Abstract
The Saccharomyces cerevisiae morphogenesis checkpoint delays mitosis in response to insults that impair actin organization and/or bud formation. The delay is due to accumulation of the inhibitory kinase Swe1p, which phosphorylates the cyclin-dependent kinase Cdc28p. Having screened through a panel of yeast mutants with defects in cell morphogenesis, we report here that the polarity establishment protein Bem2p is required for the checkpoint response. Bem2p is a Rho-GTPase activating protein (GAP) previously shown to act on Rho1p, and we now show that it also acts on Cdc42p, the GTPase primarily responsible for establishment of cell polarity in yeast. Whereas the morphogenesis role of Bem2p required GAP activity, the checkpoint role of Bem2p did not. Instead, this function required an N-terminal Bem2p domain. Thus, this single protein has a GAP-dependent role in promoting cell polarity and a GAP-independent role in responding to defects in cell polarity by enacting the checkpoint. Surprisingly, Swe1p accumulation occurred normally in bem2 cells, but they were nevertheless unable to promote Cdc28p phosphorylation. Therefore, Bem2p defines a novel pathway in the morphogenesis checkpoint.
Keywords: Bem2p/cell cycle/checkpoint/Rho-GAP/Saccharomyces cerevisiae
Introduction
In all eukaryotic cells, the G2/M transition is controlled by the cyclin-dependent kinase Cdc2 (Cdc28p in Saccharo myces cerevisiae), whose activity is in turn tightly regulated by the countervailing activities of inhibitory Wee1-family kinases (Swe1p in S.cerevisiae) and stimulatory Cdc25-family phosphatases (Mih1p in S.cerevisiae) (reviewed by Coleman and Dunphy, 1994; O’Farrell, 2001). In many cells, the Wee1/Cdc25 balance is tilted firmly in favor of Wee1 during G2, resulting in the accumulation of phosphorylated and inhibited Cdc2 complexes. An ill-defined initiating event then tilts the balance in favor of Cdc25, promoting dephosphorylation and activation of Cdc2, with consequent entry into mitosis. Checkpoint controls responding to DNA damage or incomplete DNA replication delay entry into mitosis by maintaining the G2 supremacy of the inhibitory Wee1-family kinases.
A rather different situation exists in the budding yeast S.cerevisiae, where the mitotic Cdc28p complexes do not appear to be significantly restrained by inhibitory phosphorylation (Amon et al., 1992; Sorger and Murray, 1992). In this organism, the Swe1p/Mih1p balance is tilted firmly in favor of the phosphatase Mih1p, for at least two reasons. First, Swe1p is only synthesized during late G1 phase (Sia et al., 1996) and is largely degraded by the time of mitosis (Sia et al., 1998). Secondly, even when Swe1p is stabilized by mutation of Swe1p degradation factors, Mih1p is sufficiently active to overcome Swe1p-mediated Cdc28p inhibition and cells experience only a modest G2 delay (Ma et al., 1996; McMillan et al., 1999). In combination, the degradation of Swe1p and the activity of Mih1p ensure that very little Cdc28p inhibition occurs in the unperturbed cell cycle.
In S.cerevisiae, the DNA damage and DNA replication checkpoint controls do not block Cdc28p activation (Sorger and Murray, 1992; Stueland et al., 1993), but rather act later in the cell cycle to inhibit the metaphase– anaphase transition (Yamamoto et al., 1996). In contrast, a ‘morphogenesis checkpoint’ acts to inhibit mitotic Cdc28p complexes by tilting the Swe1p/Mih1p balance in favor of Swe1p. This checkpoint pathway responds to a variety of environmental and experimental insults that impair bud formation (e.g. changes in temperature or osmolarity that depolarize actin, or drugs that depolymerize actin) (Lew and Reed, 1995; McMillan et al., 1998). The G2 delay provided by the checkpoint allows cells to recover from the insult and complete bud formation prior to undergoing nuclear division. To tilt the Swe1p/Mih1p balance in favor of Swe1p, the morphogenesis checkpoint halts the degradation of Swe1p (Sia et al., 1998) and activates a kinase cascade culminating in the MAPK Mpk1p/Slt2p, which is thought to downregulate Mih1p (Harrison et al., 2001).
We are particularly interested in understanding how the morphogenesis checkpoint detects the perturbations that impair bud formation. Studies on the DNA replication checkpoint revealed that mutations affecting components of the replication fork could render cells unable to delay the cell cycle in response to arrest of DNA replication, suggesting that the source for the checkpoint signal was the stalled replication fork itself (Araki et al., 1995; Navas et al., 1995; Sugimoto et al., 1996). By analogy, we reasoned that the morphogenesis checkpoint may monitor some ‘bud formation complex’, so that mutations in components of that complex might render the checkpoint ineffective.
Studies of yeast morphogenesis have supported a hierarchical model for bud formation in which ‘bud-site selection’ proteins act to localize the activity of ‘polarity establishment’ proteins, which in turn polarize the actin cytoskeleton and hence the secretory pathway to form a bud (reviewed by Pringle et al., 1995; Pruyne and Bretscher, 2000a,b). We tested mutant strains defective in a variety of proteins involved at each of these steps to determine whether any would be defective for the morphogenesis checkpoint. Here we report that one of these mutants, lacking the polarity establishment protein Bem2p, was uniquely defective in this regard.
Bem2p is a 246 kDa protein with a 160 amino acid Rho-GAP domain at its C-terminus (Peterson et al., 1994). Although the GAP activity of Bem2p was important for its role in polarity establishment, this activity was dispensable for its checkpoint function. Surprisingly, bem2Δ mutants were fully competent to stabilize Swe1p and to activate Mpk1p in response to actin depolymerization, but they nevertheless failed to inhibit Cdc28p and arrest the cell cycle. These studies therefore indicate the existence of a novel branch of the morphogenesis checkpoint that involves a non-enzmatyic function of the Rho- GAP Bem2p.
Results
A specific requirement for Bem2p in the morphogenesis checkpoint
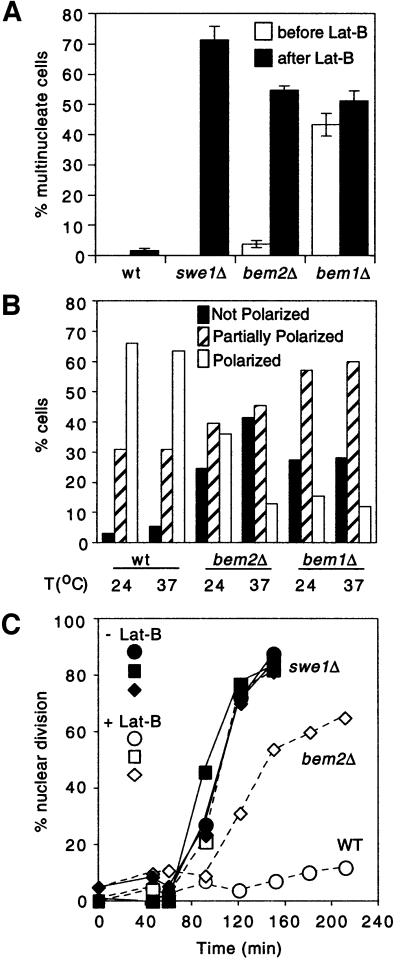
Reasoning that proteins functioning in the process of bud formation might be ideally placed to monitor the progress of bud formation, we examined checkpoint function in strains containing mutations in various genes known to be involved in that process. To assess checkpoint function, stationary phase cells were inoculated into fresh medium containing latrunculin-B (Lat-B), which partially depolymerizes actin and prevents bud formation. Wild-type cells arrest cell cycle progression in G2 under these conditions, whereas cells defective for checkpoint function (e.g. swe1Δ mutants) continue with the cell cycle and become binucleate (McMillan et al., 1998). Thus, DNA staining provides a rapid and simple assay to test whether or not mutant cells have an intact checkpoint. We found that mutants in genes encoding known bud site selection components or actin cytoskeleton components all arrested the cell cycle upon actin depolymerization (Table I). Similar results were obtained in tests of mutant strains carrying a series of alanine scanning mutations in the ACT1 gene (encoding the sole yeast actin), as well as mutations in most polarity establishment genes (Table I). The only mutants, other than swe1Δ, which displayed significant numbers of binucleate cells following Lat-B treatment were bem1Δ and bem2Δ (Figure 1A).
Table I. Yeast strains tested for morphogenesis checkpoint.
| Genotype | Description | Binucleates in Lat-B |
|---|---|---|
| Bud site selection genes | ||
| rsr1Δ | Ras-like regulator of bud site selection | – |
| bud2Δ | GAP for Rsr1p | – |
| bud5Δ | GEF for Rsr1p | – |
| rsr1Δ bud8Δ | Bud8p: landmark for bipolar budding | – |
| spa2Δ | Required for bipolar budding and shmoo formation | – |
| bni1Δ | Formin homolog | – |
|
aip3Δ2 |
= Bud6p required for bipolar budding |
– |
| Polarity establishment genes | ||
| cdc24-1a | GEF for Cdc42p | – |
| bem1Δ | Scaffold for polarity establishment proteins | +++ |
| bem2Δ | Rho GAP | +++ |
| cla4Δ | PAK kinase, Cdc42p effector | – |
| ste20Δ | PAK kinase, Cdc42p effector | – |
| gic1Δ gic2Δ | Cdc42p effectors | – |
| msb1Δ | Unknown function | – |
| msb3Δ msb4Δ | Rab GAPs | – |
| rho2Δ | Rho GTPase | – |
|
rho4Δ |
Rho GTPase |
– |
| Actin and actin-associated proteins | ||
| act1-101 | Actin | – |
| act1-102 | Actin | – |
| act1-105 | Actin | – |
| act1-108 | Actin | – |
| act1-111 | Actin | +/–b |
| act1-115 | Actin | – |
| act1-116 | Actin | – |
| act1-119 | Actin | – |
| act1-120 | Actin | +/–b |
| act1-121 | Actin | – |
| act1-122 | Actin | – |
| act1-123 | Actin | – |
| act1-124 | Actin | – |
| act1-129 | Actin | – |
| act1-132 | Actin | – |
| act1-133 | Actin | – |
| act1-135 | Actin | – |
| act1-136 | Actin | – |
| cap2Δ | Actin filament capping protein | – |
| tpm1Δ | Tropomyosin, actin filament binding | – |
| sac6Δ | Fimbrin, actin filament bundling | – |
| cof1-4 | Cofilin, actin filament severing | – |
| cof1-5 | Cofilin, actin filament severing | – |
| cof1-22 | Cofilin, actin filament severing | – |
| abp1Δ | Actin binding protein, cortical patch component | – |
| pfy1-111 | Profilin, actin monomer binding | – |
| pfy1-112 | Profilin, actin monomer binding | – |
| pfy1-116 | Profilin, actin monomer binding | – |
| myo1Δ | Type II myosin, cytokinesis | – |
| myo2-66 | Type V myosin, organelle and vesicle transport | – |
| myo4Δ | Type V myosin, mRNA localization | – |
| myo3Δ | Type I myosin, cortical patch component | – |
|
myo5Δ |
Type I myosin, cortical patch component |
– |
| Septins |
|
|
| cdc10-1a | Septin | – |
| cdc11-6a | Septin | – |
aThese strains were assayed at the restrictive temperature of 37°C.
bThese two strains showed a smaller but reproducible accumulation of binucleated cells, but this phenotype was not recapitulated upon transfer of the act1 mutations to strain BF264-15D.
