Abstract
Syncytia arising from the fusion of cells expressing the HIV-1-encoded Env gene with cells expressing the CD4/CXCR4 complex undergo apoptosis following the nuclear translocation of mammalian target of rapamycin (mTOR), mTOR-mediated phosphorylation of p53 on Ser15 (p53S15), p53-dependent upregulation of Bax and activation of the mitochondrial death pathway. p53S15 phosphorylation is only detected in syncytia in which nuclear fusion (karyogamy) has occurred. Karyogamy is secondary to a transient upregulation of cyclin B and a mitotic prophase-like dismantling of the nuclear envelope. Inhibition of cyclin-dependent kinase-1 (Cdk1) prevents karyogamy, mTOR activation, p53S15 phosphorylation and apoptosis. Neutralization of p53 fails to prevent karyogamy, yet suppresses apoptosis. Peripheral blood mononuclear cells from HIV-1-infected patients exhibit an increase in cyclin B and mTOR expression, correlating with p53S15 phosphorylation and viral load. Cdk1 inhibition prevents the death of syncytia elicited by HIV-1 infection of primary CD4 lymphoblasts. Thus, HIV-1 elicits a pro-apoptotic signal transduction pathway relying on the sequential action of cyclin B–Cdk1, mTOR and p53.
Keywords: cell death/cyclin B/mitochondria/p53/rapamycin
Introduction
Fusion of somatic cells, resulting in the formation of syncytia, is a normal process involved in the generation of myotubes, osteoclasts and the syncytiotrophoblast (Anderson, 2000). In contrast, non-physiological heterokaryon formation ultimately results in cell death, as this has been shown by overexpression of fusogenic viral glycoproteins, thereby enforcing syncytium formation (Bateman et al., 2000; Scheller and Jassoy, 2001). This applies also to the fusion of cells expressing Env, the envelope glycoprotein complex (gp120/gp41) encoded by the HIV type 1 (HIV-1) genome, with cells expressing the Env (co-)receptor, a combination of CD4 with either CXCR4 or CCR5. Syncytium formation resulting from the Env–CD4 interaction leads to apoptosis, a process thought to participate in the AIDS-associated depletion of CD4+ T lymphocytes (Lifson et al., 1986; Sodroski et al., 1986; Blaak et al., 2000; Maas et al., 2000; Mohri et al., 2001). Indeed, it appears that Env is (one of) the functionally most important apoptogenic HIV-1 product(s) (Gougeon and Montagnier, 1999; Badley et al., 2000; Selliah and Finkel, 2001). The Env complex is expressed on the surface of HIV-1-infected cells as well as on HIV-1 virions. Within this complex, gp120 mediates the primary interaction with the receptor/co-receptor complex, while gp41 participates in membrane interactions eventually leading to fusion (Eckert and Kim, 2001). The interaction between Env and the co-receptor can also mediate pro-apoptotic signals, the exact nature of which is a matter of debate. Env-elicited apoptosis may involve caspases, caspase-independent effectors, inactivation of survival signals mediated by focal adhesion complexes and calcineurin activation, as well as mitochondrial membrane permeabilization (Berndt et al., 1998; Cicala et al., 2000; Roggero et al., 2001; Vlahakis et al., 2001; Sasaki et al., 2002).
Syncytial apoptosis induced by the Env–CD4 interaction involves a precise sequence of events: (i) activation of the mammalian target of rapamycin (mTOR), a serine/threonine kinase of the phosphatidyl inositol kinase family; (ii) mTOR-mediated phosphorylation of p53 on serine 15 (p53S15); (iii) p53-dependent upregulation of the expression of Bax, which undergoes a conformational change and translocates from the cytosol to mitochondria; (iv) Bax-mediated permeabilization of mitochondrial membranes with reduction of the mitochondrial transmembrane potential (ΔΨm) and release of apoptogenic proteins from mitochondria, in particular apoptosis- inducing factor and cytochrome c; (v) cytochrome c-dependent caspase activation; and (vi) caspase-dependent nuclear chromatin condensation (Ferri et al., 2000a; Castedo et al., 2001). This sequence of events has been observed in Env-transfected HeLa cells co-cultured with CD4-transfected HeLa cells, in the absence of viral infection (Ferri et al., 2000a; Castedo et al., 2001), and has been confirmed for HIV-1-infected primary CD4+ lymphoblasts (Ferri et al., 2000a; Castedo et al., 2001; Genini et al., 2001; Petit et al., 2002).
Intrigued by these premises, we employed a combination of proteomics and hypothesis-driven research with the aim of dissecting the molecular events occurring immediately upstream of mTOR activation. Recently, we discovered that the Env–CD4 interaction not only causes fusion of the cytoplasm but results, after a lag phase, in fusion of the nucleoplasm (karyogamy) (Ferri et al., 2000b). Here, we report that Env-elicited nuclear fusion results from the abortive initiation of mitosis, involving the transient activation of cyclin B-dependent kinase-1 (Cdk1). Importantly, our results reveal the existence of a novel, pro-apoptotic signal transduction pathway elicited by HIV-1, both in vivo, in HIV-1-infected patients, and in vitro, in HIV-1-infected lymphoblasts. This lethal pathway involves the sequential action of Cdk1, mTOR and p53.
Results and discussion
p53S15 phosphorylation correlates with karyogamy in syncytia elicited by HIV-1 Env
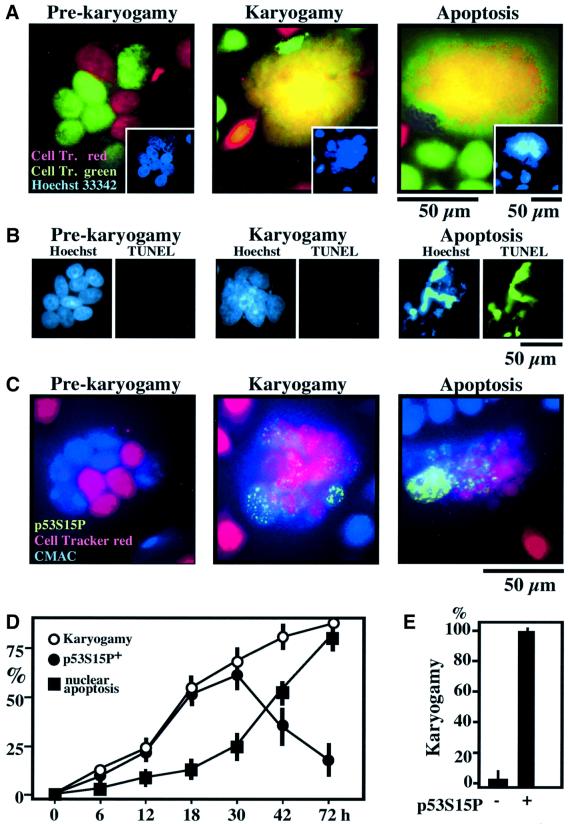
HeLa cells stably transfected with a lymphotropic HIV-1 Env gene (HeLa Env) were fused by co-culture with CD4/CXCR4-expressing HeLa cells (HeLa CD4). Fusion events, which depend on the Env–CD4 interaction (Ferri et al., 2000a,b,c; Castedo et al., 2001), were monitored by means of two stable, non-toxic CellTracker fluorescent dyes with which HeLa Env (CellTracker Green) or HeLa CD4 cells (CellTracker Red) were pre-incubated. After several hours of co-culture, juxtaposed nuclei from both cell types (red or green) could be distinguished within a common cytoplasm (Figure 1A). Subsequently, nucleoplasm fusion (karyogamy) occurred, as detectable by the blending of the two CellTracker dyes (yellow) within the nucleus. Twenty-four hours post-fusion ∼50% of syncytia induced by the Env–CD4 interaction exhibited karyogamy, and this percentage was <10% for syncytia arising from PEG-mediated cellular fusion (data not shown). Karyogamy induced by the Env–CD4/CXCR4 interaction occurred before apoptotic nuclear chromatin condensation (detected with Hoechst 33342, Figure 1A) and DNA degradation (detected by TUNEL staining, Figure 1B). Apoptosis, in turn, was only found in karyogamic syncytia (Figure 1A and B). Similar results were obtained when the two fusion partners were labeled with CellTracker Red and CMAC (blue fluorescence, Figure 1C). Karyogamy (as defined by the purple blend of red and blue fluorescence) correlated with p53S15 phosphorylation (revealed in green), as indicated by triple staining experiments (Figure 1C and E) and kinetic analyses (Figure 1D). These data suggest that illicit nuclear fusion, linked to p53S15 phosphorylation, may be involved in syncytial apoptosis.
