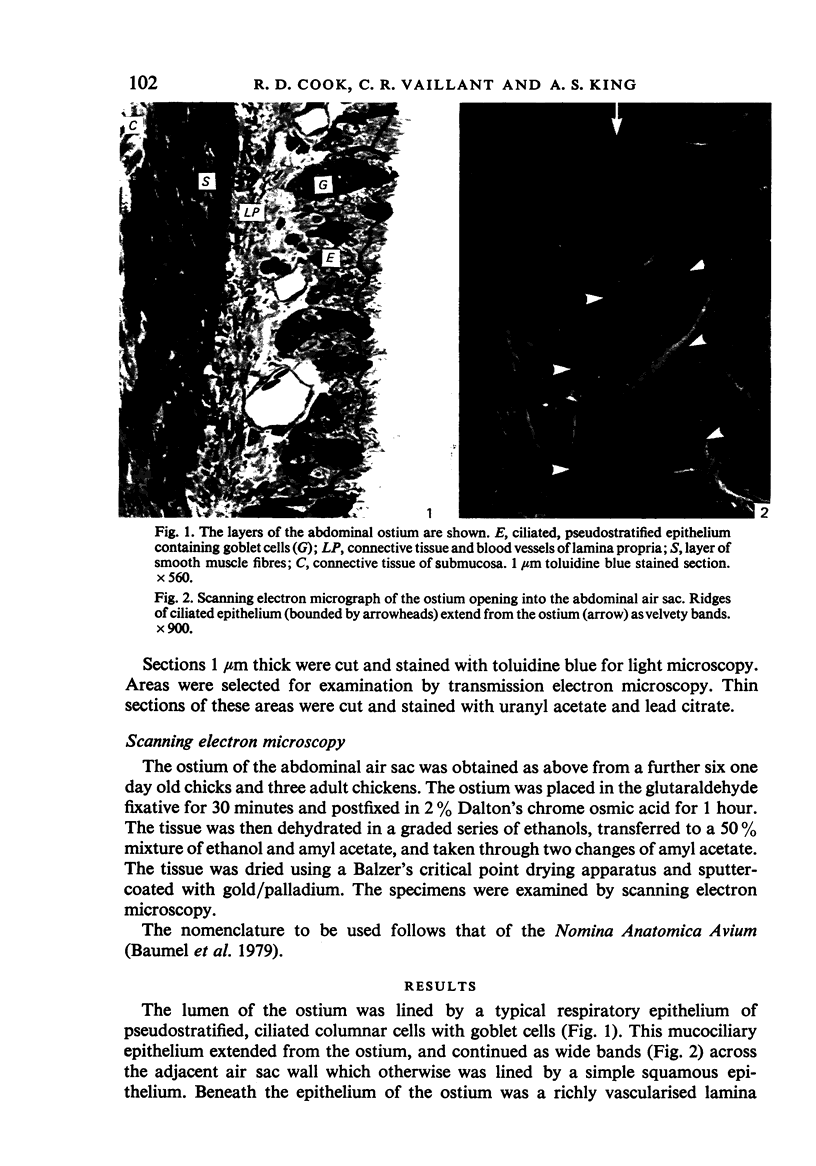
Abstract
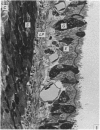
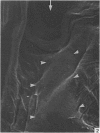
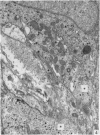
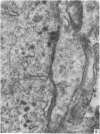
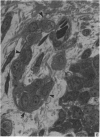
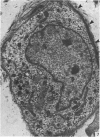
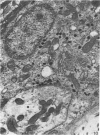
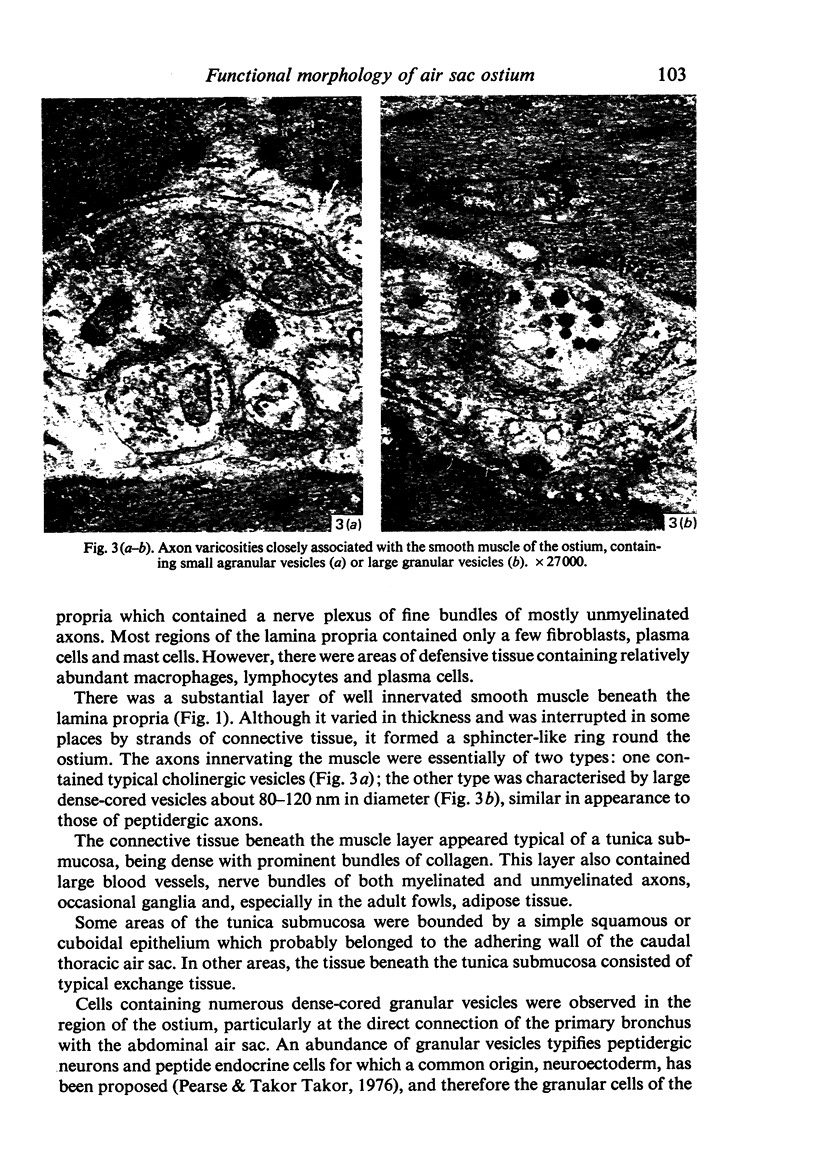
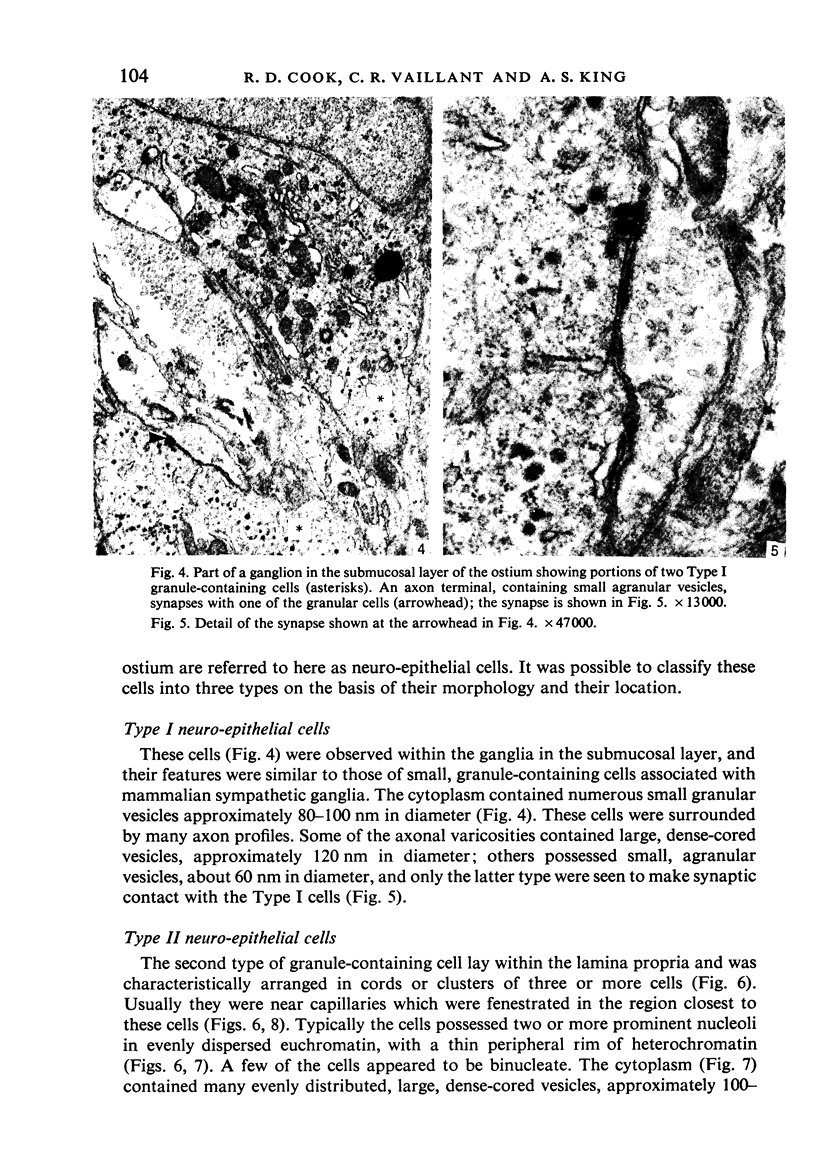
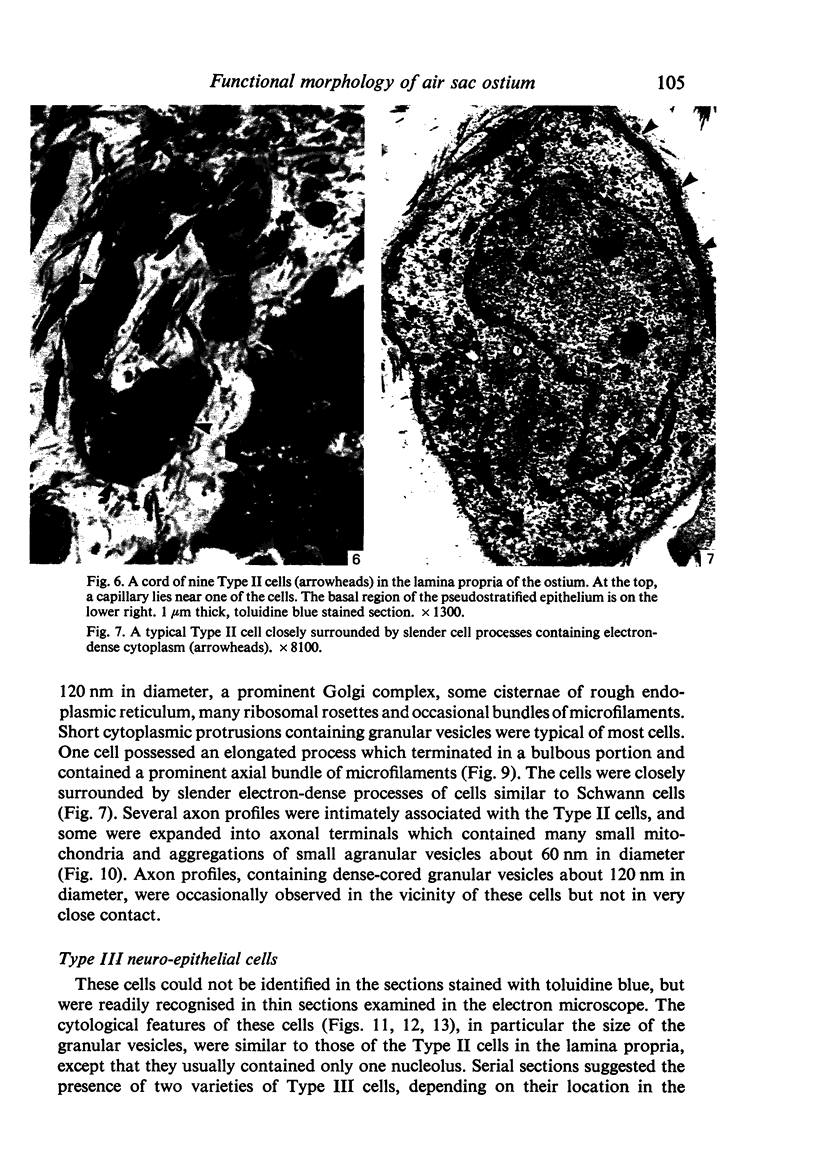
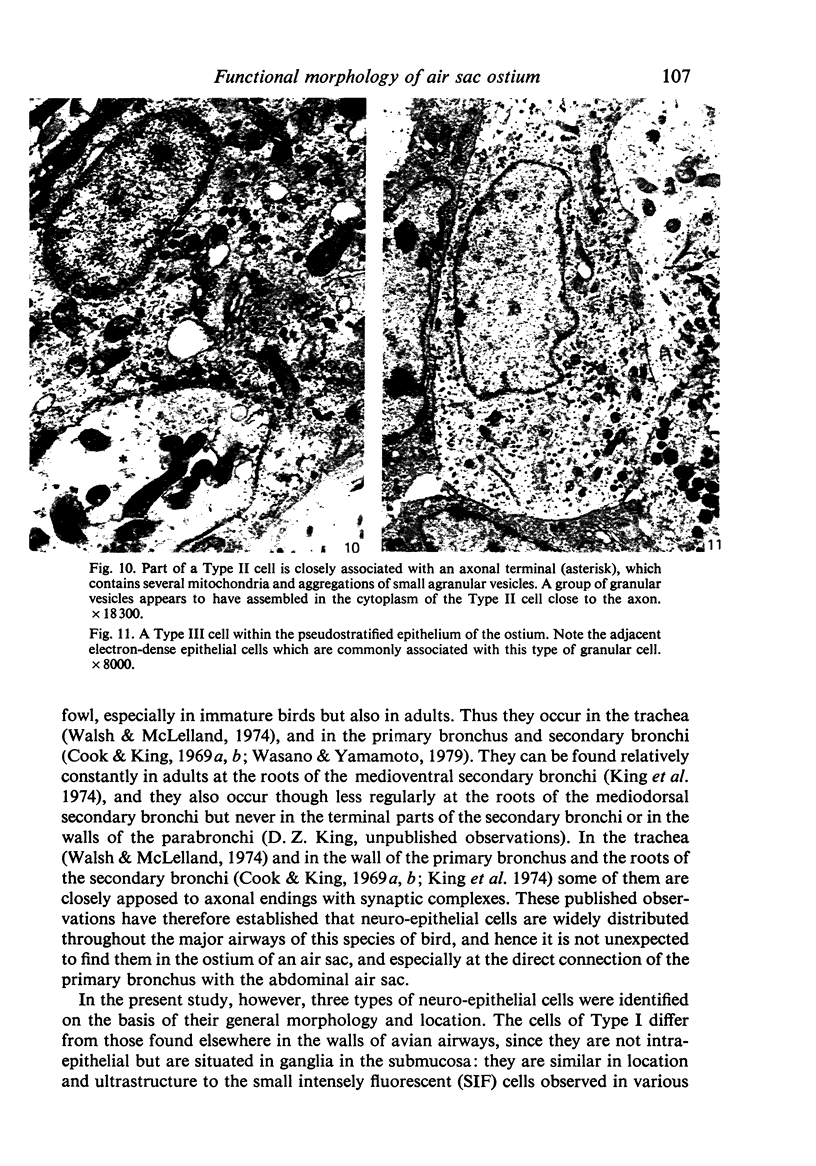
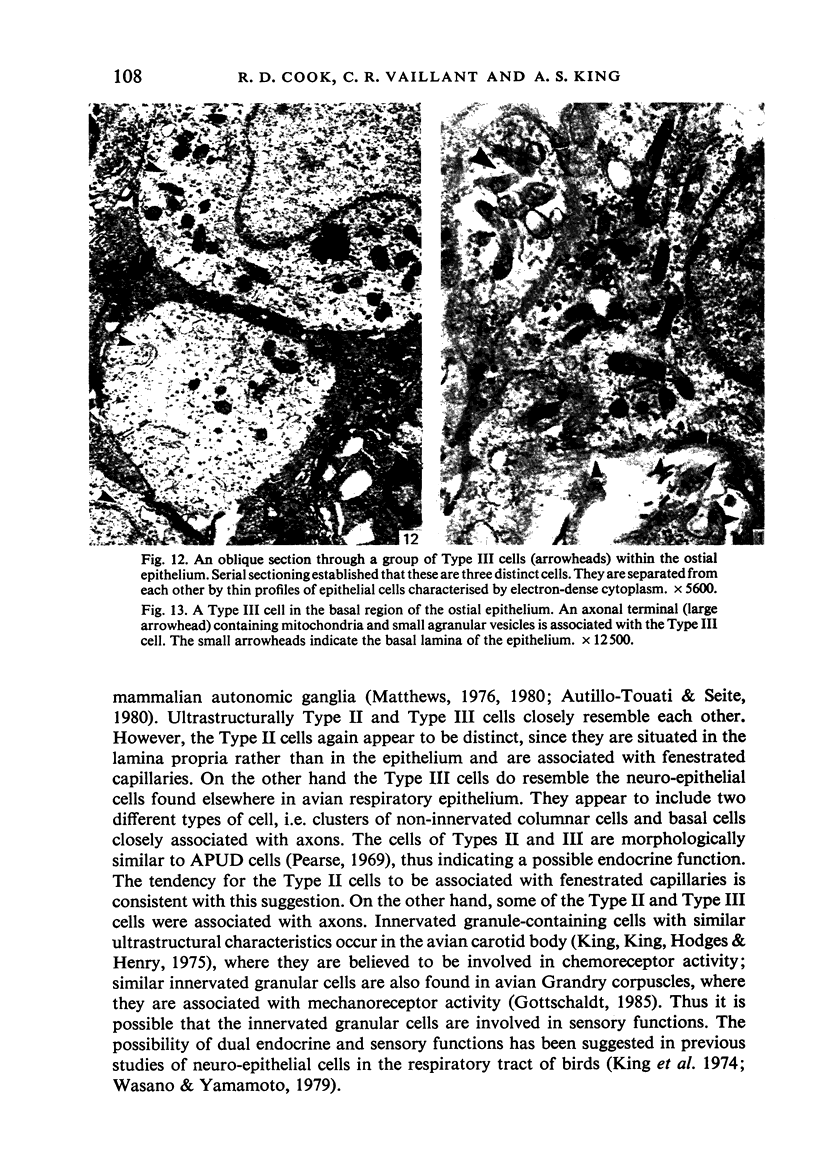
A microscopic study of the ostium of the abdominal air sac of the domestic fowl has shown that the ostium has a sphincter-like ring of well innervated smooth muscle. Three types of neuro-epithelial cell characterised by their content of numerous large granular vesicles are found in the wall of the ostium. Type I cells are present within the submucosal nerve plexus and appear to be morphologically similar to SIF cells. Type II cells occur in the lamina propria, in clusters or cords, are often associated with fenestrated capillaries, and have synaptic contact with