Fig. 1. Morphogenesis checkpoint in bem2Δ cells. (A) The indicated strains were grown to exponential phase in YEPD at 24°C and then resuspended in YEPD + 100 µM Lat-B and grown for 5 h. Cells were then fixed and stained with DAPI to visualize nuclei. Percentages of unbudded cells with multiple (>2) nuclei were scored for each strain before and after the growth in Lat-B. (B) The indicated strains were grown to exponential phase in YEPD at the indicated temperature, fixed and stained to visualize F-actin. Budded cells with an undivided nucleus were scored as polarized (all actin patches in bud), partially polarized (large majority of patches in bud), or not polarized (random distribution of actin patches). In the case of the bem1Δ mutant, a large proportion of the cells had >1 nucleus (A), and the multinucleate cells were uniformly worse in terms of actin polarity than the cells with a single nucleus. Our scoring therefore measured only the best polarized of the bem1Δ cells, and overall the polarity defect was more pronounced in bem1Δ than in bem2Δ mutants. (C) The indicated strains were synchronized in G1 with α-factor and released into fresh YEPD at 24°C. After release (30 min), cells were resuspended in 100 µM Lat-B (+) or DMSO (–) as a vehicle control. At the indicated time points, cells were fixed and stained with DAPI to score nuclear division. Strains: DLY1 (WT), DLY4021 (swe1Δ), DLY4015 (bem2Δ) and JMY1011 (bem1Δ).
The BEM1 and BEM2 genes (bud emergence) were first identified in a screen for mutants that were synthetic lethal in combination with loss of MSB1 (multicopy suppressor of budding) (Bender and Pringle, 1991), which was itself identified as a suppressor of both cdc24 and cdc42 mutants (Bender and Pringle, 1989). Subsequent studies demonstrated numerous genetic interactions between these mutations and others involved in bud site selection, polarity establishment and the actin cytoskeleton (Chant et al., 1991; Wang and Bretscher, 1995; Bender et al., 1996; Chen et al., 1996; Oehlen and Cross, 1998; Tong et al., 2001). bem1Δ and bem2Δ are among the most severely depolarized of all of the viable mutants that we tested, raising the possibility that the apparent checkpoint defect might be a consequence of the constitutively disorganized cytoskeleton exhibited by these mutants. However, further analysis showed that the basis for the observed accumulation of binucleate cells in these mutants was distinct. In particular, the bem1Δ cells, which displayed the most severe polarity defect (Figure 1A and B, see legend), managed to proliferate with a constitutively high proportion of multinucleate cells in the population even in the absence of Lat-B treatment. This proportion did not increase significantly in response to Lat-B (Figure 1A), suggesting that the checkpoint was intact in this mutant despite the severe constitutive depolarization. In contrast, the bem2Δ mutant appeared somewhat healthier and proliferated with many fewer multinucleate cells in the population, but showed a dramatic increase in the proportion of multinucleate cells following treatment with Lat-B (Figure 1A). To assess the behavior of this mutant in more detail, the kinetics of nuclear division were examined in synchronous cultures released from a pheromone-mediated G1 arrest to traverse the cell cycle in the presence or absence of Lat-B. In this experiment, the bem2Δ cells treated with Lat-B displayed only a small (∼1 h) delay in nuclear division compared with the untreated controls (Figure 1C). Wild-type cells arrested prior to nuclear division in the presence of Lat-B, whereas swe1Δ cells proceeded with nuclear division regardless of Lat-B (Figure 1C), as expected from previous work. We conclude that Bem2p, alone of all the bud formation proteins tested, is specifically required for the morphogenesis checkpoint to arrest the cell cycle in response to actin depolymerization.
GAP activity of Bem2p and the morphogenesis checkpoint
Bem2p is one of 11 proteins encoded in the yeast genome that contain a putative Rho-GAP domain. To assess which subset of these proteins might be important for checkpoint function, we assayed the effectiveness of the checkpoint in strains containing deletion alleles for each of these genes. Strikingly, only the bem2Δ strain had a significant checkpoint defect (Figure 2A), suggesting that the checkpoint role is restricted to Bem2p and not linked to general Rho-GAP activity.
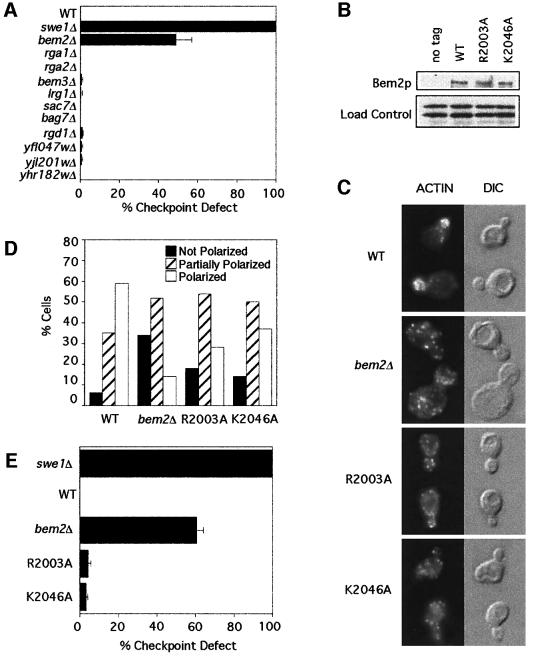
Fig. 2. Bem2p GAP activity and the morphogenesis checkpoint. (A) Homozygous diploid mutant strains of the indicated genotype were assayed for their ability to arrest the cell cycle when released from stationary phase into 100 µM Lat-B at 24°C. Percent checkpoint defect was calculated as the increase in binucleates due to Lat-B in the indicated strain as compared with the increase in the swe1Δ control strain, which was set to 100%. (B) The indicated strains were grown to exponential phase in YEPD at 30°C, pelleted and lysed. Lysates were separated by SDS–PAGE and immunoblotted with anti-myc antibody. (C) The indicated strains were grown to exponential phase in YEPD at 30°C, fixed and stained to visualize F-actin. (D) Actin polarity of samples was scored as in Figure 1B. Note that expression of wild-type BEM2 with a myc tag (WT) causes a slight polarity defect. (E) The indicated strains were assayed for checkpoint function as in (A). Strains: DLY1 (no tag), DLY4021 (swe1Δ), DLY4015 (bem2Δ), DLY4860 (WT), DLY5041 (R2003A) and DLY4862 (K2046A).
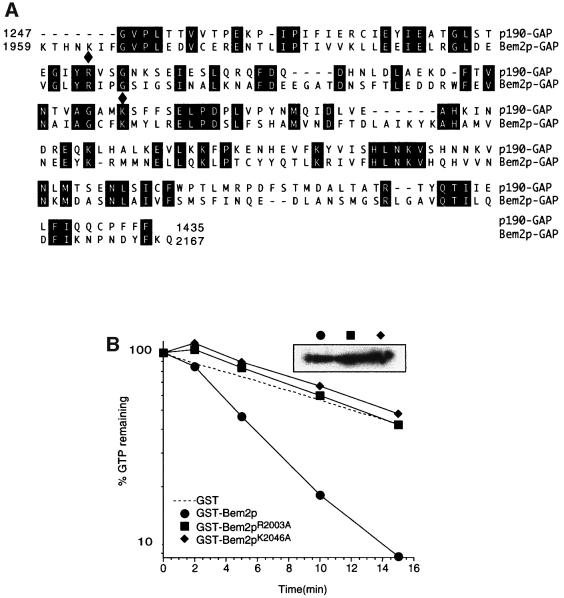
To test whether the GAP activity of Bem2p was required for its function in the checkpoint, we first generated a C-terminal truncation of Bem2p lacking the GAP domain (residues 1959–2167). This bem2-Δ1959– 2167 allele behaved as a bem2Δ null mutant both with regard to morphogenesis and with regard to checkpoint function (data not shown). Although this result is consistent with the hypothesis that the GAP domain is critical for all Bem2p functions, it is also possible that the truncation impairs the three-dimensional folding of Bem2p, indirectly inactivating some function(s) of the large non-catalytic domain. To address this possibility, we generated two single amino acid mutations in conserved residues that have been shown to be important for the activity of the mammalian p190 Rho-GAP (Figure 3A) (Li et al., 1997). The crystal structure of several mammalian Rho-GAP domains has been solved (Musacchio et al., 1996; Barrett et al., 1997), revealing that Arg1283 participates directly in catalysis as part of the ‘arginine finger’, and that Lys1321 is an important contact point for Rho binding (Musacchio et al., 1996; Barrett et al., 1997). The R1283L and K1321A mutations therefore abolish GAP activity, without disturbing the overall folding of the GAP domain. We made the analogous R2003A and K2046A mutations in BEM2 (Figure 3A). Both mutant alleles were expressed at levels comparable to those of the wild-type Bem2p (Figure 2B). The morphogenesis phenotype of these mutants was qualitatively similar to that of bem2Δ mutants, though not quite as penetrant or as severe (Figure 2C and D). However, the mutant strains were fully capable of enacting a checkpoint-mediated cell cycle arrest in response to Lat-B (Figure 2E). This result suggests that GAP activity is important for the morphogenesis role, but not for the checkpoint role, of Bem2p.
