Abstract
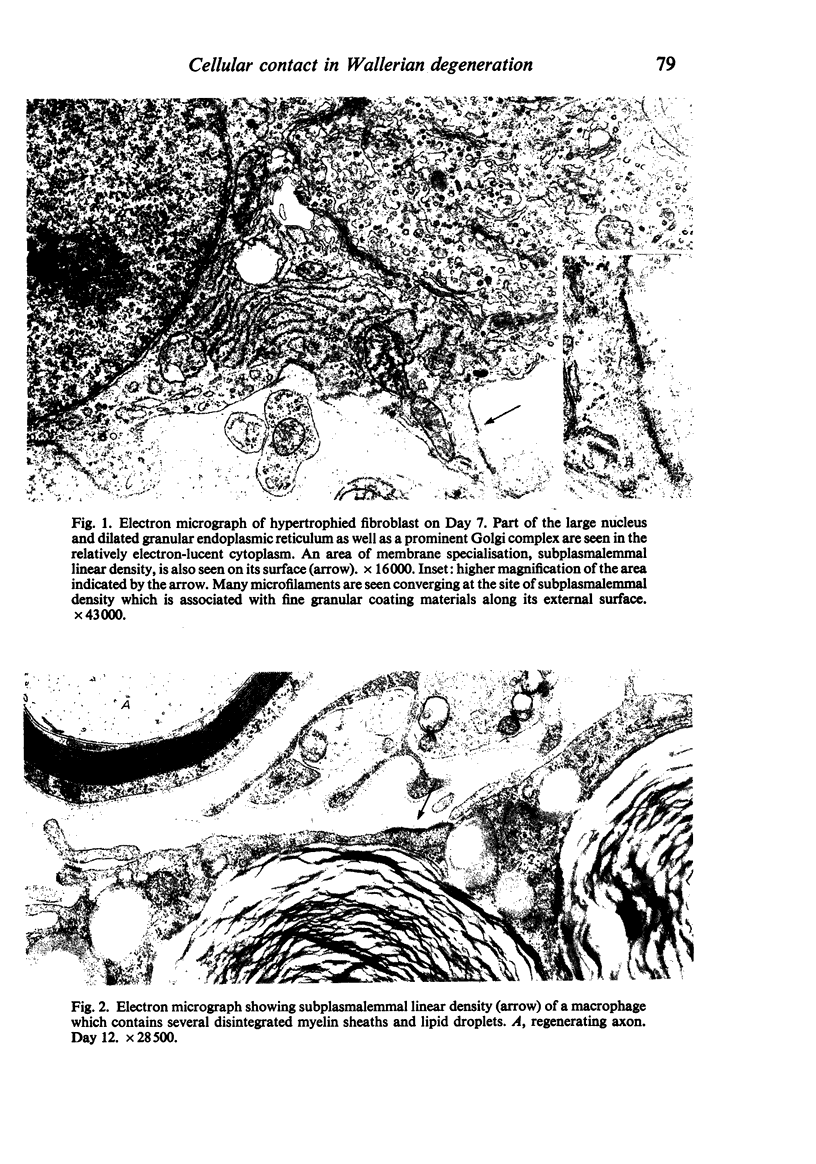
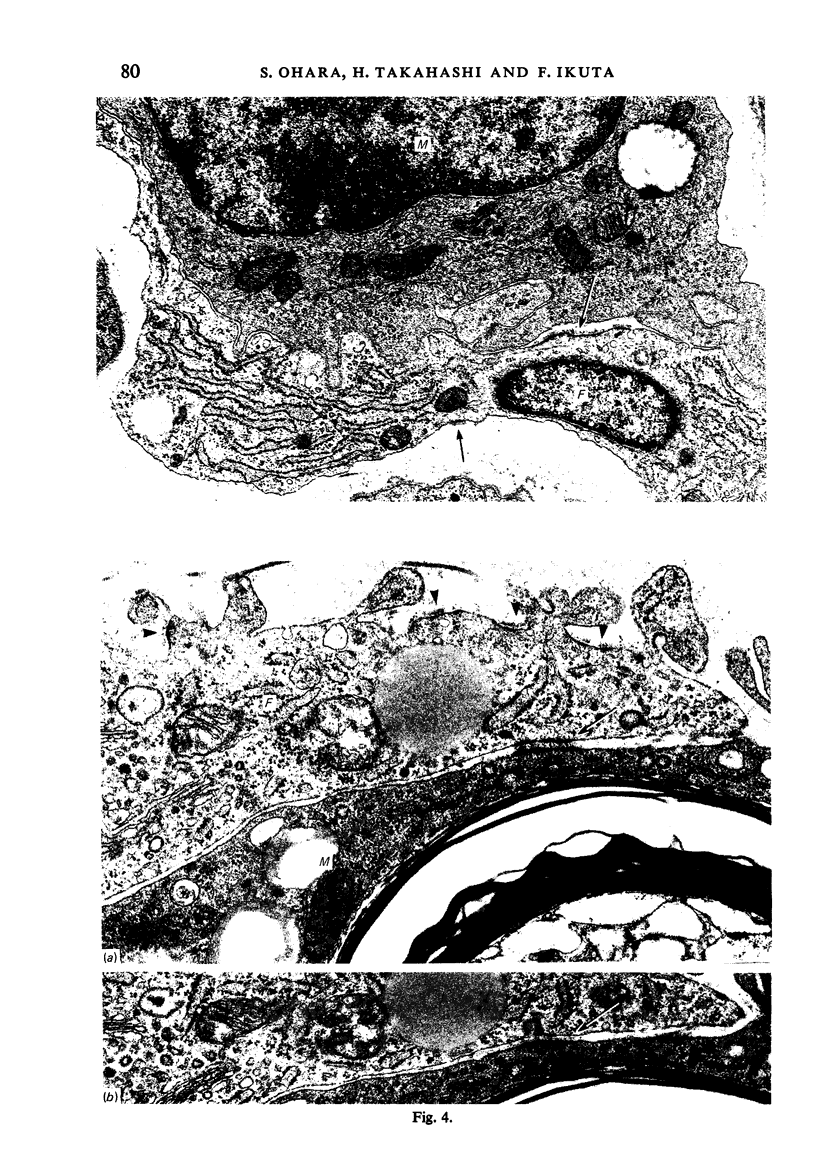
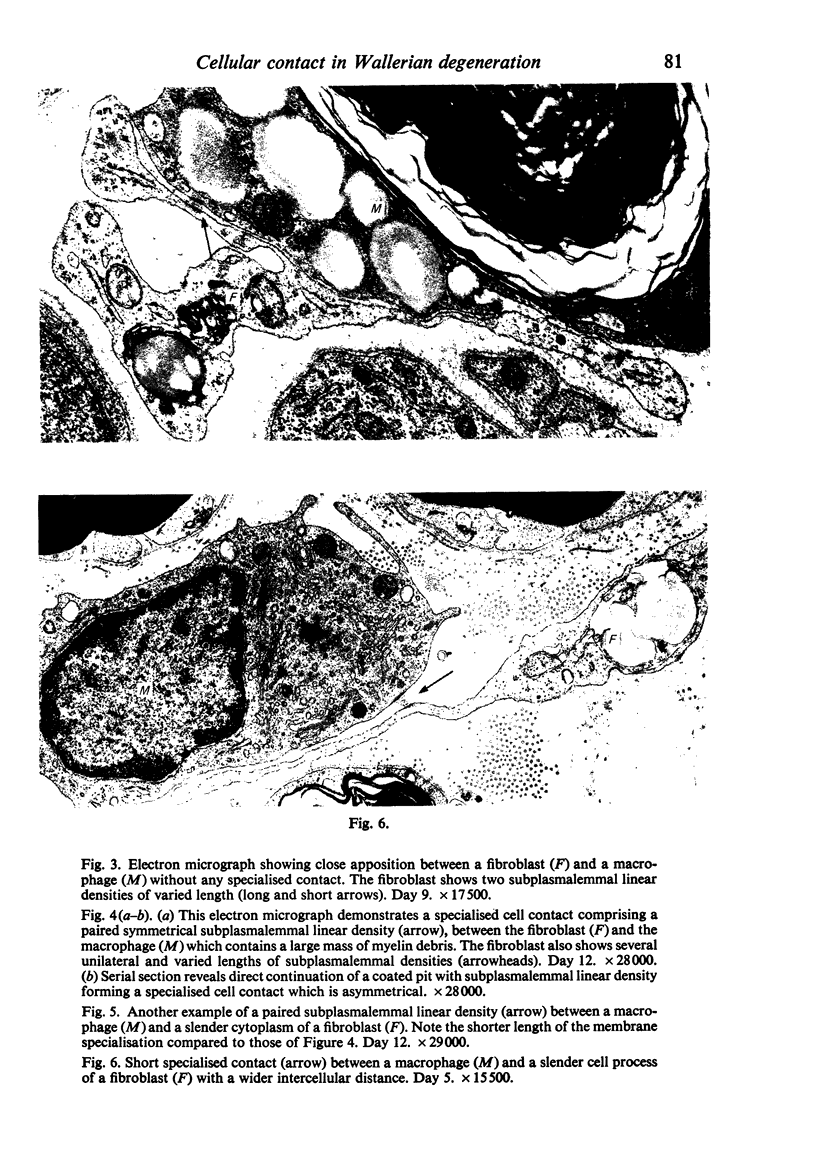
Wallerian degeneration was induced by crushing the mouse phrenic nerve at the neck. During a chronological study, a specialised cell contact was often observed between the activated endoneurial fibroblast and the macrophage at the period when the removal of myelin debris by macrophages was prominent in the endoneurium. The specialised contact was characterised by paired subplasmalemmal linear condensations with a relatively constant thickness and varied length. It was sometimes asymmetrical. In these specialised cell membrane areas the intercellular space was filled with fine granular material showing a midline denser stratum. Coated vesicles were occasionally found in association with the subplasmalemmal densities. The specialised contacts with these features are quite different from any type of previously described cell contact between fibroblasts but are morphologically identical to those reported between cells of the mononuclear phagocytotic system. The significance of specialised contacts between the fibroblasts and macrophages in Wallerian degeneration is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caputo R., Gianotti F. Junctions between histiocytes: role of coated vesicles. J Ultrastruct Res. 1979 Sep;68(3):256–264. doi: 10.1016/s0022-5320(79)90158-8. [DOI] [PubMed] [Google Scholar]
