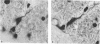
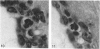
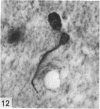
Abstract
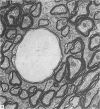
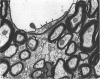
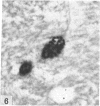
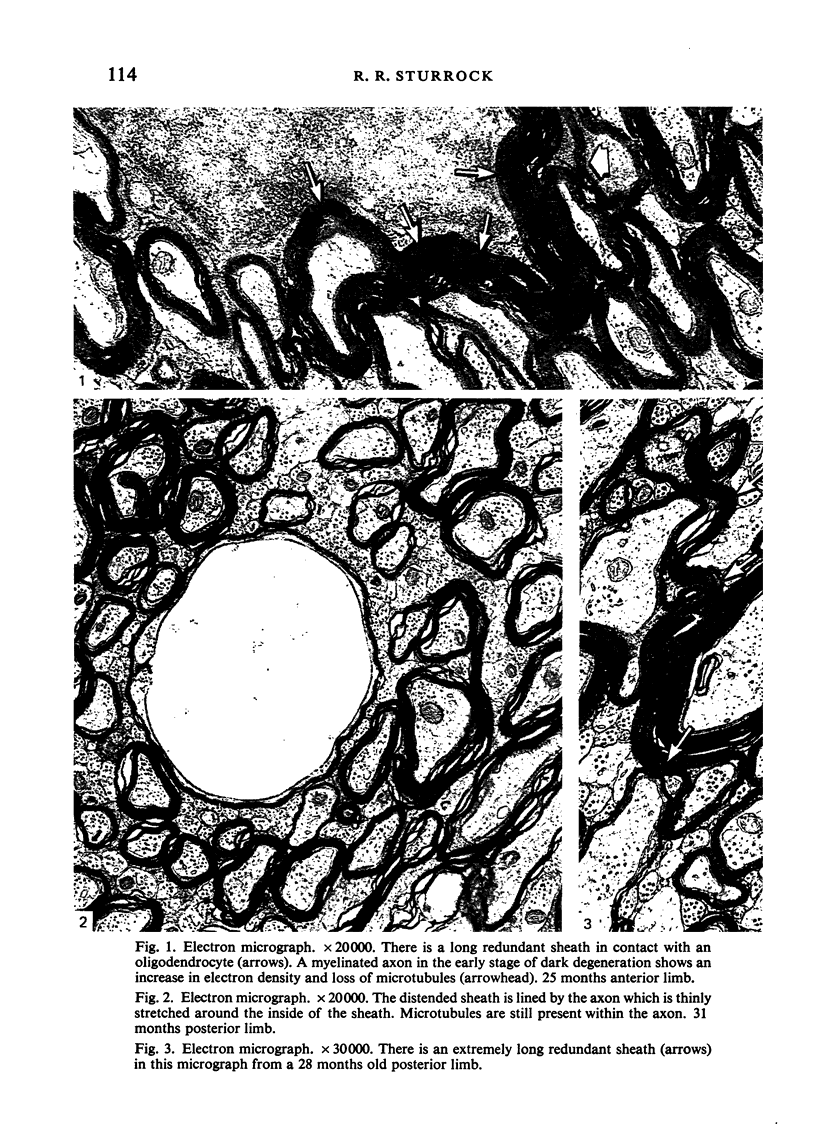
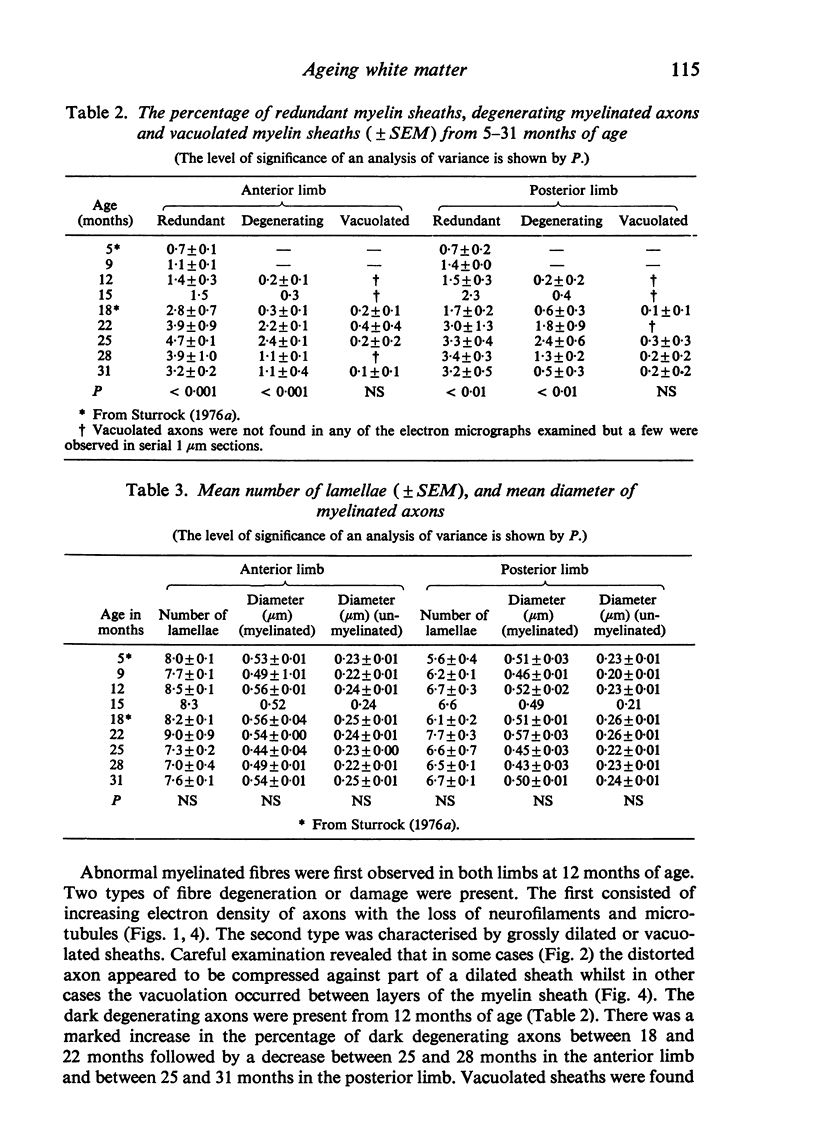
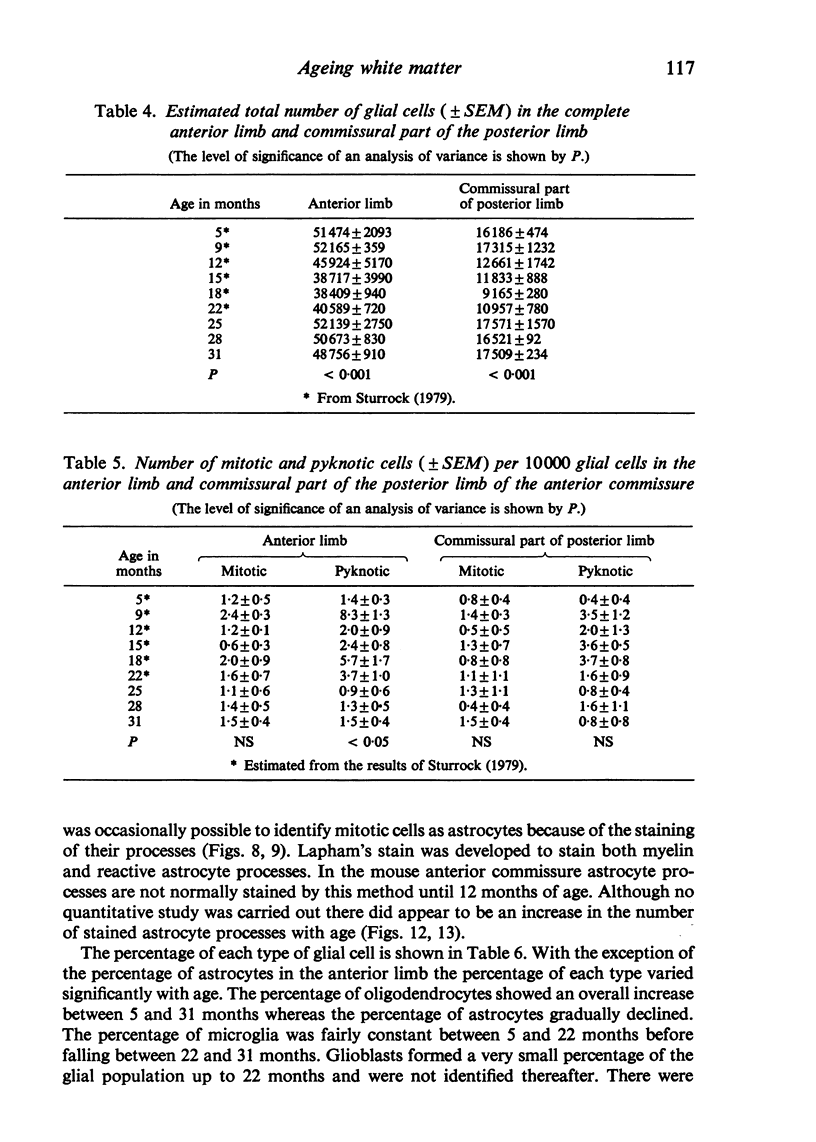
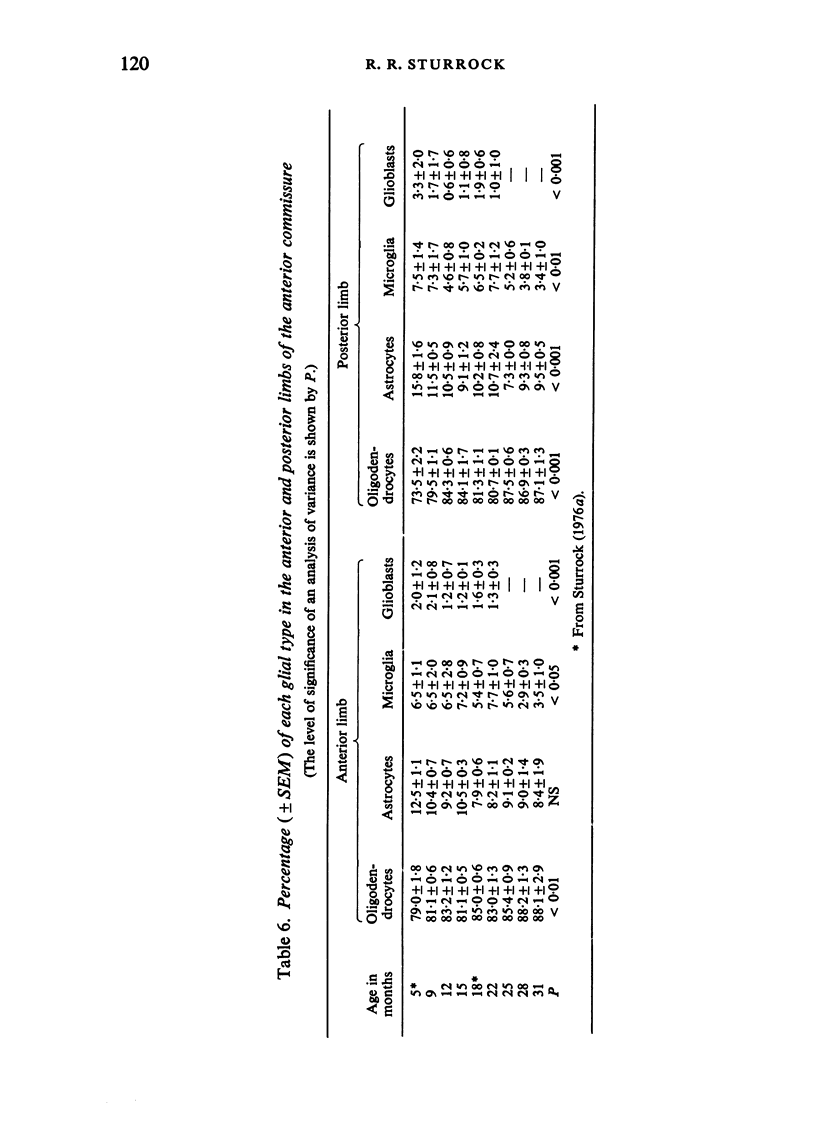
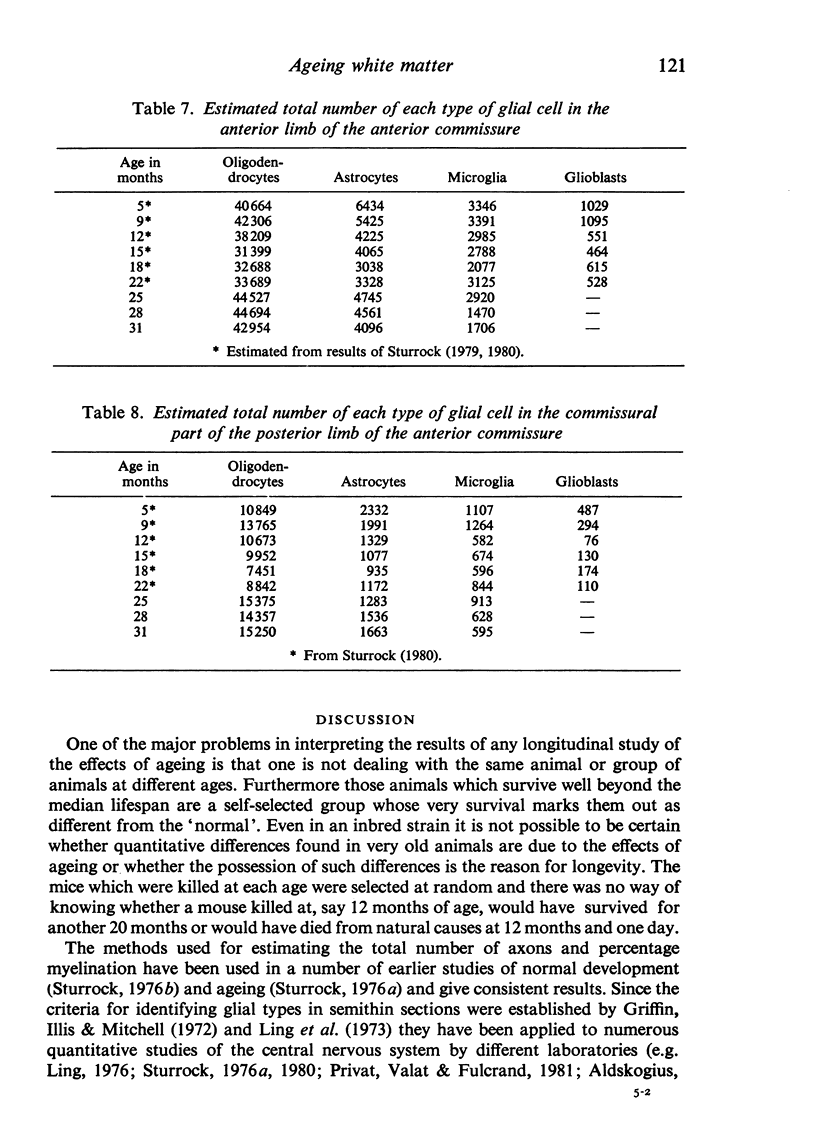
The total number of axons and the number of myelinated axons was estimated in both limbs of the anterior commissure of mouse brains from 5 to 31 months of age. The total number of glial cells and the percentage of each type of glial cell was estimated in both limbs of the anterior commissure at 25, 28 and 31 months since similar estimations had already been carried out in mice aged between 5 and 22 months. The total number of axons and the number of myelinated axons both appeared to fall between 9 and 12 months and to increase again between 22 and 25 months in the anterior limb whereas in the posterior limb the total number of axons remained constant but the number of myelinated axons increased between 22 and 28 months. Two types of abnormality were seen in myelinated fibres at all ages after 12 months. These consisted of degenerating axons enclosed in normal myelin sheaths and apparently normal axons surrounded by vacuolated sheaths. The total number of glial cells in both limbs decreased between 9 and 12 months and increased substantially between 22 and 25 months. There was a statistically significant correlation between the number of oligodendrocytes and the percentage of myelinated axons in both limbs at different ages. There was no change in the very small number of mitotic figures in the anterior commissure to account for the fluctuation in glial