Fig. 1. Karyogamy correlating with p53S15 phosphorylation in HIV-1 Env-elicited syncytia. (A) Detection of nuclear fusion. HeLa Env and HeLa CD4 cells were stained with CellTracker Red and Green, respectively, followed by co-culture for 18 h. Representative fluorescence micrographs of Hoechst 33342-counterstained syncytia exhibiting cytoplasmic fusion without nuclear fusion (‘pre-karyogamy’), nuclear fusion without apoptosis (‘karyogamy’) and nuclear fusion with apoptosis are shown. Note that karyogamy correlates with a Hoechst 33342-detectable loss of internuclear boundaries (inserts). (B) DNA degradation assessed by TUNEL staining is restricted to cells exhibiting apoptotic chromatin condensation. (C) Coincidence of karyogamy and p53S15P phosphorylation. HeLa Env and HeLa CD4 cells were pre-stained with CellTracker Red or CMAC, respectively, then fused by co-culture, fixed, permeabilized and stained for p53S15P. Representative images are shown, showing that karyogamic cells lacking signs of apoptosis are p53S15P+. (D) Kinetics of karyogamy, p53S15 phosphorylation, and nuclear apoptosis [detected as in (C)]. (E) Quantitation of karyogamy among syncytia as a function of the phosphorylation status of p53S15, 18 h after co-culture. At this stage, p53S15P can be considered as a surrogate marker of karyogamy.
Karyogamy results from an abortive entry into the mitotic prophase
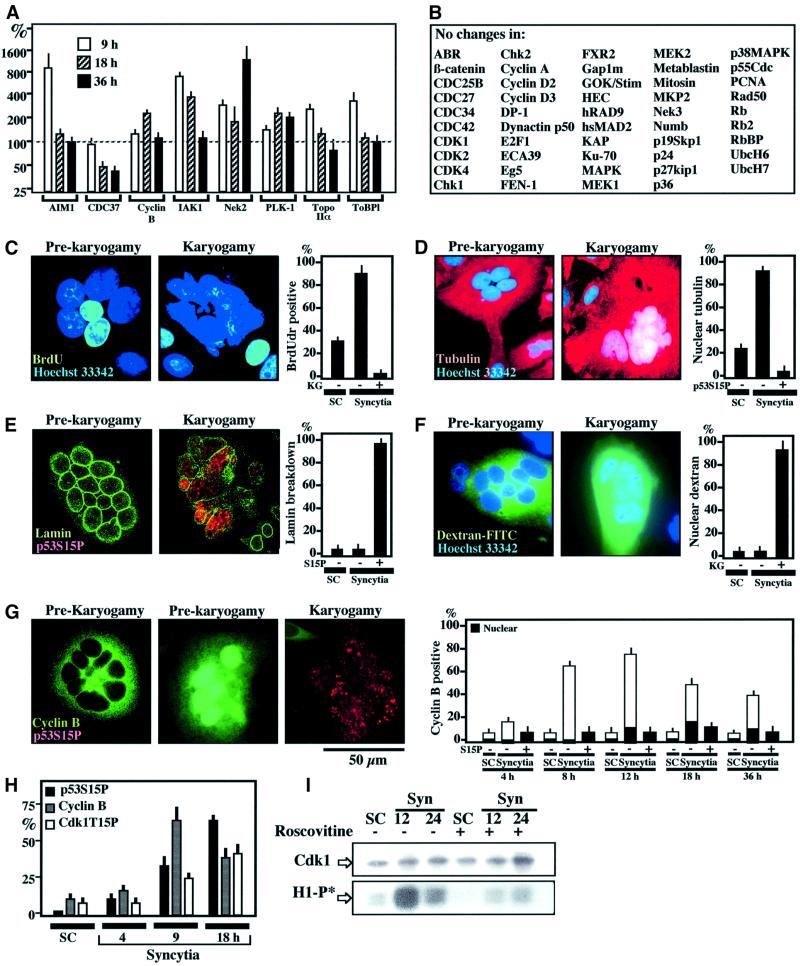
Quantitative immunoblots performed with a panel of ∼900 monoclonal antibodies revealed that a cluster of proteins accumulating at the G2/M boundary were overexpressed in HIV-1 Env-elicited syncytia, early after fusion, at 9 h (Figure 2A). This applies to AIM1 (aurora- and Ip1-like midbody-associated protein), IAK1 (IP1- and aurora-related kinase-1), PLK-1 (polo-like kinase-1), the serine-threonine kinase Nek2 and the topoisomerase IIα isoform and its binding protein (ToBP1). Among the cyclins, only cyclin B was altered, with a transient overexpression at 18 h (Figure 2A and B). Based on this information, we determined the relationship between karyogamy and cell cycle. Whereas individual nuclei from pre-karyogamic syncytia incorporated the DNA precursor BrdU, no signs of DNA synthesis could be detected in karyogamic nuclei (Figure 2C). Karyogamic nuclei stained for tubulin (Figure 2D) and exhibited a loss in lamin B staining, indicating the dissolution of the nuclear envelope (Figure 2E). Accordingly, a dextran–FITC conjugate (molecular weight 70 kDa) microinjected into the cytoplasm was excluded from nuclei of pre-karyogamic syncytia, yet did penetrate into karyogamic nuclei (Figure 2F). These features (arrest of DNA synthesis and annihilation of the barrier function of the nuclear envelope) suggest that karyogamy corresponds to the entry into the early mitotic prophase, before spindle formation and pronounced mitotic chromatin condensation occur. Cyclin B was found to accumulate in the cytosol (and sometimes in the nucleus) of syncytia before karyogamy, yet was absent from most karyogamic cells, with a peak increase ∼12 h after Env–CD4 co-cultures (Figure 2G). This suggests that the Cdk1, which is required for lamin phosphorylation/depolymerization and progres sion through mitosis (Smits and Medema, 2001), has been inactivated early after karyogamy. Accordingly, the inactivating phosphorylation of Cdk1 on Tyr15 (Kumagai and Dunphy, 1991) was found to be a relatively late event (Figure 2H). Immunoprecipitation of the cyclin B–Cdk1 complex confirmed a higher Cdk1 activity (measured on histone H1) in syncytia as compared with individual control cells, and this increase in kinase activity peaked at ∼12 h (Figure 2I).
Fig. 2. Phenotypic characterization of Env-induced karyogamy. (A) Cell cycle-relevant proteins whose expression level changes (by a factor of ≥2) upon syncytium formation as determined by quantitative immunoblots (X ± SEM, n = 3). (B) Examples of cell cycle-relevant proteins detectable in HeLa cells whose expression level varies by a factor <2. (C) 5-bromo-2′deoxyuridine (BrdU) incorporation into syncytia as a function of the karyogamy (KG) status. Sixteen-hour-old syncytia were incubated with BrdU for 2 h and then subjected to the immunodetection of BrdU into S-phase nuclei. (D) Tubulin staining of 18-h-old syncytia. Note that the tubulin network is excluded from the nucleus before karyogamy. After karyogamy, nuclei stain positively for tubulin. (E) Lamin B immunostaining of syncytia. Note that karyogamic syncytia exhibit the breakdown of lamin B. (F) Loss of nuclear envelope barrier function in karyogamy. Syncytia were microinjected into the cytoplasm with dextran-70 FITC conjugated, which is excluded from the nucleus of pre-karyogamic cells, yet penetrates into the nucleus of karyogamic syncytia. (G) Cyclin B accumulation in pre-karyogamic syncytia. Two phenotypes (12 h after fusion) are shown, namely pre-karyogamic (p53S15P+) cells exhibiting cyclin B in the cytoplasm or in the nucleus. In contrast, most karyogamic cells lack immunodetectable cyclin B. The kinetics of cyclin B accumulation is shown at different intervals. The few karyogamic (p53S15P+) cells positively staining for cyclin B demonstrate a diffuse (cytoplasmic + nuclear) staining pattern. (H) Kinetics of cyclin B accumulation and the phosphorylation of p53S15 or Cdk1T15. (I) Kinetics of Cdk1 activity. Cdk1 was immunoprecipitated at different intervals after syncytium formation and its capacity to phosphorylate histone H1 in vitro was determined (H1-P*) in the absence or presence of roscovitine (1 µM). Immunoblot detection to confirm equal loading of Cdk1 was also performed.