- Ebner H., Gebhart W. Zur Ultrastruktur der multizentrischen Reticulohistiocytose. Arch Dermatol Forsch. 1971;240(3):259–270. [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gibson J. D. The origin of the neural macrophage: a quantitative ultrastructural study of cell population changes during Wallerian degeneration. J Anat. 1979 Aug;129(Pt 1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Gilula N. B., Reeves O. R., Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972 Feb 4;235(5336):262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- Greenlee T. K., Jr, Ross R. The development of the rat flexor digital tendon, a fine structure study. J Ultrastruct Res. 1967 May;18(3):354–376. doi: 10.1016/s0022-5320(67)80124-2. [DOI] [PubMed] [Google Scholar]
- Judd P. A., Finnegan P., Curran R. C. Pulmonary sarcoidosis: A clinico-pathological study. J Pathol. 1975 Apr;115(4):191–198. doi: 10.1002/path.1711150402. [DOI] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Crystal R. G. Subplasmalemmal linear densities in cells of the mononuclear phagocyte system in lung. Am J Pathol. 1980 Jul;100(1):131–150. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Katayama M., Bourque E., Suzuki K., Suzuki K. The twitcher mouse: positive immunohistochemical staining of globoid cells with monoclonal antibody against Mac-1 antigen. Brain Res. 1985 May;352(1):49–54. doi: 10.1016/0165-3806(85)90086-0. [DOI] [PubMed] [Google Scholar]
- Korn J. H., Halushka P. V., LeRoy E. C. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980 Feb;65(2):543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. Membrane ultrastructure at mammalian intercellular junctions. Prog Biophys Mol Biol. 1973;26:45–101. doi: 10.1016/0079-6107(73)90017-5. [DOI] [PubMed] [Google Scholar]
- Oldfors A. Macrophages in peripheral nerves. An ultrastructural and enzyme histochemical study on rats. Acta Neuropathol. 1980;49(1):43–49. doi: 10.1007/BF00692218. [DOI] [PubMed] [Google Scholar]
