Abstract
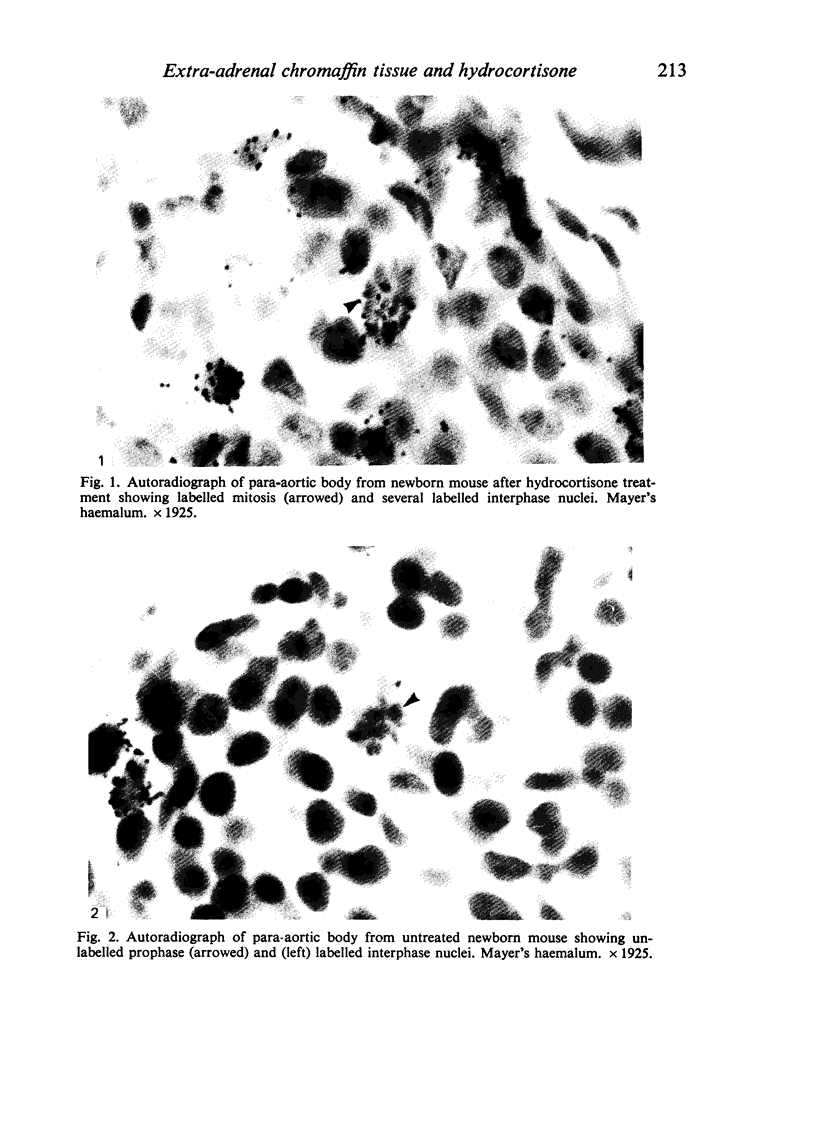
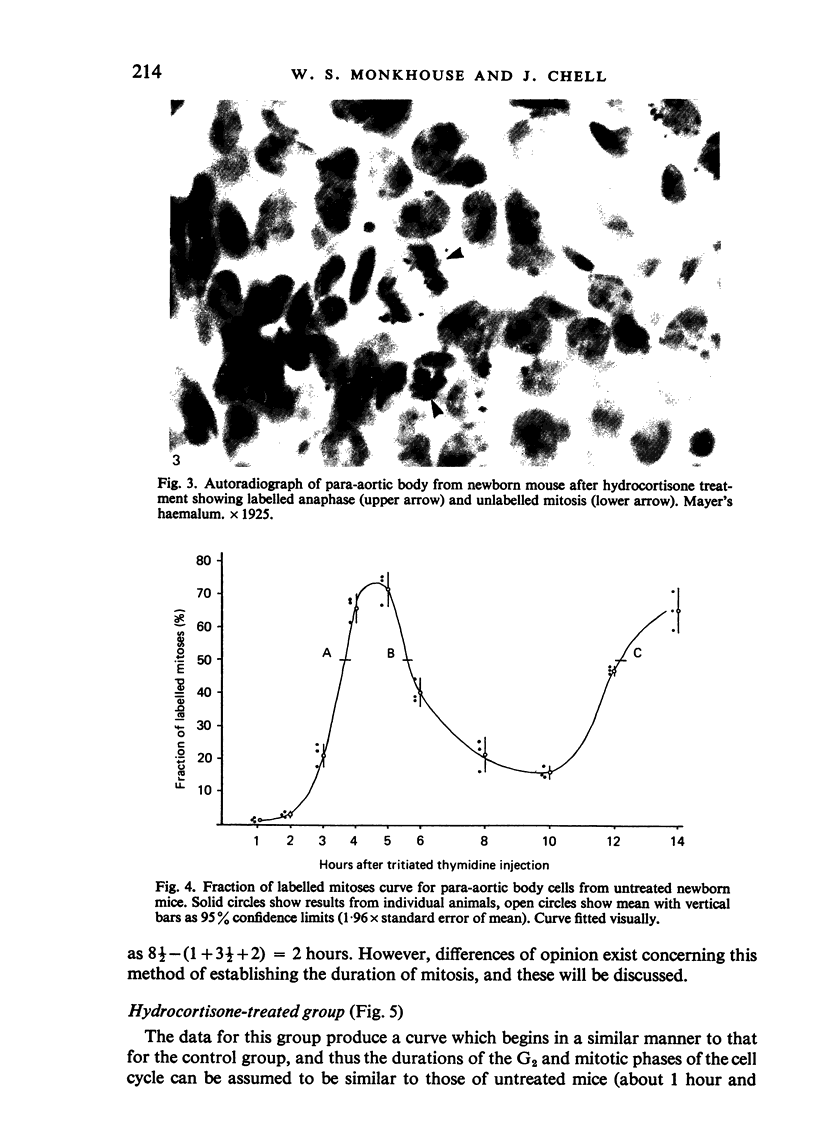
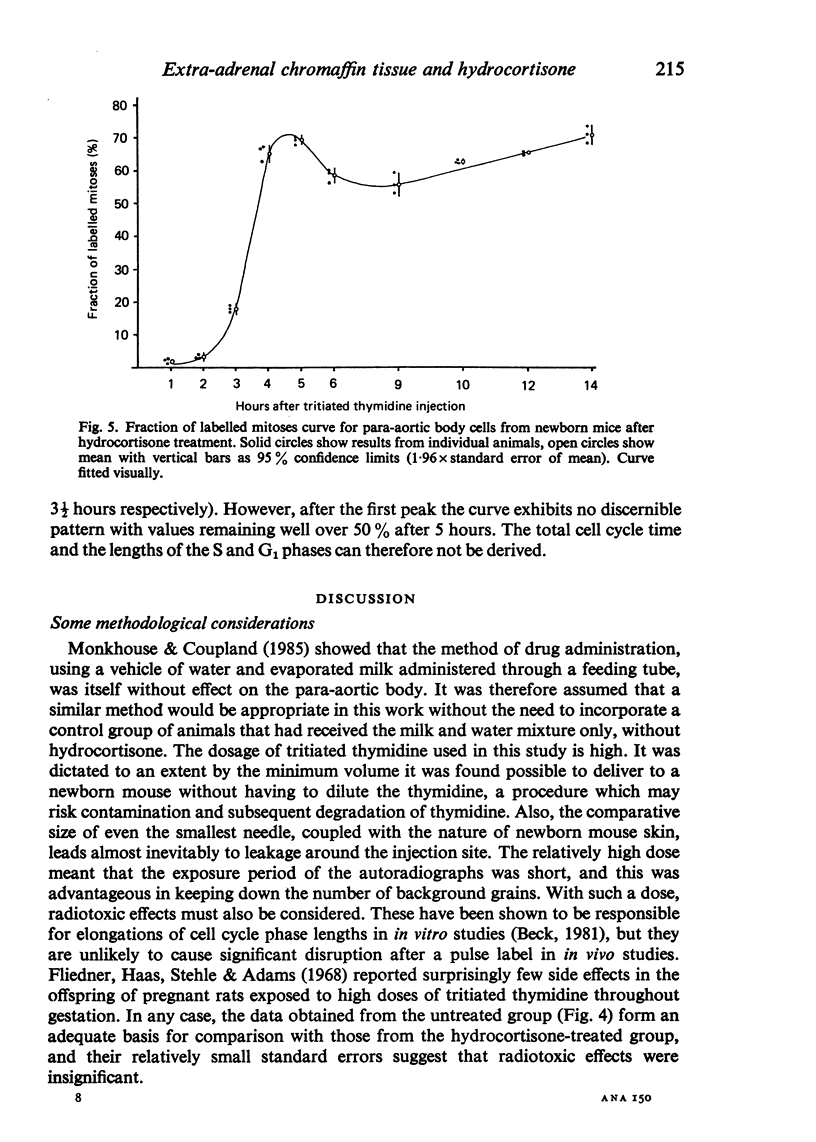
Information about the cell cycle of the mouse para-aortic body within the first 24 hours of postnatal life was derived from a fraction of labelled mitoses study. The total cell cycle time was 8 1/2 hours, being made up as follows: S phase-2 hours; G2 phase-1 hour; M phase-3 1/2 hours (by analysis of the results, not by assumption) and G1 phase-2 hours (by subtraction). Problems are discussed regarding the length of G2 and M phases and the consequences for G1. After hydrocortisone administration (40 mg/kg/day) to female mice for the last seven days of pregnancy, the pattern in newborn mice was disrupted. Values for G2 and M were similar to those of the untreated group, but no values were obtainable for the other phases of the cell cycle or for the total cell cycle time. These results after hydrocortisone treatment could be explained by the superimposition of the cell cycles of two or more different groups of cells. They are discussed with regard to the life span of the para-aortic body, and their implications are considered in the light of previously reported glucocorticoid-induced transformations of small granule cells from cervical sympathetic ganglia