Abstract
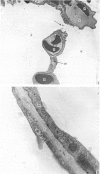
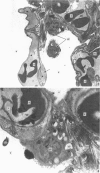
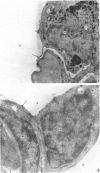
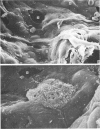
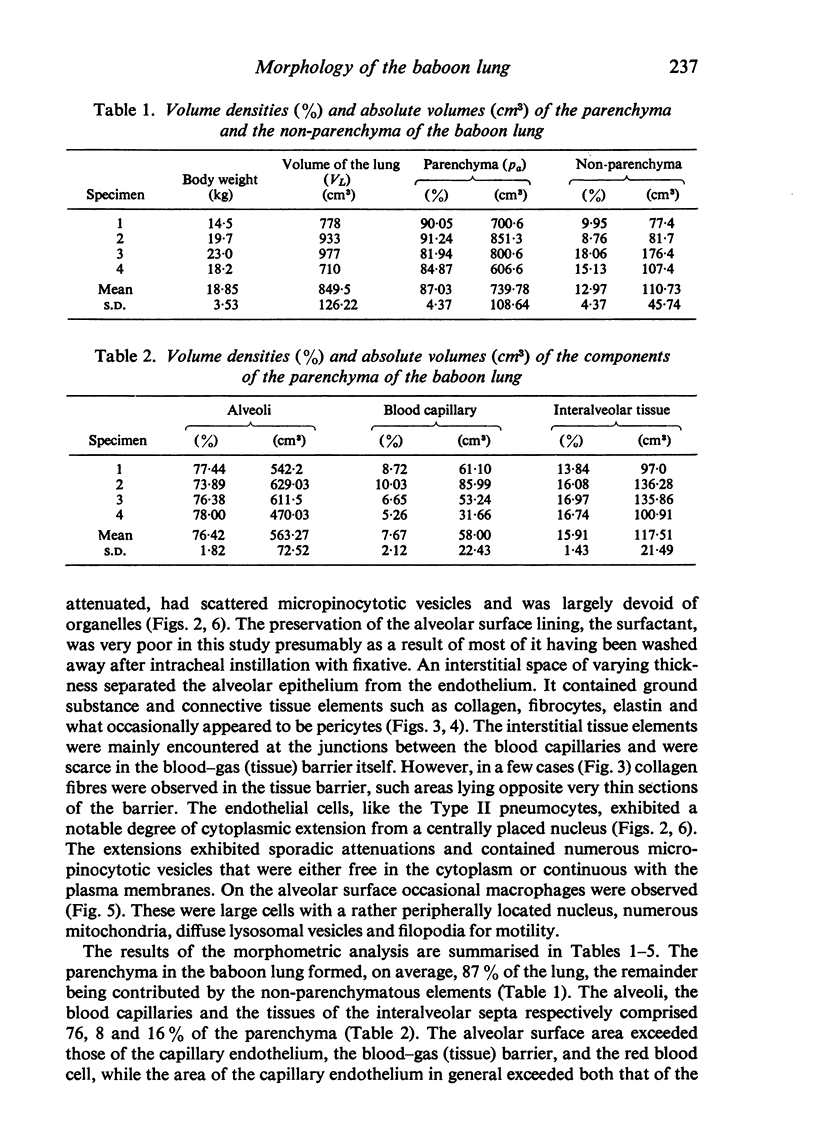
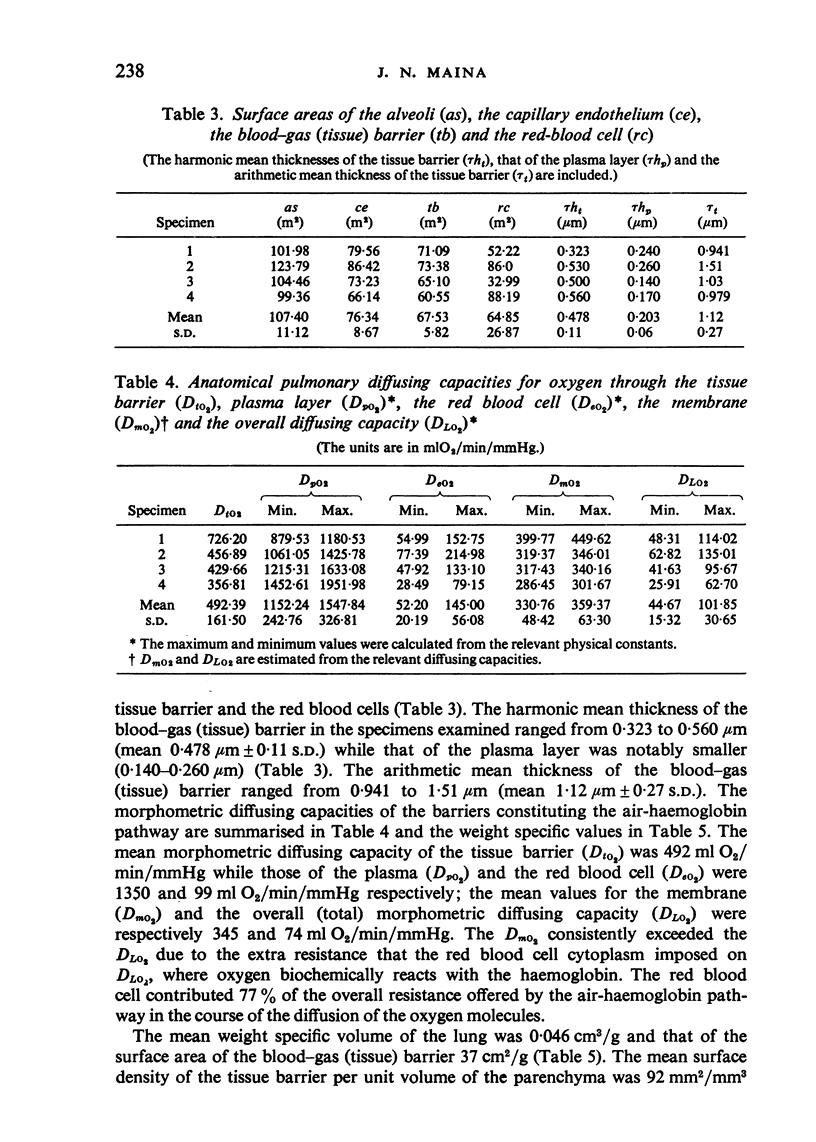
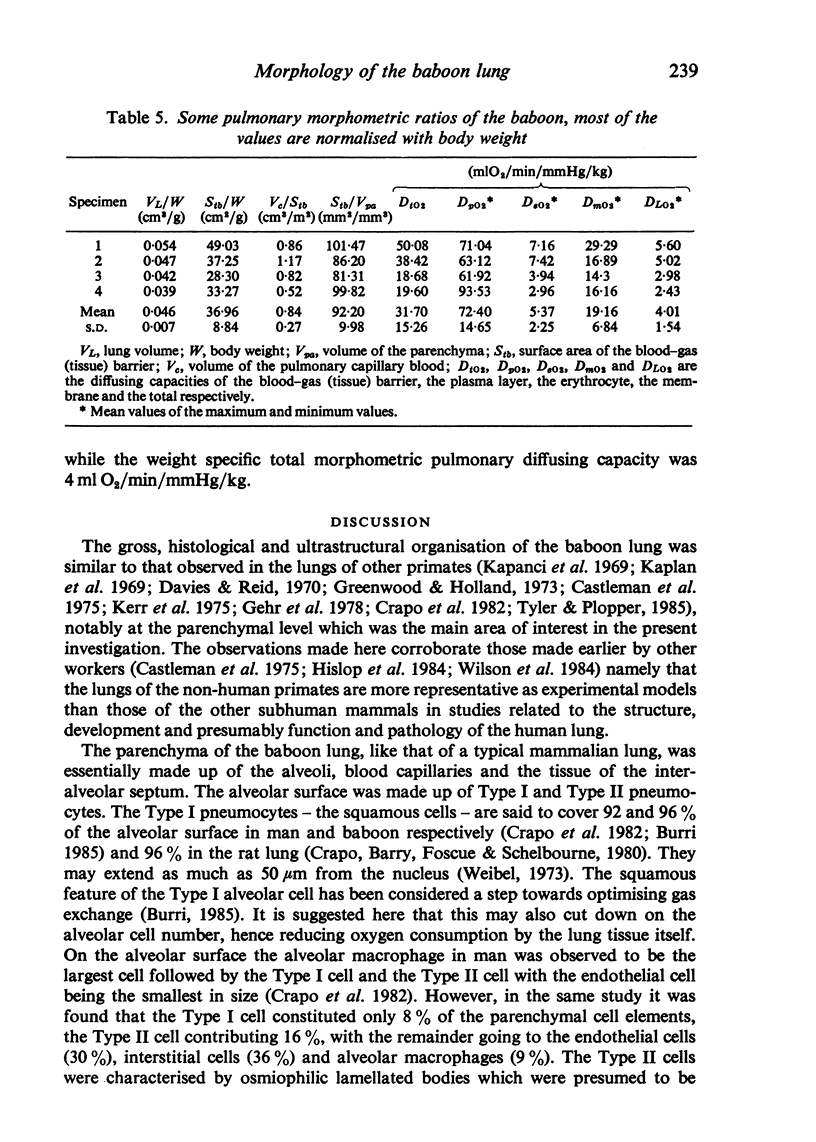
The gross, histological and ultrastructural organisation of the baboon lung was found to be similar to that of the human lung. It is suggested that, in general, the lungs of the non-human primates would serve as ideal models for the study of the human lung. The baboon lung comprises the parenchyma, the gas exchange part of the lung which consists of alveoli, blood capillaries and the tissue of the interalveolar septum, and the non-parenchyma made up of the air conducting passages like bronchi, bronchioles, larger blood vessels, connective tissue and pleura. On morphometric analysis, the parenchyma was found to constitute 87% of the lung, the rest being made up of the elements of the non-parenchyma. The alveoli, blood capillaries and the interalveolar tissue respectively constituted 76, 8 and 16% of the parenchyma. The harmonic mean thickness of the blood-gas (tissue) barrier was 0.475 micron and the arithmetic mean 1.12 micron, the ratio being 1:2.3. The weight specific