Fig. 3. Point mutations in Bem2p greatly reduce GAP activity. (A) Alignment of the Bem2p and mammalian p190 GAP domains. Residues of Bem2p homologous to critical residues of p190 are indicated by filled diamonds. (B) Cdc42p prebound to [γ-32P]GTP was incubated with GST, GST–Bem2 GAP domain, or the same domain with the R2003A or K2046A change. Radioactivity remaining bound to Cdc42p is plotted against time of incubation. The inset shows an anti-GST western blot indicating that equal levels of the different GAP domains were used in each assay. Control experiments with Cdc42p bound to [α-32P]GTP did not show a decline in bound radioactivity, demonstrating that the observed decrease reflected GTP hydrolysis, not release of GTP (data not shown).
The interpretation of this experiment relies on whether or not the mutations impaired the GAP activity of Bem2p. Although a strong argument that they must do so can be made based on structural considerations, we wished to confirm that this was indeed the case. To that end, we made recombinant GST fusion proteins containing the GAP domain of wild-type or mutant Bem2p. The physiological target Rho proteins for this domain are not entirely clear. Previous studies indicated that the Bem2p GAP domain could act on Rho1p in vitro, although the phenotype of bem2 mutants is more suggestive of an involvement with the polarity establishment GTPase Cdc42p in vivo. We have had some success in measuring Cdc42p-directed GAP activity associated with Rga1p (Gladfelter et al., 2002), and we found that the Bem2p GAP domain was also effective in accelerating GTP hydrolysis by Cdc42p (Figure 3B). Using this assay, we found that both of the mutations described above greatly reduced the in vitro GAP activity of GST–Bem2p (Figure 3). Thus, we feel confident that Bem2p GAP activity is impaired by the mutations, and therefore that GAP activity of Bem2p is not required for the morphogenesis checkpoint.
Uncoupling the morphogenesis and checkpoint roles of Bem2p
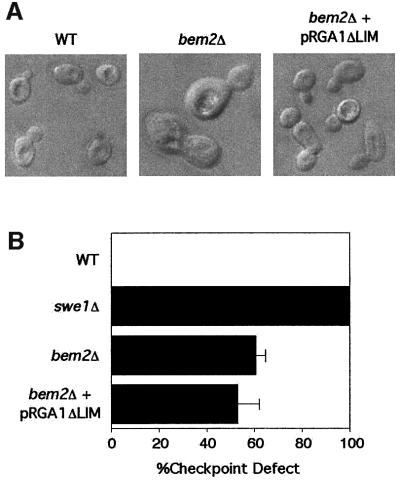
Previous studies showed that the morphogenesis defects of bem2 mutants could be largely suppressed by a dominant mutation causing the expression of a truncated derivative of the Cdc42p-GAP Rga1p, which retained the GAP domain but lacked an N-terminal region containing two LIM domains (Chen et al., 1996). This result, combined with some shared phenotypes between rga1 and bem2 mutants, and the finding that rga1Δ bem2Δ double mutants were inviable, led Chen and colleagues to conclude that these two Rho-GAPs shared some function(s) in morphogenesis. We confirmed that overexpression of N-terminally truncated RGA1 could effectively suppress the morphology defect of bem2Δ mutants in our strain background (Figure 4A). However, the truncated RGA1 was completely unable to restore checkpoint function to the bem2Δ mutant (Figure 4B). This strain therefore uncouples the morphogenesis defect from the checkpoint defect, strongly suggesting that the checkpoint defect is not an indirect effect of the perturbation of morphogenesis, but rather represents a separate function of Bem2p.
Fig. 4. Effect of overexpression of RGA1ΔLIM on cell morphology and checkpoint function in bem2Δ cells. (A) The strains DLY1 (BEM2) and DLY 4015 (bem2Δ) containing either empty plasmid (YEplac195) or RGA1ΔLIM (pDLB2129) were grown to exponential phase in dextrose-containing medium at 30°C. Images of live cells were captured using DIC microscopy. (B) The indicated strains were assayed for checkpoint function as in Figure 2A.
Mutational analysis of the N-terminal domain of Bem2p
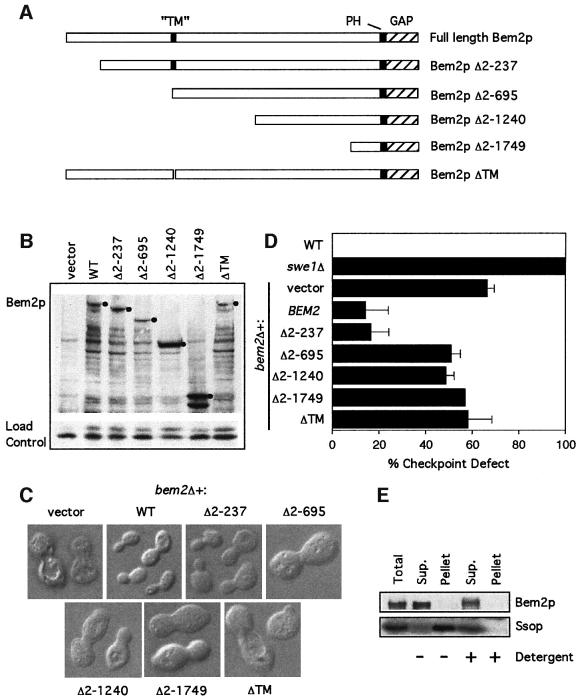
As the GAP activity of Bem2p was not required for its checkpoint role, we investigated the large non-catalytic N-terminal portion of the protein. Apart from a potential pleckstrin homology (PH) domain adjacent to the GAP domain at the C-terminus, this region does not display obvious sequence motifs, with the possible exception of a short hydrophobic stretch (residues 688–707), which could be a transmembrane domain (Figure 5A). We made a series of low-copy plasmids that express wild type or N-terminally truncated derivatives of Bem2p from its own promoter, containing a C-terminal myc tag for ease of detection. These derivatives were expressed at levels roughly comparable to those of full-length Bem2p-myc, suggesting that they encode stable proteins (Figure 5B). When the plasmids were introduced into a bem2Δ strain, we found that removal of the first 237 amino acids did not affect the ability of the Bem2p derivative to complement either the morphogenesis defect (Figure 5C) or the checkpoint defect (Figure 5D) of the mutant. However, removal of the first 695 amino acids (or more) eliminated complementation of both defects (Figure 5C and D). Amino acid 695 is centered in the middle of the putative transmembrane domain, and we found that a small internal deletion lacking amino acids 688–707 was also unable to complement bem2Δ phenotypes (Figure 5C and D).
Fig. 5. Deletion anaylsis of the N-terminal domain of Bem2p. (A) Domain structure of Bem2p, showing the C-terminal GAP domain, the PH domain and the putative transmembrane (TM) domain. Also illustrated are the regions of the protein missing in the various deletion constructs. (B) The bem2Δ strain DLY4015 containing the plasmids pRS316 (vector), pDLB768 (WT), pDLB2147 (Δ2–237), pDLB2118 (Δ2–695), pDLB2116 (Δ2–1240), pDLB2115 (Δ2–1749) or pDLB2249 (ΔTM) was grown to exponential phase in dextrose-containing media. Cell lysates from these strains were immunoblotted with an anti-myc antibody. The bands corresponding to full-length protein for each construct are marked with a filled circle. (C) Images of live cells from the same strains were captured using DIC microscopy. (D) The indicated strains were assayed for checkpoint function as in Figure 2A. (E) Cells of strain DLY4860 (BEM2-myc) were lysed and fractionated by ultracentrifugation at 250 000 g for 1 h. Pellet fractions were resuspended to the same volume as the supernatant and equal volumes were analyzed by immunoblotting with α-myc and α-Sso1/2p antibodies.
These results suggested that the putative transmembrane domain was required for proper Bem2p function, and raised the possibility that Bem2p might indeed be an integral membrane protein. To test whether this was the case, we separated a cell lysate of a strain expressing Bem2p-myc into membrane and soluble fractions by high-speed centrifugation. Whereas the control integral membrane proteins Sso1/2p were found in the pellet, Bem2p was quantitatively retained in the supernatant fraction (Figure 5E), showing that it is not an integral membrane protein. Presumably the hydrophobic region of Bem2p is serving some other essential function, either by interacting with other components or by mediating proper folding of the protein. Thus far, we have not succeeded in isolating a bem2 derivative that is defective with regard to the checkpoint function but not with regard to morphogenesis.
Bem2p levels during the cell cycle and in response to Lat-B
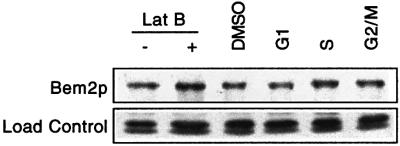
In order to determine the expression of Bem2p during the cell cycle, we arrested cells containing Bem2p-myc in G1, S or G2/M, and compared the level of Bem2p protein to that in asynchronous cells. The abundance of Bem2p did not differ significantly at different stages of the cell cycle (Figure 6). Similarly, cells that had been exposed to Lat-B did not show a significant difference in the level of Bem2p (Figure 6). Thus, if Bem2p is itself regulated by cell cycle or checkpoint cues, this regulation does not appear to be at the level of total abundance of Bem2p.
Fig. 6. Bem2p levels through the cell cycle and following treatment with Lat-B. The strain DLY4860 (BEM2-myc) was grown to exponential phase in YEPD at 30°C and then treated with 100 µM Lat-B, 30 ng/ml α-factor (G1), 0.25 M hydroxyurea (S) or 15 µg/ml nocodazole (G2/M) as indicated. Cells were then lysed and immunoblotted with an α-myc antibody.