Cdk1 is involved in karyogamy and syncytial apoptosis
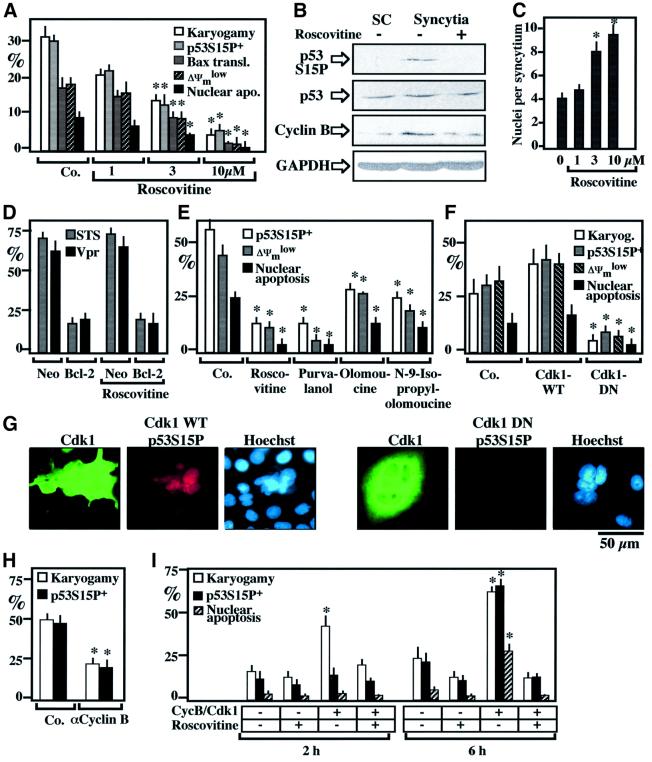
Addition of Cdk1 inhibitor roscovitine to HeLa Env/HeLa CD4 co-cultures prevented karyogamy, as well as p53S15 phosphorylation and all subsequent steps leading to apoptosis such as mitochondrial translocation of Bax, loss of the ΔΨm and apoptotic chromatin condensation (Figure 3A and B). Roscovitine allowed for the formation of larger syncytia, with more nuclei (Figure 3C). Roscovitine failed to inhibit apoptosis induced by staurosporine or by HIV-1-encoded viral protein R (Vpr), indicating that it does not act as a general apoptosis inhibitor (such as Bcl-2; Figure 3D). Chemically unrelated Cdk1 inhibitors such as purvalanol, olomoucine and N-9 isopropyl-olomoucine inhibited the Env-elicited p53S15 phosphorylation and apoptosis, much as roscovitine did (Figure 3E). These pharmacological data were corroborated at the genetic level, by transient transfection with a dominant-negative (DN) cyclin Cdk1 mutant (Figure 3F), which prevented karyogamy, p53S15 phosphorylation and apoptosis (Figure 3F and G). Moreover, microinjection of an anti-cyclin B antibody into 6-h-old syncytia inhibited karyogamy and p53S15 phosphorylation (Figure 3H). Injection of an active Cdk1–cyclin B complex into the cytoplasm of freshly formed syncytia resulted into accelerated nuclear fusion, and this effect was prevented by roscovitine (Figure 3I). This suggests that lamin phosphorylation/depolymerization induced by Cdk1 (Peter et al., 1990) is sufficient to induce karyogamy. Microinjection of Cdk1–cyclin B into syncytia also induced karyogamy and, after a lag phase, phosphorylation of p53S15 and apoptosis (Figure 3I).
Fig. 3. Involvement of Cdk1 in karyogamy and apoptosis. (A) Effects of the Cdk1 inhibitor roscovitine on karyogamy, p53S15 phosphorylation, Bax upregulation and signs of apoptosis. Values (X ± SEM, n = 3), obtained after 16 h of co-culture, are representative of seven independent experiments. Asterisks denote significant (P < 0.01, Student’s t-test) differences compared to untreated control cultures. (B) Roscovitine (10 µM)-mediated inhibition of p53S15 phosphorylation, as determined by immunoblot detection. A 1:1 mixture of HeLa CD4/HeLa Env single cells (SC) or syncytia (18 h of co-culture) generated in the absence or presence of 10 µM roscovitine were subjected to immunoblotting analysis. GAPDH was detected to confirm equal protein loading (40 µg per lane). (C) Roscovitine-mediated increase in the syncytial size (determined by counting the number of Hoechst 33342-stained nuclei per syncytium, n = 100). (D) Failure of roscovitine to act as a general apoptosis inhibitor. HeLa cells stably transfected with Bcl-2 or the Neo resistance gene only were exposed for 6 h to 4 µM staurosporine (STS) or synthetic Vpr peptide, followed by the cytofluorometric assessment of the frequency of cells incorporating the vital dye propidium iodide (X ± SEM, n = 3). (E) Comparative assessment of different pharmacological Cdk1 inhibitors. HeLa Env/HeLa CD4 syncytia were generated in the presence of 10 µM roscovitine, 3 µM purvalanol, 60 µM olomoucine or 30 µM N-9-isopropylolomoucine, and the indicated parameters were scored 24 h after initiation of co-cultures. (F) Effect of DN Cdk1 on p53S15 phosphorylation, apoptosis and syncytial size. Cells were transiently transfected 24 h before fusion with vector only (Co.), wild-type (WT) or DN Cdk1 and the indicated parameters were determined 16 h after co-culture among cells strongly staining for Cdk1-WT or Cdk1-DN. (G) Representative immunostainings of syncytia transfected with either Cdk1-WT or Cdk1-DN [as in (F)] and stained with antibodies specific for Cdk1 (which also recognizes Cdk1-DN) and p53S15P. (H) Microinjection of a neutralizing cyclin B antibody into newly formed syncytia. A cyclin B-specific monoclonal antibody (or an anti-paxillin antibody serving as a negative control) were microinjected into the cytoplasm of 6-h-old syncytia, and the frequency of karyogamy or p53S15 phosphorylation was scored 12 h later. (I) Karyogamy triggered by microinjection of cyclin B–Cdk1. Six-hour-old syncytia were microinjected with an active cyclin B–Cdk1 complex or PBS (Co.) and cultured either in the absence or in the presence of 10 µM roscovitine for the indicated period, followed by determination of karyogamy, p53S15P and nuclear apoptosis.