surface area of the blood-gas (tissue) barrier was 37 cm2/g and the surface density of the tissue barrier in the parenchyma 92 mm2/mm3. The total morphometric pulmonary diffusion per unit body weight was 4 ml O2/min/mmHg/kg and the volume of the pulmonary capillary blood per unit surface area of the tissue barrier 0.84 cm3/m2. Morphometrically the baboon lung was thus observed to be better adapted for gas exchange than that of man but less specialised than that of the smaller monkeys such as Macaca mulatta.
Full text
PDF


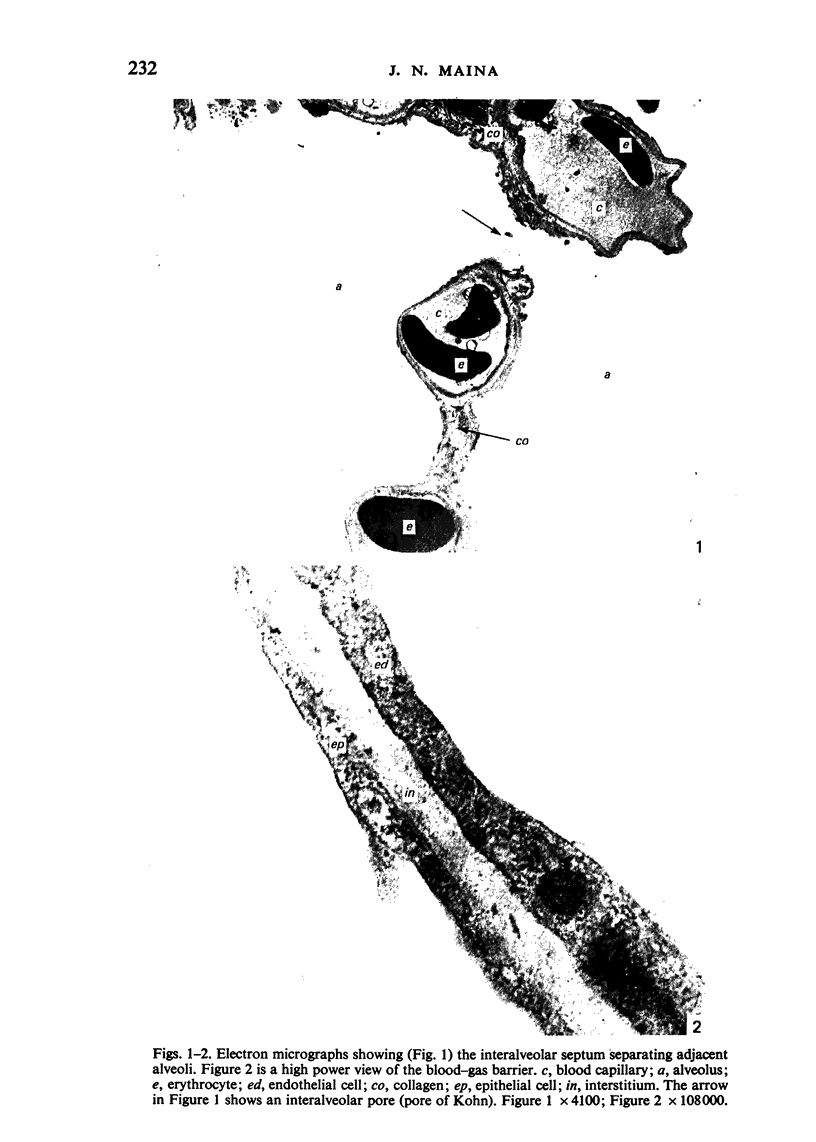
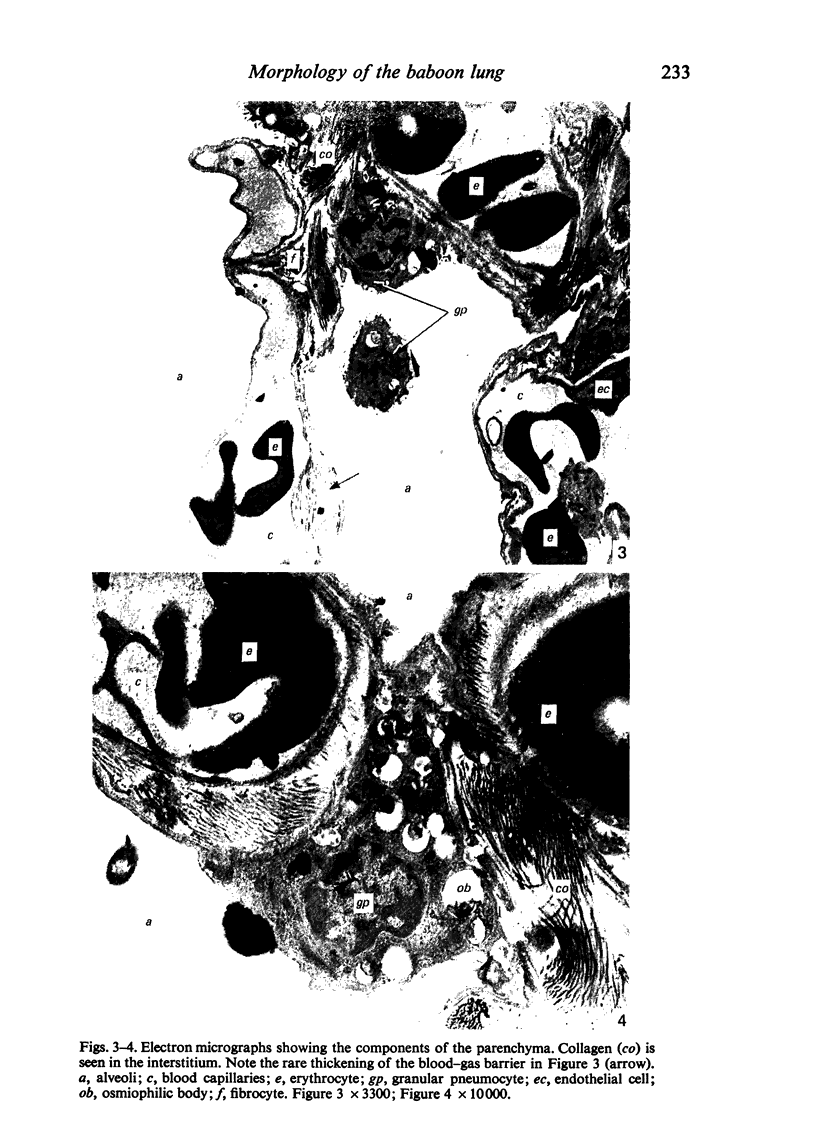
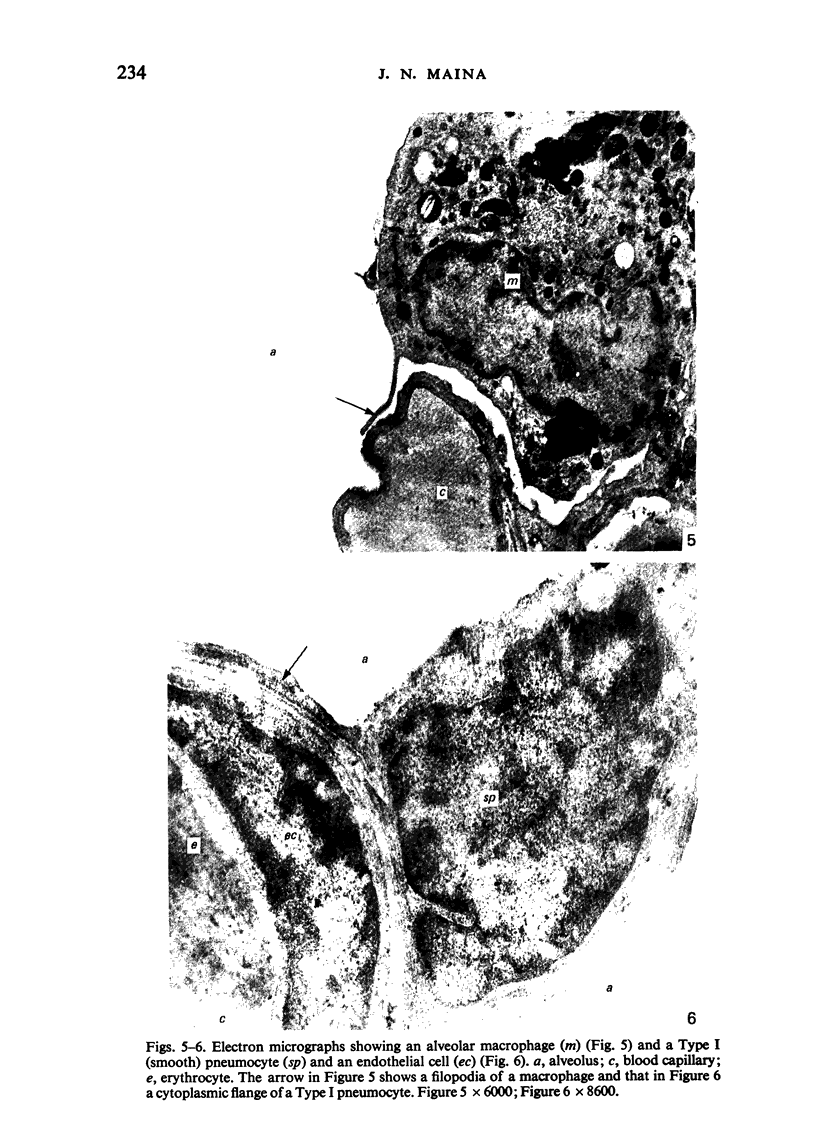
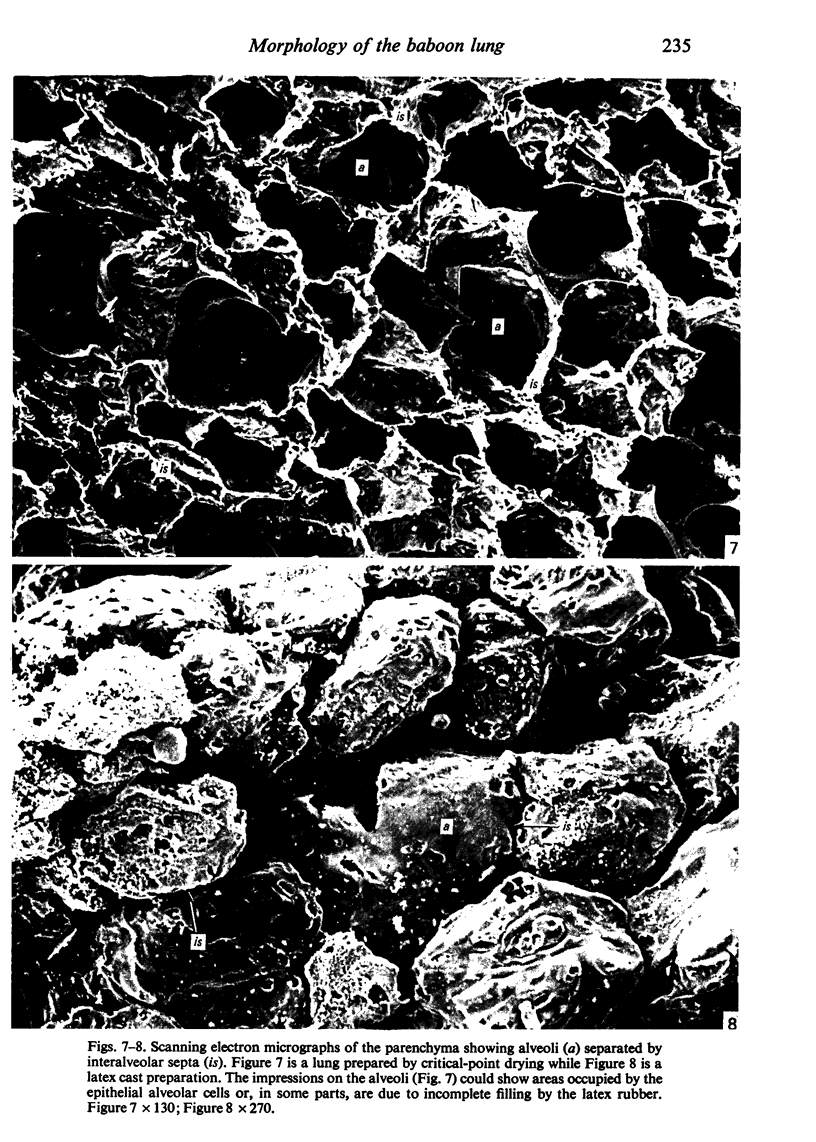
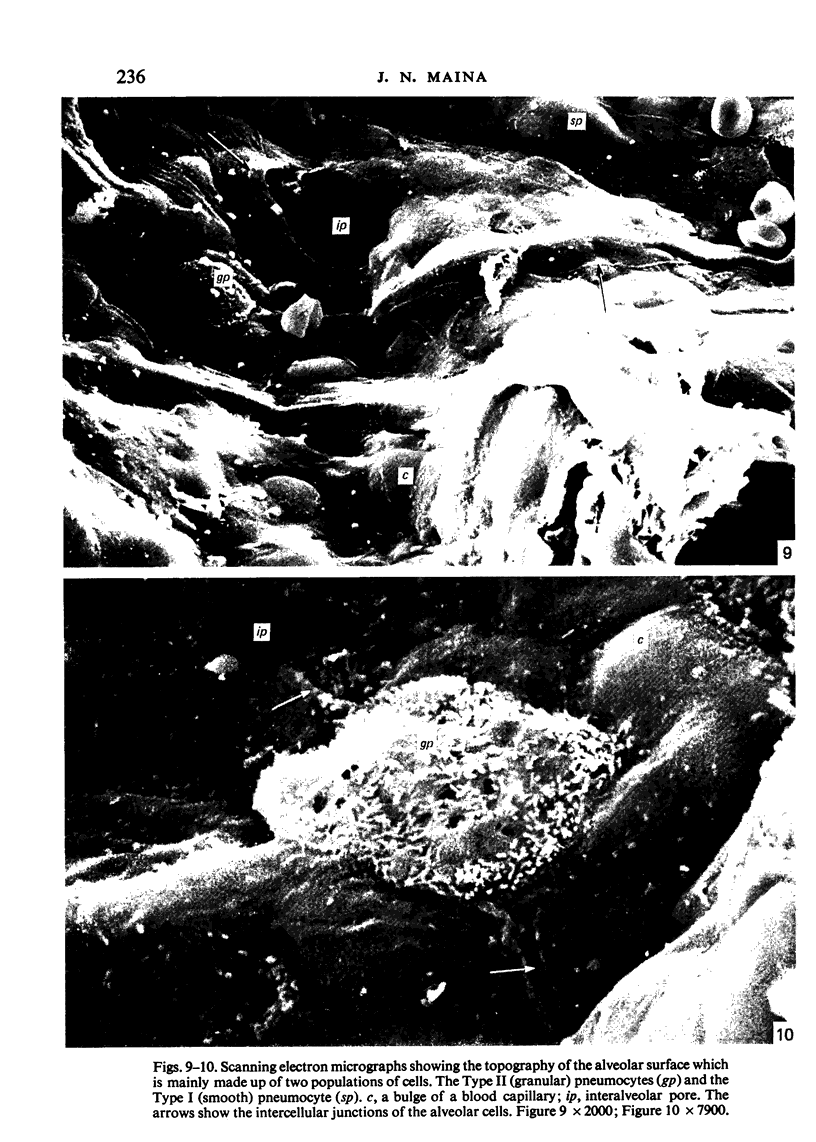





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askin F. B., Kuhn C. The cellular origin of pulmonary surfactant. Lab Invest. 1971 Sep;25(3):260–268. [PubMed] [Google Scholar]
- Breeze R. G., Wheeldon E. B. The cells of the pulmonary airways. Am Rev Respir Dis. 1977 Oct;116(4):705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Brown L. A., Bliss A. S., Longmore W. J. Effect of nutritional status on the lung surfactant system: food deprivation and caloric restriction. Exp Lung Res. 1984;6(2):133–147. doi: 10.3109/01902148409087901. [DOI] [PubMed] [Google Scholar]