number. It is postulated that there is a continuous loss and replacement of myelinated axons in both limbs of the commissure from 12 to 31 months of age and that this is possible since the amount of axon loss is so small that there is no significant phagocytic or astrocytic response. An increased requirement for myelination appears capable of bringing about an increase in the number of oligodendrocytes even in aged animals.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldskogius H. Glial cell responses in the adult rabbit dorsal motor vagal nucleus during axon reaction. Neuropathol Appl Neurobiol. 1982 Sep-Oct;8(5):341–349. doi: 10.1111/j.1365-2990.1982.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Arenella L. S., Herndon R. M. Mature oligodendrocytes. Division following experimental demyelination in adult animals. Arch Neurol. 1984 Nov;41(11):1162–1165. doi: 10.1001/archneur.1984.04050220060015. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. Ethidium bromide induced demyelination in the spinal cord of the cat. Neuropathol Appl Neurobiol. 1982 Sep-Oct;8(5):365–375. doi: 10.1111/j.1365-2990.1982.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., Coleman P. D. Stability of neuron number in cortical barrels of aging mice. J Comp Neurol. 1982 Dec 1;212(2):158–172. doi: 10.1002/cne.902120206. [DOI] [PubMed] [Google Scholar]
- David S., Miller R. H., Patel R., Raff M. C. Effects of neonatal transection on glial cell development in the rat optic nerve: evidence that the oligodendrocyte-type 2 astrocyte cell lineage depends on axons for its survival. J Neurocytol. 1984 Dec;13(6):961–974. doi: 10.1007/BF01148596. [DOI] [PubMed] [Google Scholar]