mTOR is activated downstream of Cdk1
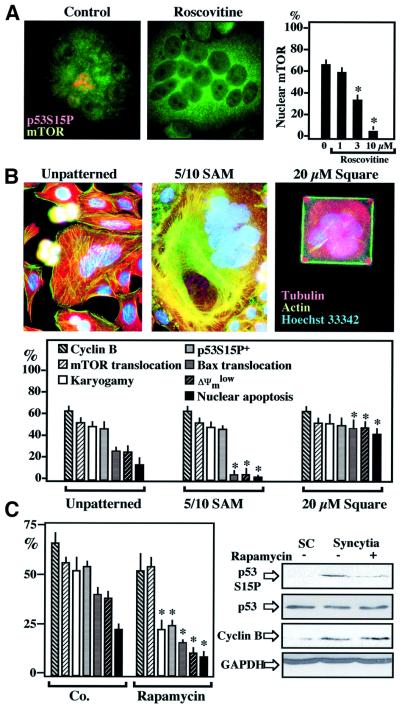
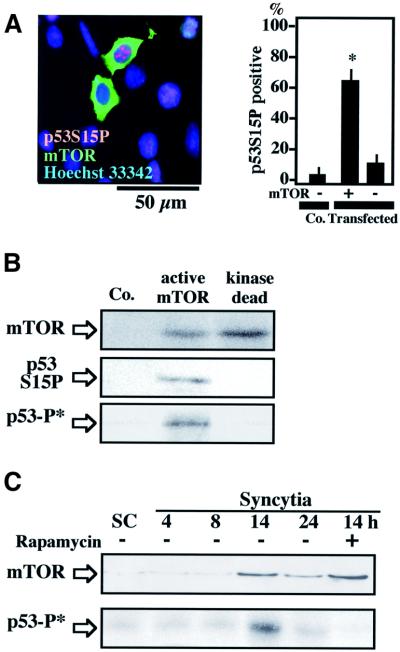
One p53S15 kinase activated by HIV-1 infection has been identified as mTOR (Castedo et al., 2001). Accordingly, mTOR, which is normally found in the cytoplasm of individual HeLa cells, was detected in nuclei from karyogamic (p53S15 phosphorylation+) syncytia (Figure 4A). Cdk1 inhibition by roscovitine, which inhibits p53S15 phosphorylation and karyogamy (Figure 3A and B), completely suppressed the nuclear relocalization of mTOR, which stayed cytoplasmic (Figure 4A). A similar inhibition of the nuclear localization of mTOR was observed upon transfection with DN-CDK1 or treatment with olomoucine (data not shown). In view of the reported intimate relationship between mTOR and cell size control (Zhang et al., 2000; Bodine et al., 2001), we tested whether external constraint on cell size of HeLa Env/HeLa CD4 heterokarya would modulate syncytial apoptosis via an effect on the subcellular localization and activity of mTOR. Syncytia cultured on adhesive squares of 20 µm diameter (Ferri et al., 2000c) exhibited a reduced number of nuclei per syncytium (2.9 ± 0.3 versus 4.7 ± 1.2 on unpatterned control substrates, 18 h after co-culture, X ± SEM, n = 100, P < 0.001), as well as increased Bax translocation and apoptosis (Figure 4B). However, the reduction of syncytial size had no effect on cyclin B accumulation, karyogamy, nuclear accumulation of mTOR and p53S15 phosphorylation (Figure 4B). Culture on a substrate specifically designed to increase spreading via extension of cell processes that attach to squares of 5 µm in diameter, separated by 10 µm of non-adhesive zones [5/10 self-assembled monolayer (SAM)] (Figure 4B) (Chen et al., 1997; Ferri et al., 2000c) resulted in an increase in the average number of nuclei per syncytium (7.2 ± 2.8, P < 0.001). In spite of the inhibition of Bax translocation and apoptosis by the 5/10 SAM, there was no effect on the nuclear relocalization of mTOR (Figure 4B). Taken together, these data suggest that, downstream of cyclin B, mTOR is activated by a pathway that is not linked to cell size control.
Fig. 4. mTOR involvement in karyogamy and p53S15 phosphorylation. (A) mTOR acts downstream of Cdk1. Untreated 24-h-old syncytia (Co.) or syncytia cultured in the continuous presence of roscovitine were subjected to immunodetection of mTOR. Note that roscovitine prevents the nuclear accumulation of mTOR as well as p53S15 phosphorylation. (B) Failure of cell size to modulate karyogamy or mTOR. Syncytia were generated on unpatterned substrates or on specialized culture substrates designed to increase cellular spreading and syncytial size (5/10 SAM) or to exert an external constraint on cell size (square-shaped SAM 20 µM in diameter). Representative staining patterns of actin and tubulin networks are shown. Note the actin stress fibers in the 5/10 SAM syncytia and the rigorously square-shaped cell cultured on the 20-µm square SAM. Twenty-four hours after co-culture, the frequency of cells exhibiting cyclin B upregulation, karyogamy, p53S15P phosphorylation, nuclear relocalization of mTOR or apoptotic parameters were determined. Asterisks indicate significant differences (P < 0.01) as compared with unpatterned control substrates. (C) Effect of rapamycin on syncytia. HeLa CD4 and HeLa Env cells were co-cultured in the absence (Co.) or presence of the mTOR inhibitor rapamycin (1 µM), followed by determination of the indicated parameters at 30 h, either by in situ fluorescence techniques (columns) or by immunoblot. The color coding is as in (B). Asterisks denote significant (P < 0.01) effects.
mTOR mediates p53S15 phosphorylation downstream of Cdk1
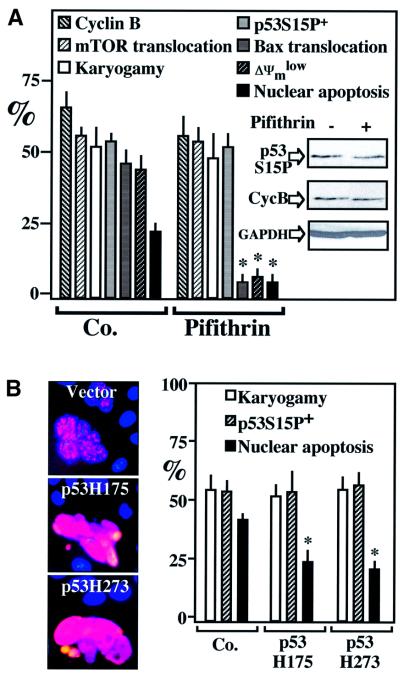
mTOR inhibition by rapamycin reduced p53S15 phosphorylation, as well as all downstream events leading to cell death including Bax translocation, ΔΨm dissipation and nuclear condensation (Figure 4C). Rapamycin had no effect on the accumulation of cyclin B preceding karyogamy. Unexpectedly, however, rapamycin reduced karyogamy in HeLa Env/HeLa CD4 heterokarya (Figure 4C), an observation that is reminiscent of that obtained in Saccharomyces cerevisiae, in which rapamycin can inhibit karyogamy (Choi et al., 2000). While these data indicate that both Cdk1 and mTOR engage in an intricate pathway regulating nuclear fusion, they do not prove that mTOR is directly phosphorylating p53S15. We therefore transfected individual HeLa cells with mTOR. Most cells that overexpress mTOR exhibited p53S15 phosphorylation (Figure 5A). mTOR immunoprecipitated with an anti-Flag antibody from a cell line stably transfected with flagged mTOR (but not that of a kinase-dead mTOR mutant) (Fang et al., 2001) phosphorylated recombinant p53 in vitro (Figure 5B). Endogenous mTOR immunoprecipitated from HeLa Env–CD4 syncytia exhibited a higher capacity to phosphorylate p53 in vitro than mTOR from individual cells, correlating with enhanced mTOR expression levels (Figure 5C). When added to HeLa Env–CD4 syncytia, the p53 inhibitor pifithrin-α (Komarov et al., 1999) had no effect on cyclin B accumulation, karyogamy or p53S15 phosphorylation, yet did prevent the mitochondrial and nuclear signs of apoptosis (Figure 6A). Similar results were obtained by transfection with DN p53 mutants, which had no effect on karyogamy nor on p53S15 phosphorylation, yet prevented apoptosis (Figure 6B). Thus, p53 operates downstream from the Cdk1/mTOR-controlled karyogamy step leading to syncytial apoptosis.