- Burri P. H. Morphology and respiratory function of the alveolar unit. Int Arch Allergy Appl Immunol. 1985;76 (Suppl 1):2–12. doi: 10.1159/000233728. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Dungworth D. L., Tyler W. S. Intrapulmonary airway morphology in three species of monkeys: a correlated scanning and transmission electron microscopic study. Am J Anat. 1975 Jan;142(1):107–121. doi: 10.1002/aja.1001420108. [DOI] [PubMed] [Google Scholar]
- Conradi C., Burri P. H., Kapanci Y., Robinson F. R., Weibel E. R. Lung changes after beryllium inhalation: ultrastructural and morphometric study. Arch Environ Health. 1971 Nov;23(5):348–358. doi: 10.1080/00039896.1971.10666020. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Davies G., Reid L. Growth of the alveoli and pulmonary arteries in childhood. Thorax. 1970 Nov;25(6):669–681. doi: 10.1136/thx.25.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman A. P. Pulmonary edema. The water-exchanging function of the lung. Circulation. 1972 Aug;46(2):390–408. doi: 10.1161/01.cir.46.2.390. [DOI] [PubMed] [Google Scholar]
- Gehr P., Bachofen M., Weibel E. R. The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respir Physiol. 1978 Feb;32(2):121–140. doi: 10.1016/0034-5687(78)90104-4. [DOI] [PubMed] [Google Scholar]