- Dunkerley G. B., Duncan D. A light and electron microscopic study of the normal and the degenerating corticospinal tract in the rat. J Comp Neurol. 1969 Oct;137(2):155–183. doi: 10.1002/cne.901370204. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Bondareff W., Dodge J. T. Hypertrophy of astroglial processes in the dentate gyrus of the senescent rat. Am J Anat. 1978 Dec;153(4):537–543. doi: 10.1002/aja.1001530405. [DOI] [PubMed] [Google Scholar]
- Gould D. H., Gustine D. L. Basal ganglia degeneration, myelin alterations, and enzyme inhibition induced in mice by the plant toxin 3-nitropropanoic acid. Neuropathol Appl Neurobiol. 1982 Sep-Oct;8(5):377–393. doi: 10.1111/j.1365-2990.1982.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Griffin R., Illis L. S., Mitchell J. Identification of neuroglia by light and electronmicroscopy. Acta Neuropathol. 1972;22(1):7–12. doi: 10.1007/BF00687546. [DOI] [PubMed] [Google Scholar]
- Griffiths I. R., McCulloch M. C., Abrahams S. Progressive axonopathy: an inherited neuropathy of boxer dogs. 2. The nature and distribution of the pathological changes. Neuropathol Appl Neurobiol. 1985 Nov-Dec;11(6):431–446. doi: 10.1111/j.1365-2990.1985.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Heumann D., Leuba G. Neuronal death in the development and aging of the cerebral cortex of the mouse. Neuropathol Appl Neurobiol. 1983 Jul-Aug;9(4):297–311. doi: 10.1111/j.1365-2990.1983.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Kelly W. R., Blakemore W. F., Jagelman S., Webb H. E. Demyelination induced in mice by avirulent Semliki Forest virus. II. An ultrastructural study of focal demyelination in the brain. Neuropathol Appl Neurobiol. 1982 Jan-Feb;8(1):43–53. doi: 10.1111/j.1365-2990.1982.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Konigsmark B. W., Murphy E. A. Volume of the ventral cochlear nucleus in man: its relationship to neuronal population and age. J Neuropathol Exp Neurol. 1972 Apr;31(2):304–316. doi: 10.1097/00005072-197204000-00006. [DOI] [PubMed] [Google Scholar]