axonal terminals containing small agranular vesicles. The cells of Types I and II are not intra-epithelial and therefore differ from the cells which have been found elsewhere in the respiratory tract of the domestic fowl and other vertebrates. Type III cells are intra-epithelial, and some of those in the basal region of the epithelium are associated with axon terminals. Type III cells are similar in ultrastructure and location to neuro-epithelial cells found elsewhere in the major airways of the domestic fowl. They also resemble cells in neuro-epithelial bodies in amphibian, reptilian and mammalian lungs, although neuro-epithelial bodies have not been found in the lung of this species of bird. The morphology of the ostium suggests that it may have a sphincter-like function, possibly regulated by the neuro-epithelial cells. The presence of a mucociliary epithelium and defensive tissue in the lamina propria indicates that the ostium is the site of defence mechanisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett T., Malmfors T. The adrenergic nervous system of the domestic fowl (Gallus domesticus [L.]). Z Zellforsch Mikrosk Anat. 1970;106(1):22–50. doi: 10.1007/BF01027715. [DOI] [PubMed] [Google Scholar]
- Bower A. J., Parker S., Molony V. An autoradiographic study of the afferent innervation of the trachea, syrinx and extrapulmonary primary bronchus of Gallus gallus domesticus. J Anat. 1978 May;126(Pt 1):169–180. [PMC free article] [PubMed] [Google Scholar]
- Carlson H. C., Beggs E. C. Ultrastructure of the abdominal air sac of the fowl. Res Vet Sci. 1973 Mar;14(2):148–150. [PubMed] [Google Scholar]
- Cook R. D., King A. S. A neurite-receptor complex in the avian lung: electron microscopical observations. Experientia. 1969 Nov 15;25(11):1162–1164. doi: 10.1007/BF01900251. [DOI] [PubMed] [Google Scholar]
- Cook R. D., King A. S. Nerves of the avian lung: electron microscopy. J Anat. 1969 Jul;105(Pt 1):202–202. [PubMed] [Google Scholar]
- Cutz E., Chan W., Sonstegard K. S. Identification of neuro-epithelial bodies in rabbit fetal lungs by scanning electron microscopy: a correlative light, transmission and scanning electron microscopic study. Anat Rec. 1978 Nov;192(3):459–466. doi: 10.1002/ar.1091920311. [DOI] [PubMed] [Google Scholar]