Stabilization of Swe1p in bem2Δ mutants treated with Lat-B
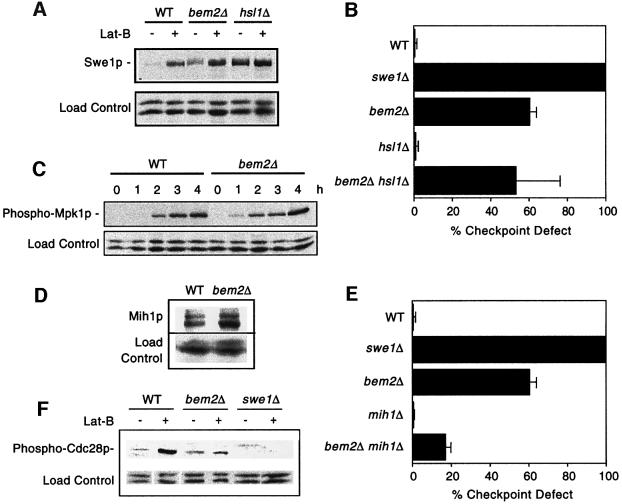
One effect of actin perturbation is the stabilization and consequent accumulation of Swe1p (Sia et al., 1998). Surprisingly, we found that proliferating bem2Δ cells expressed slightly higher amounts of Swe1p than wild-type controls (Figure 7A). Furthermore, Swe1p abundance was increased in bem2Δ mutants exposed to Lat-B, to levels equal to or exceeding those observed in wild-type cells exposed to Lat-B (Figure 7A). This result suggests that bem2Δ cells do not have a defect in Swe1p stabilization. Swe1p degradation requires the kinase Hsl1p, and in hsl1Δ mutants Swe1p is constitutively stable. We found that even bem2Δ hsl1Δ double mutants, in which Swe1p is presumably stable, failed to arrest the cell cycle in response to Lat-B (Figure 7B). We conclude that the checkpoint defect of bem2Δ mutants is not due to a failure to stabilize Swe1p.
Fig. 7. Swe1p, Mpk1p and Cdc28p regulation in bem2Δ cells. (A) The strains DLY1 (WT), DLY4021 (swe1Δ), DLY4015 (bem2Δ), JMY3-1 (hsl1Δ) and DLY4019 (bem2Δ hsl1Δ) were assayed for checkpoint function as in Figure 2A. (B) The strains DLY5330 (SWE1-myc), DLY5331 (bem2Δ SWE1-myc) and JMY1503 (hsl1Δ SWE1-myc) were grown to exponential phase and then resuspended in 100 µM Lat-B in YEPD and cultured for 2 h at 30°C. Lysates from cells before and after growth in Lat-B were immunoblotted with α-myc antibody. (C) The strains DLY1 (WT) and DLY4015 (bem2Δ) were grown to exponential phase in YEPD + 0.4 M NaCl, followed by resuspension and growth for the indicated times in the same medium supplemented with 100 µM Lat-B. Lysates were immunoblotted with anti-phospho-p44/p42 MAPK antibody to detect activated Mpk1p. (D) The strains JMY1299 (MIH1-myc) and DLY 4478 (bem2Δ MIH1-myc) were grown to exponential phase. Lysates were immunoblotted with α-myc antibody. (E) The strains DLY1 (WT), DLY4021 (swe1Δ), DLY4015 (bem2Δ), JMY3-59 (mih1Δ) and DLY3860 (bem2Δ mih1Δ) were assayed for checkpoint function as in Figure 2A. (F) The strains DLY1 (WT), DLY4015 (bem2Δ) and DLY4021 (swe1Δ) were grown to exponential phase in YEPD at 24°C and then resuspended in 100 µM Lat-B in YEPD and cultured for an additional 2 h. Lysates from cells before and after growth in Lat-B were immunoblotted with an anti-phospho-cdc2 antibody to visualize Tyr19 phosphorylated Cdc28p.
Activation of Mpk1p in bem2Δ mutants treated with Lat-B
A second effect of actin perturbation is the activation of a kinase cascade culminating in the MAPK Mpk1p, which is required to arrest the cell cycle in response to Lat-B, probably through inhibition of the phosphatase Mih1p (Harrison et al., 2001). Activation of Mpk1p can be monitored using an antibody that detects only the doubly phosphorylated (active) form of the protein. Using this reagent, we found that activation of Mpk1p in response to Lat-B occurred equally well in wild-type and in bem2Δ cells (Figure 7C). We conclude that the checkpoint defect of bem2Δ mutants is not due to a failure to activate Mpk1p.
Mih1p in bem2Δ mutants
One possible reason for the bem2Δ checkpoint defect would be that bem2Δ mutants fail to inhibit the phosphatase Mih1p. We found that there was an equivalent amount of Mih1p in wild-type and bem2Δ cells (Figure 7D), but this result does not rule out an effect of Bem2p on Mih1p activity. If the checkpoint defect of bem2Δ cells was due to a lack of Mih1p inhibition, then deleting MIH1 should eliminate the need for such inhibition, thereby making Bem2p unnecessary for proper checkpoint function. Deletion of MIH1 did partially rescue the bem2Δ checkpoint defect (Figure 7E), consistent with a role of Bem2p in Mih1p regulation. However, the fact that there was still a partial checkpoint defect in the bem2Δ mih1Δ strain suggests that Bem2p must also have an Mih1p-independent role in checkpoint function.
Cdc28p phosphorylation in bem2Δ mutants treated with Lat-B
As the experiments described above indicated that the known morphogenesis checkpoint pathways were intact in the bem2Δ mutant, we wondered whether this mutant would be able to induce the inhibitory phosphorylation at Cdc28p Tyr19 in response to Lat-B. This phosphorylation can be readily detected using a phospho-epitope-specific antibody. We found that basal levels of Cdc28p phosphorylation were elevated in bem2Δ cells (Figure 7F), perhaps due to the increased basal level of Swe1p (Figure 7A). However, bem2Δ cells were severely attenuated in their ability to phosphorylate Cdc28p in response to Lat-B (Figure 7F). Thus, it appears that bem2Δ cells are incapable of effectively phosphorylating Cdc28p, despite their ability to stabilize Swe1p and activate Mpk1p. This defect presumably accounts for their inability to arrest the cell cycle in response to actin perturbation.
Discussion
How is bud formation monitored by the morphogenesis checkpoint?
The process of bud formation begins with the inheritance by newborn cells of spatial landmarks that specify the future site of bud emergence (reviewed by Pringle et al., 1995; Pruyne and Bretscher, 2000a,b). These landmarks are then ‘interpreted’ by the GTPase Rsr1p and its regulators Bud2p and Bud5p, which promote localization of polarity establishment proteins, including Cdc24p and Cdc42p, to the appropriate site. The Cdc42p GTPase and its effectors then cause polarization of actin structures towards that site and assembly of a septin ring at that site. Polarized secretion directed by actin cables and local cell wall deposition by septin-associated proteins then collaborate to promote bud emergence. Reasoning that the checkpoint would probably monitor some structure involved in bud formation, we tested whether mutations in the genes encoding spatial landmarks, Rsr1p and its regulators, polarity establishment proteins, actin cytoskeletal proteins, or septins would cause a checkpoint defect. With the sole exception of bem2 mutants (and even in that case, many checkpoint pathways remained intact; see below), all of these strains were competent to arrest the cell cycle in response to Lat-B. This situation appears quite different from that described for the DNA replication and spindle assembly checkpoints, where mutations that affect early steps in the process (assembly of replication forks or kinetochores, respectively) preclude checkpoint function (Piatti et al., 1995; Gardner et al., 2001).
One possibility for what might be monitored by the morphogenesis checkpoint is the concentration of monomeric G-actin. The ability of actin depolymerization by Lat-B to trigger a checkpoint response in many mutant strains is consistent with this hypothesis. Conceivably, mutations and environmental stresses that delay bud formation might all increase the G-actin concentration, which would make this a useful parameter to monitor (a transient increase in G-actin has been reported following thermal or osmotic shock) (Yeh and Haarer, 1996). If that were the case, then we reasoned that interaction of G-actin with the hypothetical sensor would be critical for checkpoint sensing. Wertman et al. (1992) have generated a panel of clustered charged-to-alanine scanning mutations across the entire ACT1 gene, which are expected to disrupt interactions with the surface of actin. However, none of the viable alleles in this series disrupted checkpoint function (Table I), and we were unable to obtain any experimental support for this hypothesis.
Another possibility is that the checkpoint does indeed monitor some aspect of bud emergence, but that rather than emitting a ‘wait’ signal when bud emergence is impaired, the checkpoint emits an ‘all-clear’ signal when bud emergence is successful. Consistent with this hypothesis, it appears that degradation of Swe1p requires its targeting to the bud side of the mother-bud neck, a location that only exists following successful bud emergence (Longtine et al., 2000). If it is successful bud emergence that triggers Swe1p degradation and subsequent cell cycle progression, then a failure of bud emergence would automatically lead to Swe1p accumulation and cell cycle delay, regardless of whether mutations impaired early or late steps in bud emergence. However, it should be noted that severe actin depolymerization can cause a Swe1p-mediated arrest even after cells have made a bud, so the checkpoint cannot only respond to bud emergence (McMillan et al., 1998).
How many pathways are involved in the morphogenesis checkpoint arrest?
In addition to stabilization and accumulation of Swe1p (Sia et al., 1998), previous work indicated that an effective checkpoint arrest required activation of a kinase cascade culminating in the MAPK Mpk1p/Slt2p, which is thought to act by inhibiting the phosphatase Mih1p (Harrison et al., 2001). In this report we have shown that the Rho- GAP Bem2p is also required for an effective checkpoint arrest. Surprisingly, lack of Bem2p did not affect either Swe1p accumulation or Mpk1p activation, suggesting that a minimum of three independent pathways collaborate to induce a checkpoint arrest in response to Lat-B. As Bem2p abundance was unaffected by the checkpoint, it is not yet clear whether Bem2p actively participates in a regulated checkpoint pathway or whether constitutive Bem2p is required to allow the function of a checkpoint pathway.