- Kruger L., Maxwell D. S. Wallerian degeneration in the optic nerve of a reptile: an electron microscopic study. Am J Anat. 1969 Jul;125(3):247–269. doi: 10.1002/aja.1001250302. [DOI] [PubMed] [Google Scholar]
- LAPHAM L. W., JOHNSTONE M. A., BRUNDJAR K. H. A NEW PARAFFIN METHOD FOR THE COMBINED STAINING OF MYELIN AND GLIAL FIBERS. J Neuropathol Exp Neurol. 1964 Jan;23:156–160. [PubMed] [Google Scholar]
- Landfield P. W., Rose G., Sandles L., Wohlstadter T. C., Lynch G. Patterns of astroglial hypertrophy and neuronal degeneration in the hippocampus of ages, memory-deficient rats. J Gerontol. 1977 Jan;32(1):3–12. doi: 10.1093/geronj/32.1.3. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Electron-microscopic identification of amoeboid microglia in the spinal cord of newborn rats. Acta Anat (Basel) 1976;96(4):600–609. doi: 10.1159/000144707. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Paterson J. A., Privat A., Mori S., Leblond C. P. Investigation of glial cells in semithin sections. I. Identification of glial cells in the brain of young rats. J Comp Neurol. 1973 May 1;149(1):43–71. doi: 10.1002/cne.901490104. [DOI] [PubMed] [Google Scholar]
- Love S., Cruz-Höfling M. A., Duchen L. W. Morphological abnormalities in myelinated nerve fibres caused by Leiurus, Centruroides and Phoneutria venoms and their prevention by tetrodotoxin. Q J Exp Physiol. 1986 Jan;71(1):115–122. doi: 10.1113/expphysiol.1986.sp002962. [DOI] [PubMed] [Google Scholar]
- Ludwin S. K. Reaction of oligodendrocytes and astrocytes to trauma and implantation. A combined autoradiographic and immunohistochemical study. Lab Invest. 1985 Jan;52(1):20–30. [PubMed] [Google Scholar]
