Abstract
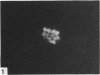
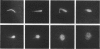
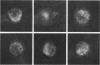
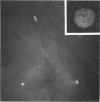
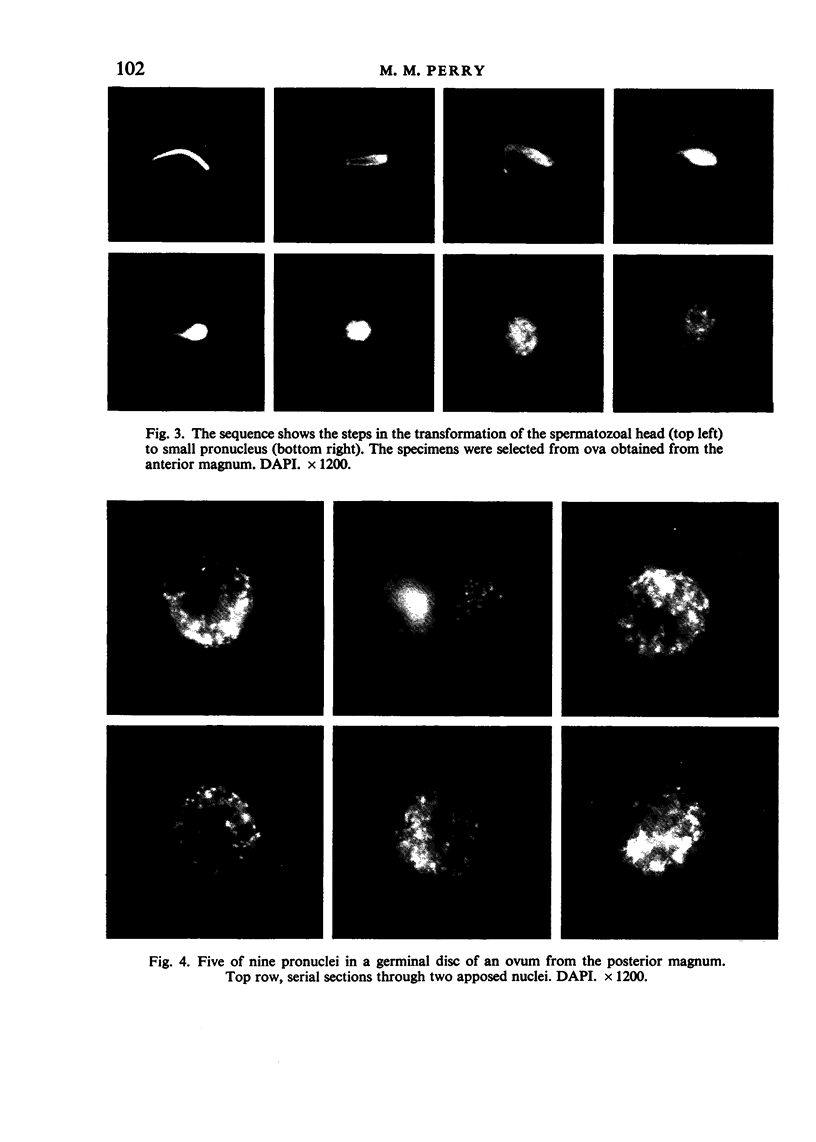
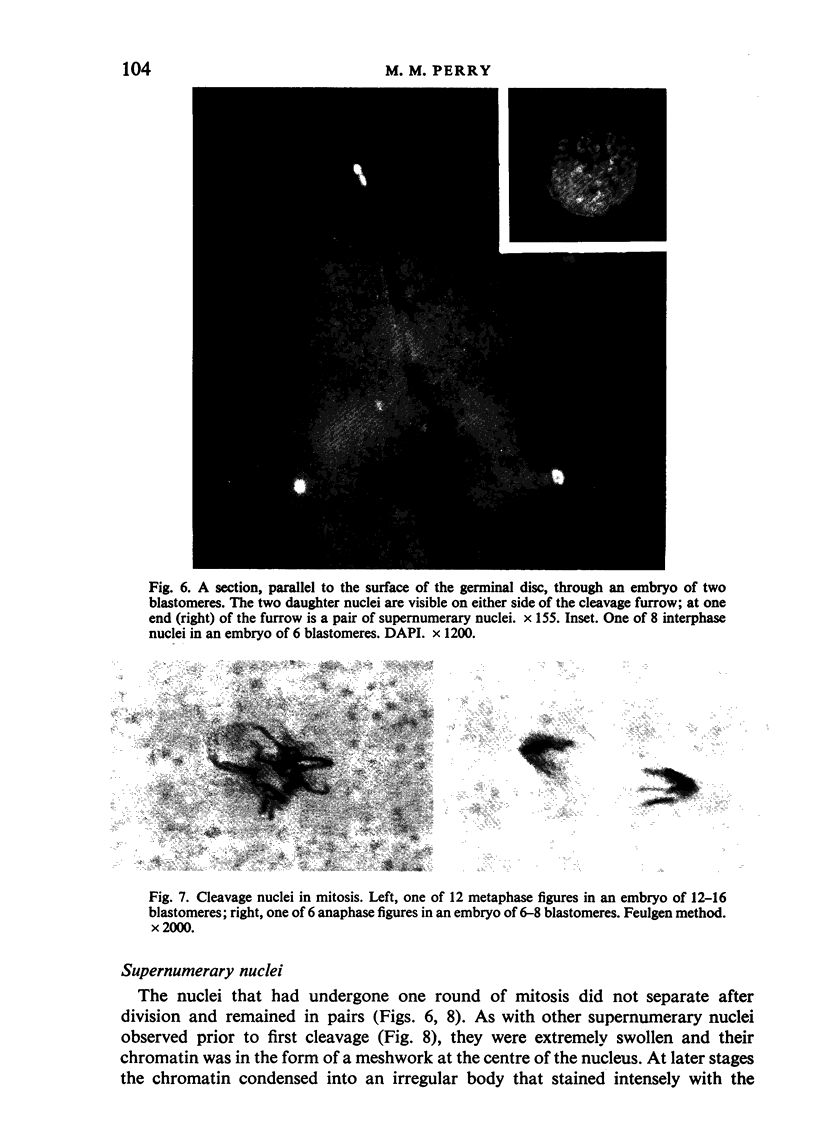
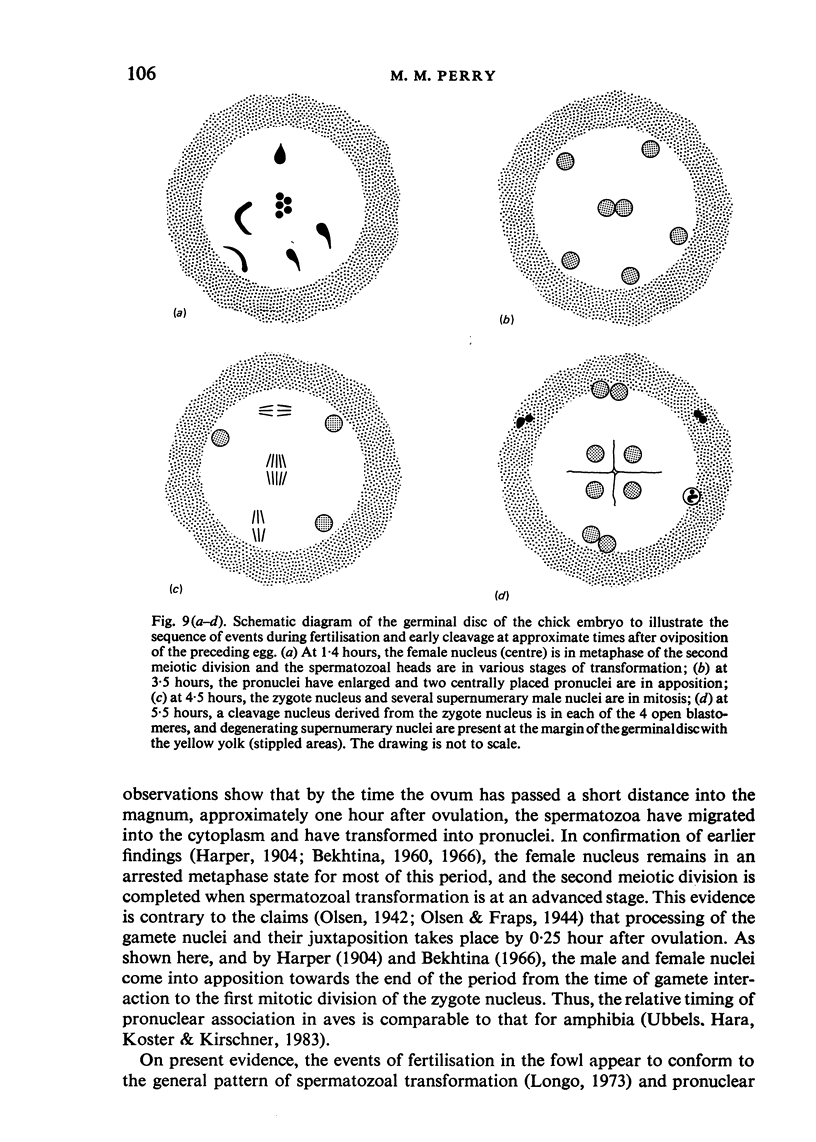
The changes in nuclear morphology from fertilisation to the 16-blastomere stage of development in the domestic fowl were examined in sections of the germinal disc stained with DAPI (4',6'-diamidino-2-phenylindole) or with the Feulgen technique. By one hour after the estimated time of ovulation, the second meiotic division of the ovum was completed and the spermatozoal heads (3-9) in the germinal disc had transformed into pronuclei. During the following two hours all the pronuclei enlarged, and two of them became juxtaposed. Mitotic figures and large pronuclei were observed at 4 hours. The zygote nucleus went through 3-4 division cycles in synchrony. The supernumerary male nuclei divided once, at most, and all showed evidence of degeneration by the 4-blastomere stage, at about five hours. Therefore, the nuclei destined to form the future chick embryo follow a pattern of behaviour during fertilisation and early cleavage similar to that of animals in general.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEKHTINA V. G. [The early stages of cleavage in the chick embryo]. Arkh Anat Gistol Embriol. 1960 Apr;38:77–85. [PubMed] [Google Scholar]