into catecholamine-storing chromaffin cells. The established hyperplastic effect of hydrocortisone on the para-aortic body is therefore not the result simply of an acceleration of the cell cycle, but it may involve the incorporation into the proliferative compartment of cells previously either moribund or nonproliferating.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck H. P. Radiotoxicity of incorporated [3H]thymidine as studied by autoradiography and flow cytometry. Consequences for the interpretation of FLM data. Cell Tissue Kinet. 1981 Mar;14(2):163–177. doi: 10.1111/j.1365-2184.1981.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Doupe A. J., Landis S. C., Patterson P. H. Environmental influences in the development of neural crest derivatives: glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci. 1985 Aug;5(8):2119–2142. doi: 10.1523/JNEUROSCI.05-08-02119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe A. J., Patterson P. H., Landis S. C. Small intensely fluorescent cells in culture: role of glucocorticoids and growth factors in their development and interconversions with other neural crest derivatives. J Neurosci. 1985 Aug;5(8):2143–2160. doi: 10.1523/JNEUROSCI.05-08-02143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliedner T. M., Haas R. J., Stehle H., Adams A. Complete labeling of all cell nuclei in newborn rats with H3-thymidine. A tool for the evaluation of rapidly and slowly proliferating cell systems. Lab Invest. 1968 Mar;18(3):249–259. [PubMed] [Google Scholar]
- Monkhouse W. S., Coupland R. E. The effect of in vivo hydrocortisone administration on the labelling index and size of the intra- and extra-adrenal chromaffin tissue of the fetal and perinatal mouse. J Anat. 1985 Jun;140(Pt 4):679–696. [PMC free article] [PubMed] [Google Scholar]
- Monkhouse W. S. The effect of in vivo hydrocortisone administration on the labelling index and size of chromaffin tissue in the postnatal and adult mouse. J Anat. 1986 Feb;144:133–144. [PMC free article] [PubMed] [Google Scholar]
- Monkhouse W. S. The effect of section thickness and embedding media on the observed S-phase labelling index of artificially selected cell populations from neonatal mouse liver and spleen. J Anat. 1985 Aug;141:201–209. [PMC free article] [PubMed] [Google Scholar]
- Tarbutt R. G., Cole R. J. Foetal erythropoiesis in genetically anaemic, flexed-tailed (f-f) mice. Cell Tissue Kinet. 1972 Nov;5(6):491–503. doi: 10.1111/j.1365-2184.1972.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem. 1966 May 25;241(10):2301–2305. [PubMed] [Google Scholar]