Why would the checkpoint require several pathways? One possibility is that instead of having a single checkpoint sensor, the morphogenesis checkpoint integrates information from multiple sensors to calibrate the appropriate response. For instance, it may be that Swe1p degradation responds to signals emanating in the vicinity of the septins at the mother-bud neck (Barral et al., 1999; Longtine et al., 2000), whereas Mpk1p responds to the status of the plasma membrane (Kamada et al., 1995) and Bem2p responds to yet another signal. Only when perturbations affected a combination of these sensors would cells mount a checkpoint response.
Regardless of whether one or several sensors are required to trigger the checkpoint, the collaboration of several independent pathways impinging on the cell cycle machinery may be essential to achieve an effective arrest. Indeed, it is clear that neither stabilization of Swe1p nor inhibition of Mih1p is sufficient to produce a prolonged cell cycle delay, although together they are quite effective (McMillan et al., 1999). Given the precedents in other systems for control of Swe1p/Mih1p homologs by post-translational means (Kumagai and Dunphy, 1992; Coleman et al., 1993; Parker et al., 1993; Wu and Russell, 1993; Mueller et al., 1995; Furnari et al., 1997; Lee et al., 2001), it seems likely that Bem2p is required for either activation of Swe1p or inhibition of Mih1p under checkpoint conditions. The bem2Δ checkpoint defect cannot be entirely accounted for by a role for Bem2p in Mih1p inhibition, because bem2Δ mih1Δ mutants are still partially defective for checkpoint-mediated arrest. Thus, we favor the hypothesis that Bem2p acts to stimulate Swe1p activity, and that without this stimulus the Swe1p that accumulates upon Lat-B treatment is unable to effectively phosphorylate Cdc28p and cause G2 arrest. Future studies will test this hypothesis by developing a quantitative assay for Swe1p specific activity.
Rho-GAP-dependent and Rho-GAP-independent roles for Bem2p
Cells lacking Bem2p have a complex phenotype that includes defects in actin polarity (Wang and Bretscher, 1995), bud site selection (Kim et al., 1994), cell wall integrity (Cid et al., 1998) and septin organization (Cid et al., 2001), as well as the morphogenesis checkpoint defect described here. As Rho-GTPases participate in all of these processes (Pringle et al., 1995; Ridley, 1995; Cabib et al., 1998; Harrison et al., 2001; Gladfelter et al., 2002), the bem2Δ defects could simply stem from an increase in the concentration of GTP-bound Rho proteins in the cell. However, we found that GAP-defective bem2 alleles were still competent to perform Bem2p’s checkpoint role. Conversely, while expression of a deregulated Rga1p Rho-GAP effectively suppressed the bem2Δ morphology defect, it could not restore checkpoint function to bem2Δ mutants. Together, these results argue that the Bem2p checkpoint function is separate from its Rho-GAP activity.
Rho-GAP domains are generally found in large proteins that often contain additional recognizable domains, including PH domains (Furukawa et al., 2001; Krugmann et al., 2002; Miura et al., 2002), GEF domains (Chuang et al., 1995), Arf-GAP domains (Krugmann et al., 2002; Miura et al., 2002), LIM domains (Chen et al., 1996), SH3 domains (Furukawa et al., 2001), WW domains (Furukawa et al., 2001) and even in one case a myosin motor (Wirth et al., 1996). However, the purpose of having these various domains in the same protein is unclear. It has been speculated that the additional domains are important for the regulation of GAP activity or localization of the GAP to different complexes within the cell (Watanabe et al., 2001), but the existence of such links has yet to be demonstrated. Our results with Bem2p suggest that it too must have at least one additional functional domain that acts in the morphogenesis checkpoint. The nature of that domain and the link, if any, between the Bem2p checkpoint function and its Rho-GAP function remains to be determined.
Materials and methods
Yeast strains and plasmids
Standard genetic and molecular biology methods were used to generate all strains and plasmids used in this study, except as indicated below. The yeast strains used are listed in Table II. The oligonucleotides used are listed in Table III. The swe1::TRP1 (Harrison et al., 2001), hsl1-Δ1::URA3 (Ma et al., 1996), SWE1-myc::HIS2 (McMillan et al., 1999), bem1::URA3 (Chenevert et al., 1992), spa2::URA3 (Gehrung and Snyder, 1990), msb1::URA3 (Bender and Pringle, 1991), cla4::TRP1 (Benton et al., 1997), ste20::TRP1 (Leberer et al., 1992), abp1::URA3 (Adams et al., 1993), tpm1::URA3 (Liu and Bretscher, 1989), sac6::LEU2 (Adams et al., 1991) and cap2::URA3 (Amatruda et al., 1990) alleles were all generated as described previously. The pfy1-111::LEU2 allele (Haarer et al., 1993) was serially backcrossed six times into the BF264-15DU background.
Table II. Yeast strains used in this study.
| Strain name | Relevant genotype | Source |
|---|---|---|
| BHY31 | a pfy1-112::LEU2 | Haarer et al. (1993) |
| BHY32 | a pfy1-116::LEU2 | Haarer et al. (1993) |
| BY4743a | a/α | Research Genetics |
| CCY1042-12B | α gic1-Δ1::LEU2 gic2-Δ2::TRP1 | Bi et al. (2000) |
| DBY7055 | a aip3Δ2::HIS3 | Amberg et al. (1997) |
| DDY336 | a act1-133::HIS3 | Wertman et al. (1992) |
| DDY337 | a act1-108::HIS3 | Wertman et al. (1992) |
| DDY338 | a act1-101::HIS3 | Wertman et al. (1992) |
| DDY339 | a act1-102::HIS3 | Wertman et al. (1992) |
| DDY340 | α act1-104::HIS3 | Wertman et al. (1992) |
| DDY341 | a act1-111::HIS3 | Wertman et al. (1992) |
| DDY343 | α act1-115::HIS3 | Wertman et al. (1992) |
| DDY344 | α act1-116::HIS3 | Wertman et al. (1992) |
| DDY346 | a act1-119::HIS3 | Wertman et al. (1992) |
| DDY347 | a act1-120::HIS3 | Wertman et al. (1992) |
| DDY348 | a act1-123::HIS3 | Wertman et al. (1992) |
| DDY349 | α act1-124::HIS3 | Wertman et al. (1992) |
| DDY351 | α act1-129::HIS3 | Wertman et al. (1992) |
| DDY352 | a act1-132::HIS3 | Wertman et al. (1992) |
| DDY353 | α act1-135::HIS3 | Wertman et al. (1992) |
| DDY356 | α act1-105::HIS3 | Wertman et al. (1992) |
| DDY357 | a act1-115::HIS3 | Wertman et al. (1992) |
| DDY654 | α act1-121::HIS3 | Wertman et al. (1992) |
| DDY655 | a act1-122::HIS3 | Wertman et al. (1992) |
| DDY1253 | α cof1-4::LEU2 | Lappalainen et al. (1997) |
| DDY1254 | α cof1-5::LEU2 | Lappalainen et al. (1997) |
| DDY1266 | α cof1-22::LEU2 | Lappalainen et al. (1997) |
| DLY1 | a bar1 | Sia et al. (1996) |
| DLY657 | a bar1 cdc24-1 | Sia et al. (1996) |
| DLY2609 | a bar1 sac6::LEU2 | This study |
| DLY2736 | a bar1 bud5::LEU2 | This study |
| DLY3368 | a bar1 rsr1::URA3 | This study |
| DLY3860 | a bar1 bem2::URA3 mih1::TRP1 | This study |
| DLY3966 | a bar1 rho4::TRP1 | This study |
| DLY4015 | a bar1 bem2::TRP1 | This study |
| DLY4019 | a bar1 bem2::TRP1 hsl1::URA3 | This study |
| DLY4021 | a bar1 swe1::TRP1 | Harrison et al. (2001) |
| DLY4478 | a bar1 bem2::TRP1 MIH1-myc::URA3 | This study |
| DLY4497 | a bar1 rho2::KANr | Harrison et al. (2001) |
| DLY4860 | a bar1 BEM2-myc::URA3 | This study |
| DLY4862 | a bar1 bem2K2046A-myc::URA3 | This study |
| DLY5041 | a bar1 bem2R2003A-myc::URA3 | This study |
| DLY5330 | a bar1 SWE1-myc::HIS2 | This study |
| DLY5331 | a bar1 bem2::TRP1 SWE1-myc::HIS2 | This study |
| DLY5516a | a/α bem2::KanMX/bem2::LEU2 | This study |
| JMY2-13 | a bar1 abp1::URA3 | This study |
| JMY2-14 | a bar1 spa2::URA3 | This study |
| JMY2-15 | a bar1 msb1::URA3 | This study |
| JMY2-26 | a myo2-66 | McMillan et al. (1998) |
| JMY3-1 | a bar1 hsl1::URA3 | This study |
| JMY3-59 | a bar1 mih1::TRP1 | This study |
| JMY1011 | a bar1 bem1::URA3 | This study |
| JMY1020 | a tpm1::URA3 | This study |
| JMY1023 | a cap2::URA3 | This study |
| JMY1066 | a pfy1-111::LEU2 | This study |
| JMY1237 | a myo5::URA3 | This study |
| JMY1239 | α myo4::URA3 | This study |
| JMY1250 | α myo3::TRP1 | This study |
| JMY1299 | a bar1 MIH1-myc::URA3 | This study |
| JMY1503 | a bar1 hsl1::URA3 SWE1-myc::HIS2 | McMillan et al. (1999) |
| JPT194-H01 | a/α cdc11-6/cdc11-6 | John Pringle |
| LH17012-H01 | a/α cdc10-1/cdc10-1 | John Pringle |
| MOSY148 | a cla4::TRP1 | This study |
| MOSY150 | a ste20::TRP1 | This study |
| RNY140 | a myo1::KANr | John Pringle |
| YBR260CΔa | a/α rgd1::KanMX/rgd1::KanMX | Research Genetics |
| YDL240WΔa | a/α lrg1::KanMX/lrg1::KanMX | Research Genetics |
| YDR379WΔa | a/α rga2::KanMX/rga2::KanMX | Research Genetics |
| YDR389WΔa | a/α sac7::KanMX/sac7::KanMX | Research Genetics |
| YEF369 | a bud2::TRP1 | John Pringle |
| YEF395 | a/α bud1::HIS3/bud1::HIS3 bud8::HIS3/bud8::HIS3 | John Pringle |
| YEF1269 | a msb3Δ::HIS3 msb4Δ::HIS3 | Bi et al. (2000) |
| YFL047WΔa | a/α yfl047w::KanMX/yfl047w::KanMX | Research Genetics |
| YHR182WΔa | a/α yhr182w::KanMX/yhr182w::KanMX | Research Genetics |
| YJL187CΔa | a/α swe1::KanMX/swe1::KanMX | Research Genetics |
| YJL201WΔa | a/α yjl201w::KanMX/yjl201w::KanMX | Research Genetics |
| YJZ427 | a bni1Δ::HIS3 | Zahner et al. (1996) |
| YOR127WΔa | a/α rga1::KanMX/rga1::KanMX | Research Genetics |
| YOR134WΔa | a/α bag7::KanMX/bag7::KanMX | Research Genetics |
| YPL115CΔa | a/α bem3::KanMX/bem3::KanMX | Research Genetics |
aStrains obtained from Research Genetics (and DLY5516, which was derived from the bem2Δ Research Genetics strain which was found to be heterozygous for the bem2Δ deletion) are in the BY4743 background (his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 lys2Δ0/LYS2 met15Δ0Δ/MET15). All other strains generated in this study are in the BF264-15DU (Richardson, 1989) background (ade1 his2 leu2-3 112 trp1-1a ura3Δns).