- Bakst M. R., Howarth B., Jr Hydrolysis of the hen's perivitelline layer by cock sperm in vitro. Biol Reprod. 1977 Oct;17(3):370–379. doi: 10.1095/biolreprod17.3.370. [DOI] [PubMed] [Google Scholar]
- Bellairs R., Lorenz F. W., Dunlap T. Cleavage in the chick embryo. J Embryol Exp Morphol. 1978 Feb;43:55–69. [PubMed] [Google Scholar]
- Coleman A. W., Maguire M. J., Coleman J. R. Mithramycin- and 4'-6-diamidino-2-phenylindole (DAPI)-DNA staining for fluorescence microspectrophotometric measurement of DNA in nuclei, plastids, and virus particles. J Histochem Cytochem. 1981 Aug;29(8):959–968. doi: 10.1177/29.8.6168681. [DOI] [PubMed] [Google Scholar]
- Emanuelsson H. Cell multiplication in the chick blastoderm up to the time of laying. Exp Cell Res. 1965 Sep;39(2):386–399. doi: 10.1016/0014-4827(65)90042-x. [DOI] [PubMed] [Google Scholar]
- Eyal-Giladi H., Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol. 1976 Apr;49(2):321–337. doi: 10.1016/0012-1606(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Fechheimer N. S. Origins of heteroploidy in chicken embryos. Poult Sci. 1981 Jul;60(7):1365–1371. doi: 10.3382/ps.0601365. [DOI] [PubMed] [Google Scholar]
- Gipson I. Electron microscopy of early cleavage furrows in the chick blastodisc. J Ultrastruct Res. 1974 Dec;49(3):331–347. doi: 10.1016/s0022-5320(74)90049-5. [DOI] [PubMed] [Google Scholar]