- Gehr P., Erni H. Morphometric estimation of pulmonary diffusion capacity in two horse lungs. Respir Physiol. 1980 Aug;41(2):199–210. doi: 10.1016/0034-5687(80)90052-3. [DOI] [PubMed] [Google Scholar]
- Gehr P., Mwangi D. K., Ammann A., Maloiy G. M., Taylor C. R., Weibel E. R. Design of the mammalian respiratory system. V. Scaling morphometric pulmonary diffusing capacity to body mass: wild and domestic mammals. Respir Physiol. 1981 Apr;44(1):61–86. doi: 10.1016/0034-5687(81)90077-3. [DOI] [PubMed] [Google Scholar]
- Gehr P., Sehovic S., Burri P. H., Claassen H., Weibel E. R. The lung of shrews: morphometric estimation of diffusion capacity. Respir Physiol. 1980 Apr;40(1):33–47. doi: 10.1016/0034-5687(80)90003-1. [DOI] [PubMed] [Google Scholar]
- Glazier J. B., Hughes J. M., Maloney J. E., West J. B. Vertical gradient of alveolar size in lungs of dogs frozen intact. J Appl Physiol. 1967 Nov;23(5):694–705. doi: 10.1152/jappl.1967.23.5.694. [DOI] [PubMed] [Google Scholar]
- Grant M. M., Sorokin S. P., Brain J. D. Lysosomal enzyme activities in pulmonary macrophages from rabbits breathing iron oxide. Am Rev Respir Dis. 1979 Nov;120(5):1003–1012. doi: 10.1164/arrd.1979.120.5.1003. [DOI] [PubMed] [Google Scholar]