Table III. Oligonucleotides used in this study.
| Oligo name | Oligonucleotide sequence |
|---|---|
| ARMO1 | GGAATTCCATATGAAAACTCATAATAAGATATTTGGGGTACC |
| ARMO2 | CCGAGCTCTTATTGCTTGAAATAATCATTTGGATTC |
| ARMO3 | GTCTAATGAAAGGTCTTCTCTGGTCTAAGAACAGGAAATCTTCAACGGCCGCGCGTTTCGGTGATGAC |
| ARMO4 | TTATTGCTTGAAATAATCATTTGGATTTGGATTCTTAATAAAATCTTGCAGGATTTTCCTGATGCGGTATTTTCTCCT |
| ARMO6 | GAAGTGGGATTGTACGCGATTCCTGGTTCCA |
| ARMO7 | TGGAACCAGGAATCGCGTACAATCCCACTTC |
| ARMO8 | GCGATCGCAGGCTGTTTCGCGATGTATTTAAGAGAG |
| ARMO9 | CTCTCTTAAATACATCGCGAAACAGCCTGCGATCGC |
| ARMO33 | AGATCTTCATTAGACTCCTGCTTCGTTCGTTATTTG |
| BUD5-1 | GCATGAGAACGGCCGTACCGCAGTTGCTGGAAGCAACTGCCTGTGTCTCTAGGCGCGTTTCGGTGATGA |
| BUD5-2 | GTTTTTAGGTAAGCCTTGGAACCTTAGCTATACTGACTTGATACGCCCTTTCCTGATGCGGTATTTTCTCCT |
| H16 | GCTCTAGAGGCTTACTACTATGCGTTCAA |
| H17 | CGAGTCGACTTGCTTGAAATAATCATTTGG |
| OJ17 | TGGACAAACCAGGATTGAAGTCAGCGAGGGTGAAGAAACCGCGCGTTTCGGTGATGAC |
| OJ18 | ATTAACGATCTTCTTGCGGGCCTGGGTAAATCTTCTCGGTTTTCCTGATGCGGTATTTTCTCCT |
| OJ37 | CAATCCAGTTATAAAACATTAATTTGCTGATCACATTTGAATCGCGCGTTTCGGTGATGAC |
| OJ38 | GTCATCATCATCATCGCCATCGTCATTATCATCATCGTCATTTCCTGATGCGGTATTTTCTCCT |
| OJ39 | CATGTCATTTGAAGTAGGAACTAAGTGTTGGTACCCTCACGCGCGTTTCGGTGATGAC |
| OJ40 | CTGACAGTAGCTAAGCCCTCTGTATTGCTGTTCTGTGTTATTTCCTGATGCGGTATTTTCTCCT |
| OJ41 | GCCAGCTAAACGCTCTGCGAATATCAAGAAAGCTACTTTTGCGCGTTTCGGTGATGAC |
| OJ67 | CATGGAGACATTTAATAGACC |
| OJ68 | CCATGAAATATCTCGAGCATGGTTTCTTCACCCTCGCTGAC |
| OJ69 | GAAGAAACCATGCTCGAGATATTTCATGGAACTGAAG |
| OJ70 | GAATTAGGATCCAGTGGACTGGATGACGCAGACG |
| RHO4-F | GTTACAGCAAACTTAAGTCAAATAGGTCCAAAAATCTCCAATAGTAACGCGCGCGTTTCGGTGATGAC |
| RHO4-R | GCCACTTTTCAAATGTTGTAAGTGCGTACAGAATGGGCAACAGAATAATCTTCTCCTGATGCGGTATTTTC |
| RSR1-2 | GGACTAATGAGAGACTATAAATTAGTAGTATTGGGTGCTGGTGGTGTCGGGCGCGTTTCGGTGATGAC |
| RSR1-3 | GTCTTTTTATCTGATATCTTGATTCATTTATAATAAAATTAAGTGACTTTTCCTGATGCGGTATTTTCTCCT |
| Z103 | GACTCTGCAGAAAATTGTTCATTGGGAGCAGTTGATGC |
| Z105 | GACTGAGCTCTTAGATTTGATATAATTAACGTGTGC |
| Z133 | GACTCCGCGGCCATTAGACTCCTGCTTCGTTATTTG |
| Z134 | GACTGAGCTCTCCACCTCCTTCCAGTTTAGGAGAGG |
| Z135 | GACTGCTAGCCACATTAGACTCCTGCTTCGTTATTTG |
| Z136 | GATCCATGGGACATTAGACTCCTGCTTCGTTATTTG |
| Z145 | TATTGGATCCAAGTCATTTCCTGTCTTTAG |
| Z146 | CCATGAAGATGACCTATGTTCAAATCTACAGCCAA |
| Z147 | TTGGCTGTAGATTTGAACATAGGTCATCTTCATGG |
| Z148 | AGAGGCGCGCGGATGATCTTGGAGGCGGCAAC |
The bem2::TRP1, bem2::URA3, bem2::LEU2, mih1::TRP1, rho4::TRP1, bud5::LEU2, rsr1::URA3, myo3::TRP1, myo4::URA3 and myo5::URA3 alleles were constructed by the one-step PCR method (Baudin et al., 1993) with plasmids pRS304 (TRP1), pRS305 (LEU2) or pRS306 (URA3) (Sikorski and Hieter, 1989) as templates. The PCR primers used were ARMO3 and ARMO4 for BEM2, OJ17 and OJ18 for MIH1, RHO4-F and RHO4-R for RHO4, BUD5-1 and BUD5-2 for BUD5, RSR1-2 and RSR2-3 for RSR1, OJ37 and OJ38 for MYO3, OJ39 and OJ40 for MYO4, and OJ41 and OJ42 for MYO5.
To create a myc-tagged version of the full-length BEM2 gene, the 3′ end of the BEM2 ORF was amplified with oligos H16 and H17. This PCR product was digested with XbaI and SalI, and ligated into pSWE1-myc (McMillan et al., 1998) cut with XbaI and SalI to excise the SWE1 coding sequence. This creates pDLB1920, encoding an in-frame fusion of the 3′ end of BEM2 and 12 myc tags. Digestion at the unique KpnI site targets integration to the genomic BEM2 locus, replacing endogenous BEM2 with BEM2-12myc. Correct integration was confirmed by PCR and western blotting. A CEN plasmid expressing full-length BEM2-12myc from its own promoter was created by ligating a KpnI–BamHI fragment of pDLB1920 (encoding the 3′ end of BEM2 and the myc tags) and a KpnI–SmaI fragment of pCC554 (gift from C.Chan) into pRS316 (Sikorski and Hieter, 1989), which had been digested with NotI, blunted with Klenow enzyme and subsequently digested with BamHI. The resulting plasmid, pDLB768, contains full-length BEM2-12myc with 995 bp of its own promoter followed by the ∼500 bp SWE1 terminator.
For expression of GST-tagged Bem2p GAP domain (amino acids 1958–2167) in bacteria, oligos ARMO1 and ARMO2 were used to amplify the GAP domain from genomic DNA, and the PCR product was cut with NdeI and SacI and ligated into pUNI-10 (Liu et al., 1998), creating pDLB1031. The R2003A and K2046A mutations were made by using overlap PCR with ARMO1 and ARMO2 as the flanking oligos, and either ARMO6 and ARMO7 (R2003A) or ARMO8 and ARMO9 (K2046A) as the internal oligos. These PCR products were similarly cloned into pUNI-10 with NdeI–SacI, creating pDLB1609 (R2003A) and pDLB1699 (K2046A). All plasmids were sequenced to confirm that the expected mutations (and no others) were present. These plasmids were then recombined with pHB2-GST (Liu et al., 1998) (directing synthesis of proteins with GST fused to the N-terminus) to form pDLB1132 (WT), pDLB1705 (R2003A) and pDLB1721 (K2046A). The plasmid to express Cdc42p as a GST fusion in bacteria, pDLB2091, has been described previously (Gladfelter et al., 2002).
To integrate the point mutants of BEM2 into the genome, a NdeI–SacI fragment from each mutant in pUNI-10 and a KpnI–BamHI fragment from pDLB768 were used to GAP repair pDLB768 cut with EcoRI. The resulting plasmids, pDLB1772 (R2003A) and pDLB1773 (K2046A), were recovered and confirmed by restriction digest. A KpnI–SalI fragment spanning the mutation sites was used to replace the correspond ing wild-type fragment in pDLB1920, yielding pDLB1921 (R2003A) and pDLB1922 (K2046A), which were then digested with KpnI to target integration at BEM2. Correct integration was confirmed by PCR and western blotting.