- Duncker H. R. The lung air sac system of birds. A contribution to the functional anatomy of the respiratory apparatus. Ergeb Anat Entwicklungsgesch. 1971;45(6):7–171. [PubMed] [Google Scholar]
- Edmondson N. A., Lewis D. J. Distribution and ultrastructural characteristics of Feyrter cells in the rat and hamster airway epithelium. Thorax. 1980 May;35(5):371–374. doi: 10.1136/thx.35.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniakowska-Witalińska L. Neuroepithelial bodies in the lung of the tree frog, Hyla arborea L. A scanning and transmission electron microscopic study. Cell Tissue Res. 1981;217(2):435–441. doi: 10.1007/BF00233593. [DOI] [PubMed] [Google Scholar]
- Hoyt R. F., Jr, Feldman H., Sorokin S. P. Neuroepithelial bodies (NEB) and solitary endocrine cells in the hamster lung. Exp Lung Res. 1982 Nov;3(3-4):299–311. doi: 10.3109/01902148209069659. [DOI] [PubMed] [Google Scholar]
- Hoyt R. F., Jr, Sorokin S. P., Feldman H. Small-granule (neuro)endocrine cells in the infracardiac lobe of a hamster lung. Number, subtypes, and distribution. Exp Lung Res. 1982 Nov;3(3-4):273–298. doi: 10.3109/01902148209069658. [DOI] [PubMed] [Google Scholar]