- Greenwood M. F., Holland P. Scanning electron microscopy of the normal and BCG-stimulated primate respiratory tract. J Reticuloendothel Soc. 1973 Mar;13(3):183–192. [PubMed] [Google Scholar]
- Hislop A., Howard S., Fairweather D. V. Morphometric studies on the structural development of the lung in Macaca fascicularis during fetal and postnatal life. J Anat. 1984 Jan;138(Pt 1):95–112. [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Assimacopoulos A., Irle C., Zwahlen A., Gabbiani G. "Contractile interstitial cells" in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol. 1974 Feb;60(2):375–392. doi: 10.1083/jcb.60.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Tosco R., Eggermann J., Gould V. E. Oxygen pneumonitis in man., Light- and electron-microscopic morphometric studies. Chest. 1972 Aug;62(2):162–169. doi: 10.1378/chest.62.2.162. [DOI] [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Kaplan H. P., Robinson F. R., Kapanci Y., Weibel E. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. I. Clinical and light microscopic studies. Lab Invest. 1969 Jan;20(1):94–100. [PubMed] [Google Scholar]
- Kerr G. R., Couture J., Allen J. R. Growth and development of the fetal Rhesus monkey. VI. Morphometric analysis of the developing lung. Growth. 1975 Mar;39(1):67–84. [PubMed] [Google Scholar]