- Parry E. W. Some electron microscope observations on the mesenchymal structures of full-term umbilical cord. J Anat. 1970 Nov;107(Pt 3):505–518. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Greenlee T. K., Jr Electron microscopy: attachment sites between connective tissue cells. Science. 1966 Aug 26;153(3739):997–999. doi: 10.1126/science.153.3739.997. [DOI] [PubMed] [Google Scholar]
- Shore R. C., Berkovitz B. K., Moxham B. J. Intercellular contacts between fibroblasts in the periodontal connective tissues of the rat. J Anat. 1981 Aug;133(Pt 1):67–76. [PMC free article] [PubMed] [Google Scholar]
- Sims D. E., Westfall J. A. Microfilament-associated adhering junctions (6 nm F-maculae adherentes) connect bovine pulmonary fibroblasts in vivo. Eur J Cell Biol. 1982 Aug;28(1):145–150. [PubMed] [Google Scholar]
- Takahashi H., Suzuki K. Globoid cell leukodystrophy: specialized contact of globoid cell with astrocyte in the brain of twitcher mouse. Acta Neuropathol. 1982;58(4):237–242. doi: 10.1007/BF00688603. [DOI] [PubMed] [Google Scholar]
- Yajima K., Fletcher T. F., Suzuki K. Sub-plasmalemmal linear density: a common structure in globoid cells and mesenchymal cells. Acta Neuropathol. 1977 Aug 31;39(3):195–200. doi: 10.1007/BF00691697. [DOI] [PubMed] [Google Scholar]
- van de Water L., 3rd, Schroeder S., Crenshaw E. B., 3rd, Hynes R. O. Phagocytosis of gelatin-latex particles by a murine macrophage line is dependent on fibronectin and heparin. J Cell Biol. 1981 Jul;90(1):32–39. doi: 10.1083/jcb.90.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]