelstein,S.J. (1996) Protein Methods, 2nd edn. Wiley-Liss Inc., New York, NY.
- Böttcher B., Wynne,S.A. and Crowther,R.A. (1997) Determination of the fold of the core protein of hepatitis B virus by electron cryo microscopy. Nature, 386, 88–91. [DOI] [PubMed] [Google Scholar]
- Chadwick C.C., Saito,A. and Fleischer,S. (1990) Isolation and characterization of the inositol trisphosphate receptor from smooth muscle. Proc. Natl Acad. Sci. USA, 87, 2132–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C.D., Huganir,R.L., Supattapone,S. and Snyder,S.H. (1989) Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature, 342, 87–89. [DOI] [PubMed] [Google Scholar]
- Frank J. (1996) Three-dimensional Electron Microscopy of Macro molecular Assemblies. Academic Press, San Diego, CA.
- Frank J., Radermacher,M., Penczek,P., Zhu,J., Li,Y., Ladjadj,M. and Leith,A. (1995) SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol., 116, 190–199. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa,S., Miyawaki,A., Wada,K., Maeda,N. and Mikoshiba,K. (1989) Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature, 342, 32–38. [DOI] [PubMed] [Google Scholar]
- Gailly P. and Colson-Van Schoor,M. (2001) Involvement of trp-2 protein in store operated influx of calcium in fibroblasts. Cell Calcium, 30, 157–165. [DOI] [PubMed] [Google Scholar]
- Go L.O., Moschella,M.C., Watras,J., Handa,K.K., Fyfe,B.S. and Marks,A.R. (1995) Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J. Clin. Invest., 95, 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorieff N. (1998) Three-dimensional structure of bovine NADH: ubiquinone oxidoreductase (complex I) at 22 Å in ice. J. Mol. Biol., 277, 1033–1046. [DOI] [PubMed] [Google Scholar]
- Hawkes P.W. (1980) Image processing based on the linear theory of image formation. In Hawkes,P.W. (ed.), Computer Processing of Electron Microscope Images. Springer-Verlag, Berlin, Germany, pp. 1–33.
- Hingorani S.R. and Agnew,W.S. (1992) Assay and purification of neuronal receptors for inositol 1,4,5-trisphosphate. Methods Enzymol., 207, 573–591. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kato,K., Kohda,K. and Mikoshiba,K. (1998) Type I inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J. Neurosci., 18, 5366–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q.-X., Chester,D.W. and Sigworth,F.J. (2001) Spherical reconstruction: a method for structure determination of membrane proteins from cryo-EM images. J. Struct. Biol., 133, 119–131. [DOI] [PubMed] [Google Scholar]
- Katayama E., Funahashi,J., Michikawa,T., Shiraishi,T., Ikemoto,T., Iino,M., Hirosawa,K. and Mikoshiba,K. (1996) Native structure and arrangement of inositol-1,4,5-trisphosphate receptor molecules in bovine cerebellar Purkinje cells as studied by quick-freeze deep-etch electron microscopy. EMBO J., 15, 4844–4851. [PMC free article] [PubMed] [Google Scholar]
- Kobertz W.R., Williams,C. and Miller,C. (2000) Hanging gondola structure of the T1 domain in a voltage-gated K+ channel. Biochemistry, 39, 10347–10352. [DOI] [PubMed] [Google Scholar]
- Lipp P., Lain,M., Tovey,S.C., Burrell,K.M., Berridge,M.J., Li,W. and Bootman,M.D. (2000) Functional InsP3 receptors that may modulate excitation–contraction coupling in the heart. Curr. Biol., 10, 939–942. [DOI] [PubMed] [Google Scholar]
- Ludtke S.J., Baldwin,P.R. and Chiu,W. (1999) EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol., 128, 82–97. [DOI] [PubMed] [Google Scholar]
- MacKrill J.J. (1999) Protein–protein interactions in intracellular Ca2+-release channel function. Biochem. J., 337, 345–361. [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Niinobe,M. and Mikoshiba,K. (1990) A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J., 9, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto A.R. (1994) Primary structure, ligand binding and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J. Biol. Chem., 269, 1222–1230. [PubMed] [Google Scholar]
- Marks A.R. (1997) Intracellular calcium-release channels: regulators of cell life and death. Am. J. Physiol., 272, H597–H605. [DOI] [PubMed] [Google Scholar]
- Michikawa T., Hamanaka,H., Otsu,H., Yamamoto,A., Miyawaki,A., Furuichi,T., Tashiro,Y. and Mikoshiba,K. (1994) Transmembrane topology and sites of N-glycosylation of inositol 1,4,5-trisphosphate receptor. J. Biol. Chem., 269, 9184–9189. [PubMed] [Google Scholar]
- Mignery G.A., Südhof,T.C., Takei,K. and de Camilli,P. (1989) Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature, 342, 192–195. [DOI] [PubMed] [Google Scholar]