- Graham C. F. The regulation of DNA synthesis and mitosis in multinucleate frog eggs. J Cell Sci. 1966 Sep;1(3):363–374. doi: 10.1242/jcs.1.3.363. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Woodland H. R. The cytoplasmic control of nuclear activity in animal development. Biol Rev Camb Philos Soc. 1968 May;43(2):233–267. doi: 10.1111/j.1469-185x.1968.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Kochav S., Ginsburg M., Eyal-Giladi H. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. II. Microscopic anatomy and cell population dynamics. Dev Biol. 1980 Oct;79(2):296–308. doi: 10.1016/0012-1606(80)90117-7. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Longo F. J. Fertilization: a comparative ultrastructural review. Biol Reprod. 1973 Sep;9(2):149–215. doi: 10.1093/biolreprod/9.2.149. [DOI] [PubMed] [Google Scholar]
- Morris T. R. The effects of ahemeral light and dark cycles on egg production in the fowl. Poult Sci. 1973 Mar;52(2):423–445. doi: 10.3382/ps.0520423. [DOI] [PubMed] [Google Scholar]
- Ubbels G. A., Hara K., Koster C. H., Kirschner M. W. Evidence for a functional role of the cytoskeleton in determination of the dorsoventral axis in Xenopus laevis eggs. J Embryol Exp Morphol. 1983 Oct;77:15–37. [PubMed] [Google Scholar]