Fig. 5. mTOR-mediated p53S15 phosphorylation. (A) Overexpression of mTOR is sufficient to cause p53S15 phosphorylation. Wild-type HeLa cells were transfected with mTOR, and the frequency of p53S15P+ cells among mTOR-overexpressing cells and vector-only transfected (Co.) cells was detected by two-color immunofluorescence staining. Asterisks denote significant (P < 0.01) effects as compared with controls. (B) In vitro phosphorylation of recombinant p53 by recombinant mTOR. mTOR was immunoprecipitated from untransfected HEK293 cells (Co.) or HEK293 cells stably transfected with active or kinase-dead FLAG-mTOR, using an anti-FLAG antibody, and assayed for its capacity to phosphorylate recombinant p53 in vitro, in the presence of [γ-32P]ATP, either by autoradiography (p53-P*) or by immunoblot with an antibody specific for p53S15P. (C) In vitro phosphorylation of recombinant p53 by mTOR expressed in syncytia. mTOR was immunoprecipitated from freshly mixed HeLa CD4 and HeLa Env cells (single cells, SC) or from co-cultures, using an mTOR-specific antibody, and its capactity to phosphorylate p53 was assessed in vitro as in (B), in the absence or presence of 1 µM rapamycin (added during cell culture as well as in vitro).
Fig. 6. p53 phosphorylation occurs downstream of cyclin B/mTOR upregulation and karyogamy. (A) Chemical inhibition of p53 by cyclic pifithrin-α. Syncytia were generated in the presence of 10 µM cyclic pifithrin-α, and the indicated parameters were determined by in situ fluorescence techniques (columns) or by immunoblotting (insert). Asterisks denote significant (P < 0.01) pifithrin-α effects. (B) Effect of DN p53. Syncytia were generated by co-culture of HeLa CD4 and HeLa Env cells previously transfected with either of two p53 mutants. Note that the level of p53S15P staining, at per-cell level, actually increased and that karyogamy and the frequency of p53S15P+ cells was not affected, while apoptosis was inhibited corresponding to the transfection efficiency (∼50%). Results are representative of three independent determinations.
mTOR and p53S15 phosphorylation in lymph nodes and peripheral blood mononuclear cells from HIV-1 patients
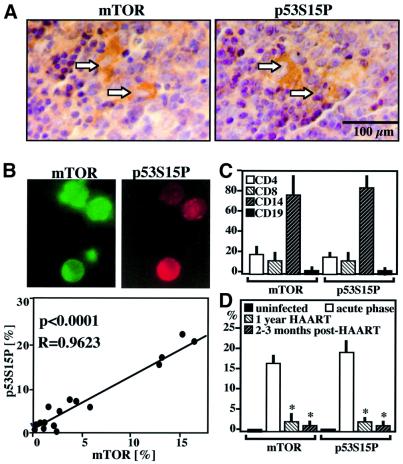
Phosphorylated p53S15 could be detected in single cells as well as in syncytia localized in the T cell area of lymph nodes from HIV-1+ patients. The same syncytia overexpress mTOR, as revealed by immunostaining of adjacent sections (Figure 7A). p53S15 phosphorylation was detected in peripheral blood mononuclear cells (PBMC) of HIV-1+ donors but not in PBMC from HIV-1– controls (<1% positive cells), correlating with the frequency of mTOR-overexpressing PBMC (Figure 7B). Such p53S15P+ or mTOR+ cells were found among all relevant PBMC populations (CD4+, CD8+, CD14+), with a preference for monocytes (CD14+) (Figure 7C). In accordance with the previous finding establishing a positive correlation between viral load and the frequency of p53S15P+ PBMC in HIV-1+ carriers (Castedo et al., 2001), untreated HIV-1+ donors exhibited far higher levels of p53S15P+ and mTOR+ cells than patients in which the number of viral copies had dropped as a result of ongoing highly active anti-retroviral therapy (HAART). This difference persisted among patients that continued to have low levels of circulating HIV-1 particles after HAART interruption (Figure 7D). In conjunction with the previously reported finding that cyclin B is overexpressed in PBMC from HIV-1 patients (Cannovo et al., 2001), these data underline the probable in vivo relevance of the HIV-1-induced lethal Cdk1/mTOR/p53 pathway.
Fig. 7. Detection of mTOR and p53S15P in HIV-1 patients. (A) Immunocytochemical localization of p53S15P and mTOR in lymph node biopsies from HIV-1+ patients. Two adjacent sections are shown. Arrows indicate positive stainings, which are virtually absent in lymph node from uninfected controls. Similar results were obtained with biopsies from three further untreated HIV-1+ donors. Less than 0.1% of the cells from normal donors stain positively for p53S15P and mTOR. (B) Immunolocalization of p53S15P (red fluorescence) and mTOR (green fluorescence) proteins in PBMC from HIV-1+ patients. Note that most cells are positive for both markers. The frequency of mTOR+ and p53S15P+ was determined among total PBMC of 21 HIV-1+ patients. Each dot represents one patient. Calculations were done following the Pearson method. (C) Frequency of mTOR+ and p53S15P+ cells among defined subsets of mononuclear cells. A subset of the patients shown in (B) was subjected to two color immunofluorescence stainings, allowing for the determination of the percentage of cells expressing mTOR+ or p53S15P+ among lymphocytes expressing CD4, CD8 or CD19, as well among CD14+ monocytes. (D) Effect of HAART on mTOR and p53S15P expression in PBMC from HIV-1+ donors. The frequency of mTOR+ and p53S15P+ PBMC was determined in uninfected controls, individuals with acute infection (untreated), patients receiving at least 12 months of HAART, as well as individuals with undetectable virus after several months of HAART interruption. Values are means ± SD. Asterisks indicate a significant reduction of the indicated parameters, as compared with untreated controls.
Cdk1 is critically involved in syncytial cell death induced by HIV-1 infection
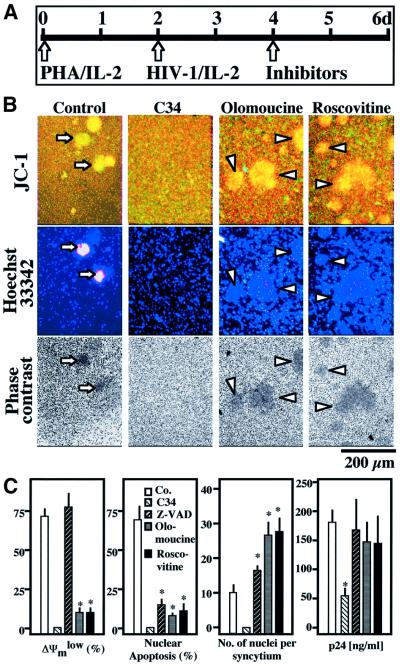
Primary CD4+ lymphoblasts from healthy donors were infected with the lymphotropic HIV-1IIIb strain in vitro, a manipulation that induces syncytium formation and subsequent apoptosis. According to one report (Wang et al., 2001), Cdk1 inhibition by roscovitine may affect HIV-1 transcription. However, addition of non-toxic doses (≤1 µM) of roscovitine 48 h after infection failed to inhibit viral replication, as determined by quantifying the production of p24 (Figure 8). Moreover, roscovitine did not reduce the HIV-1-triggered syncytium formation and rather led to the formation of giant syncytia, occasionally containing >100 nuclei (Figure 8B). Roscovitine did inhibit chromatin condensation, as well as the ΔΨm loss in HIV-1-elicited syncytia (indicated by a red–green shift in the fluorescence of JC-1-stained cells). Similar results were obtained when roscovitine was replaced by another Cdk1 inhibitor, olomoucine (Figure 8B and C). This inhibition was similar to that obtained by rapamycin (Castedo et al., 2001; data not shown). Altogether, these data confirm that the roscovitine/olomoucine-inhibited CDK1→mTOR→p53S15P pathway selectively controls apoptosis of syncytia in human primary CD4+ lymphocytes infected by HIV-1.