- Moschella M.C. and Marks,A.R. (1993) Inositol 1,4,5-trisphosphate receptor expression in cardiac myocytes. J. Cell Biol., 120, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C.L., Mignery,G.A. and Südhof,T.C. (1994) Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J. Biol. Chem., 269, 28613–28619. [PubMed] [Google Scholar]
- Orlova E.V., Serysheva,I.I., van Heel,M., Hamilton,S.L. and Chiu,W. (1996) Two structural configurations of the skeletal muscle calcium release channel. Nat. Struct. Biol., 3, 547–552. [DOI] [PubMed] [Google Scholar]
- Patel S., Joseph,S.K. and Thomas,A.P. (1999) Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium, 25, 247–264. [DOI] [PubMed] [Google Scholar]
- Perez P.J., Ramos-Franco,J., Fill,M. and Mignery,M.A. (1997) Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J. Biol. Chem., 272, 23961–23969. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto,R., Volpe,P. and Meldolesi,J. (1994) Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev., 74, 595–636. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco J., Galvan,D., Mignery,G.A. and Fill,M. (1999) Location of the permeation pathway in the recombinant type 1 inositol 1,4,5-trisphosphate receptor. J. Gen. Physiol., 114, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud J.-L., Pitard,B. and Levy,D. (1995) Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta, 1231, 223–246. [DOI] [PubMed] [Google Scholar]
- Samsò M. and Wagenknecht,T. (1998) Contributions of electron microscopy and single-particle techniques to the determination of the ryanodine receptor three-dimensional structure. J. Struct. Biol., 121, 172–180. [DOI] [PubMed] [Google Scholar]
- Sato C., Sato,M., Iwasaki,A., Doi,T. and Engel,A. (1998) The sodium channel has four domains surrounding a central pore. J. Struct. Biol., 121, 314–325. [DOI] [PubMed] [Google Scholar]
- Serysheva I.I., Orlova,E.V., Chiu,W., Sherman,M.B., Hamilton,S.L. and van Heel,M. (1995) Electron cryomicroscopy and angular recon stitution used to visualize the skeletal muscle calcium release channel. Nat. Struct. Biol., 2, 18–24. [DOI] [PubMed] [Google Scholar]
- Serysheva I.I., Schatz,M., van Heel,M., Chiu,W. and Hamilton,S.L. (1999) Structure of the skeletal muscle calcium release channel activated with Ca2+ and AMP-PCP. Biophys. J., 77, 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova O., Kolmakova-Partensky,L. and Grigorieff,N. (2001) Three-dimensional structure of a voltage-gated potassium channel at 2.5 nm resolution. Structure, 9, 215–220. [DOI] [PubMed] [Google Scholar]
- Südhof T.C., Newton,C.L., Archer,B.T.,III, Ushkaryov,Y.A. and Mignery,G.A. (1991) Structure of a novel InsP3 receptor. EMBO J., 10, 3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower E.C., Mobasheri,H., Dargan,S., Marius,P., Lea,E.J.A. and Dawson,A. (2000) Interaction of luminal calcium and cytosolic ATP in the control of type 1 inositol(1,4,5)-trisphosphate receptor channels. J. Biol. Chem., 275, 36049–36055. [DOI] [PubMed] [Google Scholar]
- Thrower E.C., Park,H.Y., So,S.H., Yoo,S.H. and Ehrlich,B.E. (2002) Activation of the inositol 1,4,5-trisphosphate receptor by the calcium storage protein chromogranin A. J. Biol. Chem., 277, 15801–15806. [DOI] [PubMed] [Google Scholar]
- van Heel M. (1987) Similarity measures between images. Ultra microscopy, 21, 95–100. [Google Scholar]
- van Heel M., Harauz,G., Orlova,E.V., Schmidt,R. and Schatz,M. (1996) A new generation of the IMAGIC image processing system. J. Struct. Biol., 116, 17–24. [DOI] [PubMed] [Google Scholar]
- Wilcox R.A., Primrose,W.U., Nahorski,S.R. and Challiss,R.A. (1998) New developments in the molecular pharmacology of the myo-inositol 1,4,5-trisphosphate receptor. Trends Pharmacol. Sci., 19, 467–475. [DOI] [PubMed] [Google Scholar]
- Williams A.J., West,D.J. and Sitsapesan,R. (2001) Light at the end of the Ca2+-release channel tunnel: structures and mechanisms involved in ion translocation in ryanodine receptor channels. Q. Rev. Biophys., 34, 61–104. [DOI] [PubMed] [Google Scholar]
- Yoshida Y. and Imai,S. (1997) Structure and function of inositol 1,4,5-trisphosphate receptor. Jpn J. Pharmacol., 74, 125–137. [DOI] [PubMed] [Google Scholar]
- Zhou Z.H., Baker,M.L., Jiang,W., Bougherty,M., Jakana,J., Dong,G., Lu,G. and Chiu,W. (2001) Electron cryo-microscopy and bio-informatics suggest protein fold models for rice dwarf virus. Nat. Struct. Biol., 8, 868–873. [DOI] [PubMed] [Google Scholar]