To create a version of RGA1 lacking the N-terminal LIM domains, oligos Z105 and Z103 were used to generate a PCR fragment containing 350 bp of the RGA1 promoter along with the start codon, and this fragment was used to replace the SacI–PstI fragment from full-length RGA1 (containing the promoter as well as the first 164 amino acids) in pDLB1537 (Gladfelter et al., 2002), yielding the 2 µm plasmid pDLB2129.
To create the MIH1-myc::URA3 allele, a fragment containing the promoter region of MIH1 was amplified using oligos OJ67 and OJ68, and cloned into pRS306 (Sikorski and Hieter, 1989) using KpnI–XhoI. Then a second fragment containing the 5′ end of the MIH1 gene was amplified with oligos OJ69 and OJ70, and cloned into the resulting plasmid using XhoI–BamHI. The resulting plasmid was then linearized with XhoI and a 400 bp XhoI–SalI fragment containing 12 tandem copies of the myc epitope (P.Russell, The Scripps Research Institute) was inserted. This creates pJM1030, which was linearized with BglII to target integration at MIH1, confirmed by PCR and western blotting.
The N-terminal deletion series of BEM2 was created by using PCR to amplify a 650 bp region of the BEM2 promoter along with the start codon, followed by various restriction sites. The 5′ oligo used to create these fragments was Z134. Fragments ending with NcoI (oligo Z136), NheI (oligo Z135), SacII (oligo Z133) and BglII (oligo ARMO33) sites were amplified and ligated into pDLB768, which had been cut with SacI and the appropriate second enzyme, creating plasmids that contained the BEM2 promoter driving synthesis of Bem2p missing residues 2–237 (pDLB2147), 2–695 (pDLB2118), 2–1240 (pDLB2116) and 2–1749 (pDLB2115).
To create a BEM2 plasmid missing amino acids 688–707, overlap PCR was used with internal oligos (Z146 and Z147) that loop out this region flanked by oligos (Z145 and Z148) that amplify an ∼1 kb fragment. This fragment was cut with BamHI and BssHII and ligated into pDLB768, from which an analogous fragment was removed, creating the plasmid pDLB2249.
Media, growth conditions and cell synchrony
Yeast media (YEPD rich media, synthetic medium lacking specific nutrients and sporulation medium) have been described previously (Guthrie and Fink, 1991). Synchronous release of cells from α-factor arrest was carried out as described previously (McMillan et al., 1999). When indicated, log phase cultures (107 cells/ml) were resuspended in fresh medium containing 100 µM Lat-B (BioMol Research Laboratories Inc., Plymouth Meeting, PA) or 1% dimethylsulfoxide (DMSO; vehicle control).
Preparation of lysates and western blotting
Cell pelleting and lysis were carried out as previously described (McMillan et al., 1999). Detection of diphospho-Mpk1p and tyrosine phosphorylated Cdc28p has been described previously (Harrison et al., 2001). Detection of Sso1/2p was carried out as described previously (Lehman et al., 1999).
Morphogenesis checkpoint assay
The morphogenesis checkpoint assay has been described previously (Harrison et al., 2001).
Fluorescence staining and microscopy
To visualize nuclear DNA, cells were fixed in 70% ethanol for >1 h, harvested by centrifugation and resuspended in 0.3 µg/ml DAP (Sigma). Visualization of F-actin was accomplished by incubating fixed cells in 0.1 U/ml Alexa Fluor 568 phalloidin (Molecular Probes, Eugene, OR) for 20 min, followed by washing three times with PBS. Cells were then viewed on an Axioscop apparatus (Zeiss, Thornwood, NY) equipped with epifluorescence and differential interference contrast (DIC) optics. Images were captured using a cooled model charge-coupled device (CCD) camera (Princeton Instruments, Princeton, NJ).
Cell fractionation
Cell fractionation was carried out as described previously (Lehman et al., 1999), with the following modifications. To obtain sufficient levels of protein, 4.5 × 109 cells were harvested by centrifugation and washed in 3 ml of 10 mM Tris pH 7.5 and 10 mM sodium azide. Cells were then spheroplasted by incubation for 40 min in 7 ml of spheroplast buffer [0.1 mg/ml yeast lytic enzyme (ICN Biomedicals), 100 mM Tris pH 7.5, 10 mM sodium azide, 1.2 M sorbitol, 21 mM β-mercaptoethanol] at 37°C. Spheroplasts were then lysed in 3 ml of TEAE/sorbitol (10 mM TEA, 1 mM EDTA, 0.8 M sorbitol) with a 7 ml glass Dounce homogenizer. The homogenates were then cleared of intact cells and debris by centrifugation (450 g) for 30 min at 4°C. The supernatant was removed, divided into two and diluted 1:1 in either TEAE/sorbitol or TEAE/sorbitol/2% Triton X-100. The supernatant was then spun at 55 000 r.p.m. (250 000 g) in a TSL55 rotor for a Beckman l7-65 ultracentrifuge. All pellets were normalized to the volume of the supernatant fractions and 20 µl of each sample were run on an 8% SDS–polyacrylamide gel.
Acknowledgments
Acknowledgements
We thank B.Haarer, D.Botstein, D.Drubin, J.Pringle, F.Cross, A.Bretscher, A.Bender, M.Grunstein and A.Adams for providing plasmids and strains. We thank P.Brennwald for a generous gift of Sso1p antibody. Thanks also to the members of the Lew, Pringle and Kornbluth laboratories for stimulating discussions. This work was funded by grants from the NIH (GM53050) and the Leukemia and Lymphoma Society to D.J.L.
References
- Adams A.E.M., Botstein,D. and Drubin,D.G. (1991) Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature, 354, 404–408. [DOI] [PubMed] [Google Scholar]
- Adams A.E., Cooper,J.A. and Drubin,D.G. (1993) Unexpected combinations of null mutations in genes encoding the actin cytoskeleton are lethal in yeast. Mol. Biol. Cell, 4, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda J.F., Cannon,J.F., Tatchell,K., Hug,C. and Cooper,J.A. (1990) Disruption of the actin cytoskeleton in yeast capping protein mutants. Nature, 344, 352–354. [DOI] [PubMed] [Google Scholar]
- Amberg D.C., Zahner,J.E., Mulholland,J.W., Pringle,J.R. and Botstein,D. (1997) Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol. Biol. Cell, 8, 729–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A., Surana,U., Muroff,I. and Nasmyth,K. (1992) Regulation of p34CDC28 tyrosine phosphorylation is not required for entry into mitosis in S.cerevisiae. Nature, 355, 368–371. [DOI] [PubMed] [Google Scholar]
- Araki H., Leem,S.-H., Phongdara,A. and Sugino,A. (1995) Dpb11, which interacts with DNA polymerase II(e) in Saccharomyces cerevisiae, has a dual role in S-phase progression and at a cell cycle checkpoint. Proc. Natl Acad. Sci. USA, 92, 11791–11795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y., Parra,M., Bidlingmaier,S. and Snyder,M. (1999) Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev., 13, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. et al. (1997) The structure of the GTPase-activating domain from p50rhoGAP. Nature, 385, 458–461. [DOI] [PubMed] [Google Scholar]
- Baudin A., Ozier-Kalogeropoulos,O., Denouel,A., Lacroute,F. and Cullin,C. (1993) A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res., 21, 3329–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A. and Pringle,J.R. (1989) Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl Acad. Sci. USA, 86, 9976–9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A. and Pringle,J.R. (1991) Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol., 11, 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender L., Shuen Lo,H., Lee,H., Kokojan,V., Peterson,J. and Bender,A. (1996) Associations among PH and SH3 domain-containing proteins and Rho-type GTPases in yeast. J. Cell Biol., 133, 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton B.K., Tinkelenberg,A., Gonzalez,I. and Cross,F.R. (1997) Cla4p, a Saccharomyces cerevisiae Cdc42p-activated kinase involved in cytokinesis, is activated at mitosis. Mol. Cell. Biol., 17, 5067–5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E., Chiavetta,J.B., Chen,H., Chen,G.C., Chan,C.S. and Pringle,J.R. (2000) Identification of novel, evolutionarily conserved Cdc42p-interacting proteins and of redundant pathways linking Cdc24p and Cdc42p to actin polarization in yeast. Mol. Biol. Cell, 11, 773–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Drgonova,J. and Drgon,T. (1998) Role of small G proteins in yeast cell polarization and wall biosynthesis. Annu. Rev. Biochem., 67, 307–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J., Corrado,K., Pringle,J.R. and Herskowitz,I. (1991) Yeast BUD5, encoding a putative GDP–GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. Cell, 65, 1213–1224. [DOI] [PubMed] [Google Scholar]
- Chen G.C., Zheng,L. and Chan,C.S. (1996) The LIM domain-containing Dbm1 GTPase-activating protein is required for normal cellular morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenevert J., Corrado,K., Bender,A., Pringle,J. and Herskowitz,I. (1992) A yeast gene (BEM1) necessary for cell polarization whose product contains two SH3 domains. Nature, 356, 77–79. [DOI] [PubMed] [Google Scholar]
- Chuang T.H., Xu,X., Kaartinen,V., Heisterkamp,N., Groffen,J. and Bokoch,G.M. (1995) Abr and Bcr are multifunctional regulators of the Rho GTP-binding protein family. Proc. Natl Acad. Sci. USA, 92, 10282–10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid V.J., Cenamor,R., Sanchez,M. and Nombela,C. (1998) A mutation in the Rho1-GAP-encoding gene BEM2 of Saccharomyces cerevisiae affects morphogenesis and cell wall functionality. Microbiology, 144, 25–36. [DOI] [PubMed] [Google Scholar]
- Cid V.J., Adamikova,L., Sanchez,M., Molina,M. and Nombela,C. (2001) Cell cycle control of septin ring dynamics in the budding yeast. Microbiology, 147, 1437–1450. [DOI] [PubMed] [Google Scholar]
- Coleman T.R. and Dunphy,W.G. (1994) Cdc2 regulatory factors. Curr. Opin. Cell Biol., 6, 877–882. [DOI] [PubMed] [Google Scholar]
- Coleman T.R., Tang,Z. and Dunphy,W.G. (1993) Negative regulation of the Wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell, 72, 919–929. [DOI] [PubMed] [Google Scholar]