Fig. 8. Involvement of Cdk1 in apoptosis of syncytia induced by HIV-1 infection. (A) Experimental set-up. After an initial stage (48 h) stimulation with PHA and interleukin-2 (IL-2), primary CD4+ lymphoblasts were infected with HIV-1IIIB. After 48 h, 1 µM AZT (which blocks viral RNA synthesis), 500 nM C34 (a gp41-derived peptide that blocks Env-mediated fusion), 50 µM Z-VAD.fmk, (a pan-caspase inhibitor) or either of two Cdk1 inhibitors (10 µM olomoucine or 1 µM roscovitine) were added to the cells. After a further 48 h culture period, virological and apoptotic parameters were assessed. (B) Representative fluorescence micrographs. Cells were stained with the ΔΨm-sensitive dye JC-1 (red fluorescence indicates a high ΔΨm, green fluorescence a low ΔΨm) and Hoechst 33342 for the detection of chromatin condensation. Arrows indicate apoptotic syncytia, while arrow heads mark syncytia with a high ΔΨm and no signs of nuclear apoptosis. (C) Quantitative determinations. The frequency of cells exhibiting signs of apoptosis, the number of nuclei per syncytium, and production of the viral protein p24 were determined. Note that no syncytia were found in the presence of AZT or C34. Results (mean values ± SEM) are representative of four independent experiments. Asterisks denote significant effects as compared with control cultures (P < 0.01).
Conclusions
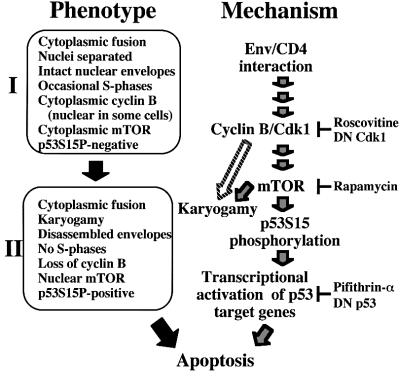
Syncytia elicited by HIV-1 Env progress to apoptosis in a stepwise fashion. After an initial stage (‘stage I’; Figure 9), during which nuclei are separated by intact envelopes, cyclin B accumulates in the cytoplasm, mTOR remains a predominantly cytoplasmic protein and p53S15 is non-phosphorylated. Later, syncytia adopt a different phenotype (‘stage II’; Figure 9), with nuclear fusion (karyogamy), dismantled nuclear envelopes, downregulated cyclin B, mTOR in the nucleus and p53S15 phosphorylation (Figures 1 and 2). Altogether, these changes resemble ‘mitotic catastrophe’ (Pines, 1999), an apoptosis-like process that begins in prophase, after dissolution of the nuclear membrane, and is associated with the entry of Cdk1 and cyclin B into the nucleus, which can be found in a minority of stage I cells (Figure 2G). Molecular ordering of the process has been achieved using a battery of chemical inhibitors (of Cdk1, mTOR, p53), DN constructs (of Cdk1 and p53) and positive manipulations (microinjection of Cdk1–cyclin B, transfection with mTOR) (Figures 3–8). Upon syncytium formation, cyclin B is upregulated in a transient fashion, thereby triggering Cdk1-dependent activation of lamin disassembly (which facilitates karyogamy) and nuclear accumulation of mTOR. mTOR, which contributes to karyogamy, then phosphorylates p53S15, resulting in the transcriptional activation of apoptogenic p53 target genes (Figure 9). That mTOR (or a closely associated kinase) directly phosphorylates p53 is suggested by the facts that mTOR co-immunoprecipitates with p53 in syncytia but not in normal cells (data not shown), that mTOR translocates to the nuclei (Figure 4A) and that immunoprecipitated mTOR from mTOR-transfected normal cells or from syncytia can phosphorylate recombinant p53 in vitro (Figure 5). Intriguingly, physiological syncytia (myotubes, osteoclasts, syncytiotrophoblasts) can only form after differentiation-associated irreversible cell cycle arrest, and do not undergo karyogamy (Bamberger et al., 1999; Kitzmann and Fernandez, 2001; Okahashi et al., 2001). Thus, maintenance of a post-mitotic status might condition the survival of physiological syncytia, which would contrast with Env-induced syncytia, in which progression to early mitosis automatically triggers cell death.
Fig. 9. Mechanisms of Env-triggered apoptosis. After fusion, cells adopt two preponderant phenotypes (stages I and II, left) before they undergo apoptosis, as shown in Figures 1 and 2. The hypothetical molecular order of events (right) is based on the experiments shown in Figures 3–7.
This scenario, which has been elaborated in co-cultures of two HeLa cell lines expressing either Env or CD4 (Figures 1–6), may be relevant to in vivo HIV-1 infection, based on several observations. Cyclin B is increased among PBMC from HIV-1-infected patients, a phenomenon that has been attributed to T cell activation (Cannovo et al., 2001), but which might also be triggered by the interaction between Env and its (co-)receptors. A significant correlation could be found between mTOR, p53S15P expression, and the virological status of patients (Figure 7). Therapeutic inhibition of HIV-1 replication decreased the expression of cyclin B in PBMC (Cannovo et al., 2001) and, as shown here, reduced the frequency of both mTOR+ and p53S15P+ PBMC (Figure 7). Primary CD4 lymphoblasts infected by HIV-1 upregulate cyclin B (Kolesnitchenko et al., 1995), and Cdk1 inhibition prevented syncytial cell death induced by HIV-1 infection (Figure 8). Taken together, these data underscore the probable pathophysiological relevance of the novel pro-apoptotic Cdk1–cyclin B→mTOR→p53 pathway delineated in this paper.
Future work will unravel the upstream signal(s) accounting for the HIV-1-stimulated cyclin B–Cdk1 activation, and thus identify the triggers that cause PBMC from HIV-1 carriers to follow a pattern of pro-apoptotic signal transduction which mimics that of Env-elicited syncytia. It remains also an open question through which exact molecular mechanisms Cdk1 activates mTOR, and why Cdk1 activation is transient, leading to an abortive, catastrophic entry into cell cycle. It may be anticipated that the answers to these questions will yield further insights into the pathway by which HIV-1-encoded proteins trigger the devastating apoptosis of immune cells.
Materials and methods
Cell lines, transfection and microinjection
HeLa cells stably transfected with the Env gene of HIV-1 LAI (HeLa Env) and HeLa cells transfected with CD4 (HeLa CD4) were cultured alone or together (1:1 ratio), at a density of 1–1.5 × 103 cells/mm at the beginning of co-cultures, resulting in progressive cell fusion (with >50% of cells within syncytia within 12 h, when monolayers are near to confluence) (Ferri et al., 2000a). Transfection with pcDNA3.1 vector only, wild-type (WT) or DN Cdk1 mutant (van den Heuvel and Harlow, 1993), p53 DN plasmids (gift from T.Soussi), Myc-tagged mTOR/FRAP constructs (Kim and Chen, 2000) was performed by electroporation (Loeffler et al., 2001) 24 h before co-culture of HeLa CD4 and HeLa Env cells. HeLa Neo and HeLa Bcl-2 cells (gift from Dr V.Goldmacher) were exposed to staurosporine or synthetic Vpr1-96 peptide (Jacotot et al., 2000). In some experiments, all syncytia growing on a pre-marked V-shaped area of a coverslip (>200 per experiment) were microinjected into the cytoplasm (Jacotot et al., 2001), with phosphate-buffered saline (PBS) only (pH 7.2), recombinant human cyclin B–Cdk1 (500 U/µl; Promega) (Xie et al., 1998), a monoclonal antibody (mAb) specific for cyclin B, or an isotype-(IgG1) matched paxillin mAb (BD Transduction Laboratories) (both at 200 ng/µl). Alternatively, cells were cultured with cyclic pifithrin-α, Cdk1 inhibitors (Calbiochem) or rapamycin (Sigma).