- Naranjo N., Green E. Use of reduced silver staining to show loss of connections in aged rat brain. Brain Res Bull. 1977 Jan-Feb;2(1):71–74. doi: 10.1016/0361-9230(77)90029-6. [DOI] [PubMed] [Google Scholar]
- Paterson J. A. Dividing and newly produced cells in the corpus callosum of adult mouse cerebrum as detected by light microscopic radioautography. Anat Anz. 1983;153(2):149–168. [PubMed] [Google Scholar]
- Peters A., Feldman M. L., Vaughan D. W. The effect of aging on the neuronal population within area 17 of adult rat cerebral cortex. Neurobiol Aging. 1983 Winter;4(4):273–282. doi: 10.1016/0197-4580(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Privat A., Valat J., Fulcrand J. Proliferation of neuroglial cell lines in the degenerating optic nerve of young rats. A radioautographic study. J Neuropathol Exp Neurol. 1981 Jan;40(1):46–60. [PubMed] [Google Scholar]
- Spencer P. S., Thomas P. K. The examination of isolated nerve fibres by light and electron microscopy, with observations on demyelination proximal to neuromas. Acta Neuropathol. 1970;16(3):177–186. doi: 10.1007/BF00687357. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Thomas P. K. Ultrastructural studies of the dying-back process. II. The sequestration and removal by Schwann cells and oligodendrocytes of organelles from normal and diseases axons. J Neurocytol. 1974 Dec;3(6):763–783. doi: 10.1007/BF01097197. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. A comparative quantitative and morphological study of ageing in the mouse neostriatum, indusium griseum and anterior commissure. Neuropathol Appl Neurobiol. 1980 Jan-Feb;6(1):51–68. doi: 10.1111/j.1365-2990.1980.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. A quantitative histological study of the indusium griseum and neostriatum in elderly mice. J Anat. 1986 Dec;149:195–203. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. A quantitative lifespan study of changes in cell number, cell division and cell death in various regions of the mouse forebrain. Neuropathol Appl Neurobiol. 1979 Nov-Dec;5(6):433–456. doi: 10.1111/j.1365-2990.1979.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. Age related changes in cellularity, mitotic activity and pyknotic cell number in the mouse subependymal layer. J Anat. 1985 Aug;141:19–26. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. Changes in neurologia and myelination in the white matter of aging mice. J Gerontol. 1976 Sep;31(5):513–522. doi: 10.1093/geronj/31.5.513. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. Development of the mouse anterior commissure. Part I. A comparison of myelination in the anterior and posterior limbs of the anterior commissure of the mouse brain. Zentralbl Veterinarmed C. 1976 Mar;5(1):54–67. doi: 10.1111/j.1439-0264.1976.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. Electron microscopic evidence for mitotic division of oligodendrocytes. J Anat. 1981 May;132(Pt 3):429–432. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R. Identification of mitotic cells in the central nervous system by electron microscopy of re-embedded semithin sections. J Anat. 1984 Jun;138(Pt 4):657–673. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R., Rao K. A. A quantitative histological study of neuronal loss from the locus coeruleus of ageing mice. Neuropathol Appl Neurobiol. 1985 Jan-Feb;11(1):55–60. doi: 10.1111/j.1365-2990.1985.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Veronesi B. A rodent model of organophosphorus-induced delayed neuropathy: distribution of central (spinal cord) and peripheral nerve damage. Neuropathol Appl Neurobiol. 1984 Sep-Oct;10(5):357–368. doi: 10.1111/j.1365-2990.1984.tb00366.x. [DOI] [PubMed] [Google Scholar]