- Hung K. S., Chapman A. L., Mestemacher M. A. Scanning electron microscopy of bronchiolar neuroepithelial bodies in neonatal mouse lungs. Anat Rec. 1979 Apr;193(4):913–926. doi: 10.1002/ar.1091930412. [DOI] [PubMed] [Google Scholar]
- King A. S., King D. Z., Hodges R. D., Henry J. Synaptic morphology of the carotid body of the domestic fowl. Cell Tissue Res. 1975 Oct 27;162(4):459–473. doi: 10.1007/BF00209346. [DOI] [PubMed] [Google Scholar]
- King A. S., McLelland J., Cook R. D., King D. Z., Walsh C. The ultrastructure of afferent nerve endings in the avian lung. Respir Physiol. 1974 Oct;22(1-2):21–40. doi: 10.1016/0034-5687(74)90045-0. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Cokelaere M., Theunynck P. Neuro-epithelial bodies in the respiratory mucosa of various mammals. A light optical, histochemical and ultrastructural investigation. Z Zellforsch Mikrosk Anat. 1972;135(4):569–592. doi: 10.1007/BF00583438. [DOI] [PubMed] [Google Scholar]
- Lucas A. M. Avian functional anatomic problems. Fed Proc. 1970 Sep-Oct;29(5):1641–1648. [PubMed] [Google Scholar]
- Matthews M. R. Ultrastructural studies relevant to the possible functions of small granule-containing cells in the rat superior cervical ganglion. Adv Biochem Psychopharmacol. 1980;25:77–86. [PubMed] [Google Scholar]
- Pearse A. G., Takor T. T. Neuroendocrine embryology and the APUD concept. Clin Endocrinol (Oxf) 1976;5 (Suppl):229S–244S. doi: 10.1111/j.1365-2265.1976.tb03832.x. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Rogers D. C., Haller C. J. Innervation and cytochemistry of the neuroepithelial bodies in the ciliated epithelium of the toad lung (Bufo marinus). Cell Tissue Res. 1978 Dec 29;195(3):395–410. doi: 10.1007/BF00233885. [DOI] [PubMed] [Google Scholar]
- Scheuermann D. W., De Groodt-Lasseel M. H., Stilman C., Meisters M. L. A correlative light-, fluorescence- and electron-microscopic study of neuroepithelial bodies in the lung of the red-eared turtle, Pseudemys scripta elegans. Cell Tissue Res. 1983;234(2):249–269. doi: 10.1007/BF00213767. [DOI] [PubMed] [Google Scholar]