- Kerr G. R., Helmuth A. C. Growth and development of the fetal Rhesus monkey. V. Fatty acids of phospholipids in fetal lung. Biol Neonate. 1974;25(1-2):10–22. doi: 10.1159/000240674. [DOI] [PubMed] [Google Scholar]
- Lechner A. J. Pulmonary design in a microchiropteran bat (Pipistrellus subflavus) during hibernation. Respir Physiol. 1985 Mar;59(3):301–312. doi: 10.1016/0034-5687(85)90135-5. [DOI] [PubMed] [Google Scholar]
- Maina J. N., King A. S. Correlations between structure and function in the design of the bat lung: a morphometric study. J Exp Biol. 1984 Jul;111:43–61. doi: 10.1242/jeb.111.1.43. [DOI] [PubMed] [Google Scholar]
- Maina J. N. Morphometrics of the avian lung. 3. The structural design of the passerine lung. Respir Physiol. 1984 Mar;55(3):291–307. doi: 10.1016/0034-5687(84)90052-5. [DOI] [PubMed] [Google Scholar]
- Meban C. Thickness of the air-blood barriers in vertebrate lungs. J Anat. 1980 Sep;131(Pt 2):299–307. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. The alveolar brush cell in rat lung--a third pneumonocyte. J Ultrastruct Res. 1968 Apr;23(1):71–80. doi: 10.1016/s0022-5320(68)80032-2. [DOI] [PubMed] [Google Scholar]