- Furnari B., Rhind,N. and Russell,P. (1997) Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science, 277, 1495–1497. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Kawasoe,T., Daigo,Y., Nishiwaki,T., Ishiguro,H., Takahashi,M., Kitayama,J. and Nakamura,Y. (2001) Isolation of a novel human gene, ARHGAP9, encoding a rho-GTPase activating protein. Biochem. Biophys. Res. Commun., 284, 643–649. [DOI] [PubMed] [Google Scholar]
- Gardner R.D., Poddar,A., Yellman,C., Tavormina,P.A., Monteagudo,M.C. and Burke,D.J. (2001) The spindle checkpoint of the yeast Saccharomyces cerevisiae requires kinetochore function and maps to the CBF3 domain. Genetics, 157, 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrung S. and Snyder,M. (1990) The SPA2 gene of Saccharomyces cerevisiae is important for pheromone-induced morphogenesis and efficient mating. J. Cell Biol., 111, 1451–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter A.S., Bose,I., Zyla,T.R., Bardes,E.S. and Lew,D.J. (2002) Septin ring assembly involves cycles of GTP loading and hydrolysis by Cdc42p. J. Cell Biol., 156, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C. and Fink,G.R. (eds) (1991) Guide to yeast genetics and molecular biology. Methods Enzymol., 194, 933. [PubMed] [Google Scholar]
- Haarer B.K., Petzold,A.S. and Brown,S.S. (1993) Mutational analysis of yeast profilin. Mol. Cell. Biol., 13, 7864–7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J.C., Bardes,E.S., Ohya,Y. and Lew,D.J. (2001) A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol., 3, 417–420. [DOI] [PubMed] [Google Scholar]
- Kamada Y., Jung,U.S., Piotrowski,J. and Levin,D.E. (1995) The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev., 9, 1559–1571. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Francisco,L., Chen,G.C., Marcotte,E. and Chan,C.S. (1994) Control of cellular morphogenesis by the Ip12/Bem2 GTPase-activating protein: possible role of protein phosphorylation. J. Cell Biol., 127, 1381–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugmann S. et al. (2002) Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol. Cell, 9, 95–108. [DOI] [PubMed] [Google Scholar]
- Kumagai A. and Dunphy,W.G. (1992) Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell, 70, 139–151. [DOI] [PubMed] [Google Scholar]
- Lappalainen P., Fedorov,E.V., Fedorov,A.A., Almo,S.C. and Drubin,D.G. (1997) Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J., 16, 5520–5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Dignard,D., Harcus,D., Thomas,D.Y. and Whiteway,M. (1992) The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein bg subunits to downstream signaling components. EMBO J., 11, 4815–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Kumagai,A. and Dunphy,W.G. (2001) Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol. Biol. Cell, 12, 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman K., Rossi,G., Adamo,J.E. and Brennwald,P. (1999) Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol., 146, 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D.J. and Reed,S.I. (1995) A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J. Cell Biol., 129, 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Zhang,B. and Zheng,Y. (1997) Structural determinants required for the interaction between Rho GTPase and the GTPase-activating domain of p190. J. Biol. Chem., 272, 32830–32835. [DOI] [PubMed] [Google Scholar]
- Liu H. and Bretscher,A. (1989) Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell, 57, 233–242. [DOI] [PubMed] [Google Scholar]
- Liu Q., Li,M.Z., Leibham,D., Cortez,D. and Elledge,S.J. (1998) The univector plasmid-fusion system, a method for rapid construction of recombinant DNA without restriction enzymes. Curr. Biol., 8, 1300–1309. [DOI] [PubMed] [Google Scholar]
- Longtine M.S., Theesfeld,C.L., McMillan,J.N., Weaver,E., Pringle,J.R. and Lew,D.J. (2000) Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell. Biol., 20, 4049–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X.-J., Lu,Q. and Grunstein,M. (1996) A search for proteins that interact genetically with histone H3 and H4 amino termini uncovers novel regulators of the Swe1 kinase in Saccharomyces cerevisiae. Genes Dev., 10, 1327–1340. [DOI] [PubMed] [Google Scholar]
- McMillan J.N., Sia,R.A.L. and Lew,D.J. (1998) A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J. Cell Biol., 142, 1487–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J.N., Longtine,M.S., Sia,R.A., Theesfeld,C.L., Bardes,E.S., Pringle,J.R. and Lew,D.J. (1999) The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol. Cell. Biol., 19, 6929–6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K., Jacques,K.M., Stauffer,S., Kubosaki,A., Zhu,K., Hirsch,D.S., Resau,J., Zheng,Y. and Randazzo,P.A. (2002) ARAP1: a point of convergence for Arf and Rho signaling. Mol. Cell., 9, 109–119. [DOI] [PubMed] [Google Scholar]
- Mueller P.R., Coleman,T.R. and Dunphy,W.G. (1995) Cell cycle regulation of a Xenopus Wee1-like kinase. Mol. Biol. Cell, 6, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Cantley,L.C. and Harrison,S.C. (1996) Crystal structure of the breakpoint cluster region-homology domain from phosphoinositide 3-kinase p85 α subunit. Proc. Natl Acad. Sci. USA, 93, 14373–14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas T.A., Zhou,Z. and Elledge,S.J. (1995) DNA polymerase ε links the DNA replication machinery to the S phase checkpoint. Cell, 80, 29–39. [DOI] [PubMed] [Google Scholar]
- O’Farrell P.H. (2001) Triggering the all-or-nothing switch into mitosis. Trends Cell Biol., 11, 512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlen L.J. and Cross,F.R. (1998) Potential regulation of Ste20 function by the Cln1–Cdc28 and Cln2–Cdc28 cyclin-dependent protein kinases. J. Biol. Chem., 273, 25089–25097. [DOI] [PubMed] [Google Scholar]
- Parker L.L., Walter,S.A., Young,P.G. and Piwnica-Worms,H. (1993) Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature, 363, 736–738. [DOI] [PubMed] [Google Scholar]
- Peterson J., Zheng,Y., Bender,L., Myers,A., Cerione,R. and Bender,A. (1994) Interactions between the bud emergence proteins Bem1p and Bem2p and Rho-type GTPases in yeast. J. Cell Biol., 127, 1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti S., Lengauer,C. and Nasmyth,K. (1995) Cdc6 is an unstable protein whose de novo synthesis in G1 is important for the onset of S phase and for preventing a ‘reductional’ anaphase in the budding yeast Saccharomyces cerevisiae. EMBO J., 14, 3788–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J.R., Bi,E., Harkins,H.A., Zahner,J.E., De Virgilio,C., Chant,J., Corrado,K. and Fares,H. (1995) Establishment of cell polarity in yeast. Cold Spring Harb. Symp. Quant. Biol., 60, 729–744. [DOI] [PubMed] [Google Scholar]
- Pruyne D. and Bretscher,A. (2000a) Polarization of cell growth in yeast. J. Cell Sci., 113, 571–585. [DOI] [PubMed] [Google Scholar]
- Pruyne D. and Bretscher,A. (2000b) Polarization of cell growth in yeast. J. Cell Sci., 113, 365–375. [DOI] [PubMed] [Google Scholar]
- Ridley A.J. (1995) Rho-related proteins: actin cytoskeleton and cell cycle. Curr. Opin. Genet. Dev., 5, 24–30. [DOI] [PubMed] [Google Scholar]
- Sia R.A.L., Herald,H.A. and Lew,D.J. (1996) Cdc28 tyrosine phosphorylation and the morphogenesis checkpoint in budding yeast. Mol. Biol. Cell, 7, 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia R.A.L., Bardes,E.S.G. and Lew,D.J. (1998) Control of Swe1p degradation by the morphogenesis checkpoint. EMBO J., 17, 6678–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R.S. and Hieter,P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics, 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P.K. and Murray,A.W. (1992) S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34CDC28. Nature, 355, 365–368. [DOI] [PubMed] [Google Scholar]
- Stueland C.S., Lew,D.J., Cismowski,M.J. and Reed,S.I. (1993) Full activation of p34CDC28 histone H1 kinase activity is unable to promote entry into mitosis in checkpoint-arrested cells of the yeast Saccharomyces cerevisiae. Mol. Cell. Biol., 13, 3744–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Shimomura,T., Hashimoto,K., Araki,H., Sugino,A. and Matsumoto,K. (1996) Rfc5, a small subunit of replication factor C complex, couples DNA replication and mitosis in budding yeast. Proc. Natl Acad. Sci. USA, 93, 7048–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A.H. et al. (2001) Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science, 294, 2364–2368. [DOI] [PubMed] [Google Scholar]
- Wang T. and Bretscher,A. (1995) The rho-GAP encoded by BEM2 regulates cytoskeletal structure in budding yeast. Mol. Biol. Cell, 6, 1011–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D., Abe,M. and Ohya,Y. (2001) Yeast Lrg1p acts as a specialized RhoGAP regulating 1,3-β-glucan synthesis. Yeast, 18, 943–951. [DOI] [PubMed] [Google Scholar]
- Wertman K.F., Drubin,D.G. and Botstein,D. (1992) Systematic mutational analysis of the yeast ACT1 gene. Genetics, 132, 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J.A., Jensen,K.A., Post,P.L., Bement,W.M. and Mooseker,M.S. (1996) Human myosin-IXb, an unconventional myosin with a chimerin-like rho/rac GTPase-activating protein domain in its tail. J. Cell Sci., 109, 653–661. [DOI] [PubMed] [Google Scholar]
- Wu L. and Russell,P. (1993) Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature, 363, 738–741. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Guacci,V. and Koshland,D. (1996) Pds1p, an inhibitor of anaphase in budding yeast, plays a critical role in the APC and checkpoint pathway(s). J. Cell Biol., 133, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. and Haarer,B.K. (1996) Profilin is required for the normal timing of actin polymerization in response to thermal stress. FEBS Lett., 398, 303–307. [DOI] [PubMed] [Google Scholar]
- Zahner J.E., Harkins,H.A. and Pringle,J.R. (1996) Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 1857–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]