Fluorescence staining of live cells and immunofluorescence
HeLa CD4 cells or HeLa Env cells were labeled with either of three fluorochromes (Molecular Probes) for long-term cell labeling: CellTracker Red, CellTracker Green (15 µM, 30 min at 37°C in complete medium), or CMAC (7-amino-4-chloromethylcoumarin; 15 µM, 30 min in serum-free medium) and washed extensively before co-culture. Live cells were stained with the potentiometric dye 5,5′,6,6′-tetrachloro-1,1′, 3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) and/or Hoechst 33342 (2 µM; Sigma) for 30 min at 37°C (Ferri et al., 2000a). A rabbit antiserum specific for p53S15P (Cell Signaling Technology, MA) was used on paraformaldehyde (4% w:v) and picric acid-fixed (0.19% v:v) cells and revealed with a goat anti-rabbit IgG conjugated to phycoerythrine (PE) or FITC (Southern Biotechnology, Birmingham, AL). Cells were also stained for the detection of cyclin B (mAb from BD Transduction Laboratories), lamin B (mAb from Oncogene), Cdk1T15P (Cell Signaling Technology). 5-bromo-2′deoxy-uridine (BrdU) and Tunel staining was performed with detection kits from Roche.
Quantitation of protein expression levels, immunoblots and in vitro kinase assays
Protein samples were prepared from HeLa Env and HeLa CD4 single cells mixed at a 1:1 ratio in lysis buffer (0 control) or from HeLa Env–CD4 syncytia obtained after 9, 18 or 36 h of co-culture. These samples were processed by the PowerBlot facility (Becton Dickinson, Lexington, KY), to measure the expression level of ∼900 different signal-transducing proteins using a combination of SDS–PAGE (5–15% gradient), immunoblotting with specific mAbs revealed by a secondary goat anti-mouse horse radish peroxidase, acquisition of chemiluminescence data by a CDD camera, and computerized processing of densitometric data, in triplicate, after normalization on the mean protein expressions. Changes ≥2 were well above background variations (generally <1.5) observed in these assays (http://www.translab.com/TOC.shtml). Aliquots of protein extracts (40 µg) were subjected to immunoblots using antibodies specific for cyclin B, p53 (BD Transduction Laboratories), p53S15P, or glyceraldehyde-3-phosphated dehydrogenase (GAPDH; mAb from Chemicon). The capacity of immunoprecipitated cyclin B–Cdk1 to phosphorylate histone H1 was assessed (Pines and Hunter, 1994), and a similar protocol was employed to measure the phosphorylation of recombinant p53 (Santa Cruz) by mTOR immunoprecipitated from HeLa cells using an mTOR-specific mAb (Santa Cruz SC8315) or from HEK293 cells stably transfected with pCDNA3-FLAG-mTOR(S2035T) (active mTOR) or pCDNA3-FLAG-mTOR (S2035T, D2357E) (kinase-dead mTOR) lacking the regulatory N-terminus (1362C) (Kim and Chen, 2000; Fang et al., 2001).
Preparation of SAMs
An elastomeric stamp with a relief of the predetermined pattern was used to transfer hexadecanethiol (which is compatible with protein absorption) to designated regions of gold-coated glass coverslips. The slides were then immersed in 2 mM tri(ethylene glycol)-terminated alkanethiol (which resists the absorption of proteins) reacting with the bare regions of gold remaining after the printing process (Mrksich et al., 1996). Cover slips were pre-coated with human fibronectin (25 µg/ml in PBS, pH 7.2, 2 h at room temperature; Sigma), blocked with bovine serum albumin (10 mg/ml, 20 min at room temperature; Sigma), and rinsed with culture medium containing 1% of fetal calf serum (FCS) before addition of cells (cultured in 1% FCS).
Patients’ samples
Peripheral blood samples were obtained from four healthy and 25 HIV-1-infected individuals (mean age 36 years). Seven patients were naive for HAART with a plasma viral load >100 000 copies/ml; seven patients were HAART-treated with a viral load <5000 copies/ml, and 11 patients were under 2 months of antiretroviral therapy interruption with <50 copies/ml. None of the HIV-1+ patients received interferons, glucocorticoids, was positive for hepatitis B or C, or exhibited signs of autoimmunity. PBMC were fixed with 4% formaldehyde in PBS, pH 7.2. Plasma HIV-1 RNA levels were determined by the bDNA procedure (Versant HIV RNA 3.0) according to the manufacturer’s instructions (Bayer). Biopsies of axillary lymph nodes, obtained from healthy donors and HIV-1+ asymptomatic patients, naive for antiretroviral therapy, were fixed with 10% formalin, dehydrated and paraffin embedded. Deparaffinized tissue sections were subjected to immunocytochemistry. Cells were stained with anti-p53S15P (revealed with goat anti-rabbit IgG Alexa Fluor 595) and anti-mTOR (revealed with goat anti-mouse IgG Alexa Fluor 488; Molecular Probes, OR).
HIV-1 infection
Primary CD4+ lymphoblast (10 × 106) cells (Castedo et al., 2001) were incubated with the HIV-1IIIB (500 ng of p24) for 4 h at 37°C. After washing out unabsorbed virus, cells (106 cells/ml) were cultured in RPMI medium containing 20% FCS and 10 U/ml interleukin-2. Forty-eight hours after infection, when the first syncytia appeared in the infected cultures, Cdk1 inhibitors were added. Viral replication was evaluated by measuring the level of HIV-1 core protein p24 in the supernatant (Innogenetics ELISA kit, Barcelona, Spain).
Acknowledgments
Acknowledgements
We thank Dr Thierry Soussi (Institut Curie, Paris, France), Dr Sander van den Heuvel (Harvard Medical School, Charlestown, MA) for cDNA constructs, Nathanael Larochette (CNRS, Villejuif, France) for assistance, and the NIH AIDS reagents program (Bethesda, MD) for cell lines. This work has been supported by a special grant from LNC, as well as grants from ANRS, FRM, European Commission (QLG1-CT-1999-00739) (to G.K.) and the Italian Ministery of Public Health (to M.P.).