- Perry S. F. Quantitative anatomy of the lungs of the red-eared turtle, Pseudemys scripta elegans. Respir Physiol. 1978 Dec;35(3):245–262. doi: 10.1016/0034-5687(78)90001-4. [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Barry B. E., O'Neil J. J., Raub J. A., Pratt P. C., Crapo J. D. Morphologic changes in the lung during the lifespan of Fischer 344 rats. Am J Anat. 1982 Jun;164(2):155–174. doi: 10.1002/aja.1001640206. [DOI] [PubMed] [Google Scholar]
- Schneeberger-Keeley E. E., Karnovsky M. J. The ultrastructural basis of alveolar-capillary membrane permeability to peroxidase used as a tracer. J Cell Biol. 1968 Jun;37(3):781–793. doi: 10.1083/jcb.37.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. P., Brain J. D. Pathways of clearance in mouse lungs exposed to iron oxide aerosols. Anat Rec. 1975 Mar;181(3):581–625. doi: 10.1002/ar.1091810304. [DOI] [PubMed] [Google Scholar]
- Thurlbeck W. M. Postnatal human lung growth. Thorax. 1982 Aug;37(8):564–571. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler N. K., Plopper C. G. Morphology of the distal conducting airways in rhesus monkey lungs. Anat Rec. 1985 Mar;211(3):295–303. doi: 10.1002/ar.1092110310. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R., KNIGHT B. W. A MORPHOMETRIC STUDY ON THE THICKNESS OF THE PULMONARY AIR-BLOOD BARRIER. J Cell Biol. 1964 Jun;21:367–396. doi: 10.1083/jcb.21.