- Sorokin S. P., Hoyt R. F., Jr, Grant M. M. Development of neuroepithelial bodies in fetal rabbit lungs. I. Appearance and functional maturation as demonstrated by high-resolution light microscopy and formaldehyde-induced fluorescence. Exp Lung Res. 1982 Nov;3(3-4):237–259. doi: 10.3109/01902148209069656. [DOI] [PubMed] [Google Scholar]
- Walsh C., McLelland J. Granular "endocrine" cells in avian respiratory epithelia. Cell Tissue Res. 1974;153(2):269–276. doi: 10.1007/BF00226615. [DOI] [PubMed] [Google Scholar]
- Wasano K. Neuro-epithelial bodies in the lung of the rat and the mouse. Arch Histol Jpn. 1977;40 (Suppl):207–219. doi: 10.1679/aohc1950.40.supplement_207. [DOI] [PubMed] [Google Scholar]
- Wasano K., Yamamoto T. A scanning and transmission electron-microscopic study on neuro-epithelial bodies in the neonatal mouse lung. Cell Tissue Res. 1981;216(3):481–490. doi: 10.1007/BF00238645. [DOI] [PubMed] [Google Scholar]
- Wasano K., Yamamoto T. APUD-type recepto-secretory cells in the chicken lung. Cell Tissue Res. 1979 Sep 3;201(2):197–205. doi: 10.1007/BF00235057. [DOI] [PubMed] [Google Scholar]