References
- Anderson J.M. (2000) Multinucleated giant cells. Curr. Opin. Hematol., 7, 40–47. [DOI] [PubMed] [Google Scholar]
- Badley A.D., Pilon,A.A., Landay,A. and Lynch,D.H. (2000) Mechanisms of HIV-associated lymphocyte apoptosis. Blood, 96, 2951–2964. [PubMed] [Google Scholar]
- Bamberger A., Sudahl,S., Bamberger,C.M., Schulte,H.M. and Loning,T. (1999) Expression patterns of the cell-cycle inhibitor p27 and the cell-cycle promoter cyclin E in the human placenta throughout gestation: implications for the control of proliferation. Placenta, 20, 401–406. [DOI] [PubMed] [Google Scholar]
- Bateman A., Bullough,F., Murphy,S., Emiliusen,L., Lavillette,D., Cosset,F.L., Cattaneo,R., Russel,S.J. and Vile,R.G. (2000) Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res., 60, 1492–1497. [PubMed] [Google Scholar]
- Berndt C., Möpps,B., Angermüller,S., Gierschik,P. and Krammer,P.H. (1998) CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ cells. Proc. Natl Acad. Sci. USA, 95, 12556–12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaak H., van’t Wout,A.B., Brouwer,M., Hoolbrink,B., Hovenkamp,E. and Schuitemaker,H. (2000) In vivo HIV-1 infection of CD45RA+ CD4+ T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4+ T cell decline. Proc. Natl Acad. Sci. USA, 97, 1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine S.C. et al. (2001) Akt/mTOR pathway is a crucial regulator of skeletal hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biol., 3, 1014–1019. [DOI] [PubMed] [Google Scholar]
- Cannovo G. et al. (2001) Abnormal intracellular kinetics of cell-cycle-dependent proteins in lymphocytes from patients infected with human immunodeficiency virus: a novel biologic link between immune activation, accelerated T-cell turnover and high levels of apoptosis. Blood, 97, 1756–1764. [DOI] [PubMed] [Google Scholar]
- Castedo M. et al. (2001) Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12–rapamycin-associated protein-mediated p53 phosphorylation. J. Exp. Med., 194, 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.S., Mrksich,M., Huang,S., Whitesides,G.M. and Ingber,D.E. (1997) Geometric control of cell life and death. Science, 276, 1425–1428. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Adames,N.R., Chan,T.-F., Zng,C., Cooper,J.A. and Zheng,X.F.S. (2000) TOR signaling regulates microtubule structure and function. Curr. Biol., 10, 861–864. [DOI] [PubMed] [Google Scholar]
- Cicala C., Arthos,J., Rubbert,A., Selig,S., Wildt,K., Cohen,O.J. and Fauci,A.S. (2000) HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4+ T cells. Proc. Natl Acad. Sci. USA, 97, 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert D.M. and Kim,P.S. (2001) Design of potent inhibitors of HIV-1 entry from the fp41 N-peptide region. Proc. Natl Acad. Sci. USA, 98, 11187–11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Vilella-Bach,M., Bachmann,R., Flanigan,A. and Chen,J. (2001) Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science, 294, 1942–1945. [DOI] [PubMed] [Google Scholar]
- Ferri K.F. et al. (2000a) Apoptosis control in syncytia induced by the HIV-1-envelope glycoprotein complex. Role of mitochondria and caspases. J. Exp. Med., 192, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri K.F., Jacotot,E., Geuskens,M. and Kroemer,G. (2000b) Apoptosis and karyogamy in syncytia induced by HIV-1–ENV/CD4 interaction. Cell Death Differ., 7, 1137–1139. [DOI] [PubMed] [Google Scholar]
- Ferri K.F., Jacotot,E., LeDuc,P., Geuskens,M., Ingber,D.E. and Kroemer,G. (2000c) Apoptosis of syncytia induced by HIV-1– Envelope glycoprotein complex. Influence of cell shape and size. Exp. Cell Sci., 261, 119–126. [DOI] [PubMed] [Google Scholar]
- Genini D. et al. (2001) HIV induced lymphocyte apoptosis by a p53-initiated, mitochondrion-mediated mechanism. FASEB J., 15, 5–6. [DOI] [PubMed] [Google Scholar]
- Gougeon M.L. and Montagnier,L. (1999) Programmed cell death as a mechanism of CD4 and CD8 T cell depletion in AIDS—Molecular control and effect of highly active anti-retroviral therapy. Ann. N. Y. Acad. Sci., 887, 199–212. [DOI] [PubMed] [Google Scholar]
- Jacotot E. et al. (2000) The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med., 191, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacotot E. et al. (2001) Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 Vpr and Bcl-2. J. Exp. Med., 193, 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E. and Chen,J. (2000) Cytoplasmic-nuclear shuttling of FKBP12–rapamycin-associated protein is involved in rapamycin-sensitive signaling and translation initiation. Proc. Natl Acad. Sci. USA, 97, 14340–14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann M. and Fernandez,A. (2001) Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell. Mol. Life Sci., 58, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnitchenko V. et al. (1995) Human immunodeficiency virus 1 envelope-initiated G2-phase programmed cell death. Proc. Natl Acad. Sci. USA, 92, 11889–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarov P.G., Komarova,E.A., Kondratov,R.V., Christov-Tselkov,K., Coon,J.S., Chernov,M.V. and Gudkov,A.V. (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science, 285, 1733–1737. [DOI] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1991) The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell, 64, 903–914. [DOI] [PubMed] [Google Scholar]
- Lifson J.D., Reyes,G.R., McGrath,M.S., Stein,B.S. and Engleman,E.G. (1986) AIDS retrovirus-induced cytopathology: giant cell formation and involvement of CD4 antigen. Science, 232, 1123–1127. [DOI] [PubMed] [Google Scholar]
- Loeffler M., Daugas,E., Susin,S.A., Zamzami,N., Métivier,D., Nieminen,A.-L., Brothers,G., Penninger,J.M. and Kroemer,G. (2001) Dominant cell death induction by extramitochondrially targeted apoptosis inducing factor. FASEB J., 15, 758–767. [DOI] [PubMed] [Google Scholar]
- Maas J.J., Gange,S.J., Schuitemaker,G., Coutinho,R.A., van Leeuwen,R. and Margolick,J.B. (2000) Strong association between failure of T cell homeostasis and the syncytium-inducing phenotype among HIV-1-infected men in the Amsterdam Cohort Study. AIDS, 16, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Mohri H. et al. (2001) Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med., 194, 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrksich M., Chen,C.S., Xia,Y., Dike,L., Ingber,D.E. and Whitesides,G.M. (1996) Controlling cell attachment on contoured surfaces with self-assembled monolayers of alkanethiolates on gold. Proc. Natl Acad. Sci. USA, 93, 10775–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Murase,Y., Koseki,T., Sato,T., Yamato,K. and Nishihara,T. (2001) Osteoclast differentiation is associated with transient upregulation of cyclin-dependent kinase inhibitors. J. Cell. Biochem., 80, 339–345. [PubMed] [Google Scholar]
- Peter M., Nakagawa,J., Doree,M., Labbe,J.C. and Nigg,E.A. (1990) In vitro dissassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell, 61, 591–602. [DOI] [PubMed] [Google Scholar]
- Petit F., Arnoult,D., Lelievre,J.D., Parseval,L.M., Hance,A.J., Schneider,P., Corbeil,J., Ameisen,J.C. and Estaquier,J. (2002) Productive HIV-1 infection of primary CD4+ T cells induces mitochondrial membrane permeabilization leading to caspase-independent cell death. J. Biol. Chem., 277, 1477–1487. [DOI] [PubMed] [Google Scholar]
- Pines J. (1999) Cell cycle: checkpoint on the nuclear frontier. Nature, 397, 104–105. [DOI] [PubMed] [Google Scholar]
- Pines J. and Hunter,T. (1994) The differential localization of human cyclins A and B is due to a cytoplasmic retention signal in cyclin B. EMBO J., 13, 3772–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggero R., Robert-Hebmann,V., Harrington,S., Roland,J., Verne,L., Jaleco,S., Devaux,C. and Biard-Piechaczyk,M. (2001) Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochndrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J. Virol., 75, 7637–7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Miyazaki,K., Koga,Y., Kimura,G., Nomoto,K. and Yoshida, H. (2002) Calcineurin-dependent mitochondrial disturbances in calcium-induced apoptosis of human immunodeficiency virus gp160-expressing CD4+ cells. J. Virol., 76, 416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C. and Jassoy,C. (2001) Syncytium formation amplifies apoptotic signals: a new view on apoptosis in HIV infection in vitro. Virology, 282, 48–55. [DOI] [PubMed] [Google Scholar]
- Selliah N. and Finkel,T.H. (2001) Biochemical mechanisms of HIV induced T cell apoptosis. Cell Death Differ., 8, 127–136. [DOI] [PubMed] [Google Scholar]
- Smits V.A. and Medema,R.H. (2001) Checking out the G2/M transition. Biochim. Biophys. Acta, 1519, 1–12. [DOI] [PubMed] [Google Scholar]
- Sodroski J.G., Goh,W.C., Rosen,A., Campbell,K. and Haseltine,W.A. (1986) Role of the HTLV/LAV envelope in syncytia formation and cytopathicity. Nature, 322, 470–474. [DOI] [PubMed] [Google Scholar]
- van den Heuvel S. and Harlow,E. (1993) Distinct roles for cyclin dependent kinases in cell cycle control. Science, 262, 2050–2054. [DOI] [PubMed] [Google Scholar]
- Vlahakis S.R., Algeciras-Schimnich,A., Bou,G., Heppelmann,C.J., Villasis-Keever,A., Collman,R.C. and Paya,C.V. (2001) Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J. Clin. Invest., 107, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. et al. (2001) Inhibition of human immunodeficienct virus type 1 transcrition by chemic cyclin dependent kinase inhibitors. J. Virol., 75, 7266–7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.H., Schendel,S., Matsuyama,S. and Reed,J.C. (1998) Acidic pH promotes dimerization of Bcl-2 family proteins. Biochemistry, 37, 6410–6418. [DOI] [PubMed] [Google Scholar]
- Zhang H., Stallock,J.P., Ng,J.C., Reinhard,C. and Neufeld,T.P. (2000) Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev., 14, 2712–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]