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N. S., Thurlbeck W. M. Scanning electron microscopy of the lung. Hum Pathol. 1970 Jun;1(2):227–231. doi: 10.1016/s0046-8177(70)80036-3. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Morphological basis of alveolar-capillary gas exchange. Physiol Rev. 1973 Apr;53(2):419–495. doi: 10.1152/physrev.1973.53.2.419. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Morphometric estimation of pulmonary diffusion capacity. I. Model and method. Respir Physiol. 1970;11(1):54–75. doi: 10.1016/0034-5687(70)90102-7. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. Morphometric estimation of pulmonary diffusion capacity. V. Comparative morphometry of alveolar lungs. Respir Physiol. 1972 Mar;14(1):26–43. doi: 10.1016/0034-5687(72)90015-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Plopper C. G., Hyde D. M. The tracheobronchial epithelium of the bonnet monkey (Macaca radiata): a quantitative ultrastructural study. Am J Anat. 1984 Sep;171(1):25–40. doi: 10.1002/aja.1001710104. [DOI] [PubMed] [Google Scholar]
- Winkler G. C., Cheville N. F. Morphometry of postnatal development in the porcine lung. Anat Rec. 1985 Apr;211(4):427–433. doi: 10.1002/ar.1092110409. [DOI] [PubMed] [Google Scholar]