Abstract
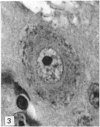
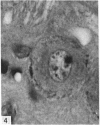
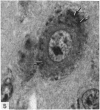
The number of neurons and neuronal nuclear diameter was estimated in the mesencephalic and motor nuclei of the mouse trigeminal nerve at 6, 9, 12, 15, 22, 25, 28 and 31 months of age. Analyses of variance showed that neuronal number decreased significantly (P less than 0.01) in the mesencephalic nucleus between 25 and 31 months, but, although lowest at 31 months of age in the motor nucleus, the decrease in neuronal number in the motor nucleus was not statistically significant. Neuronal nuclear diameter increased significantly in both nuclei between 25 and 31 months, being obvious first in the mesencephalic nucleus. The main histological features of ageing were a marked accumulation of lipofuscin granules in neurons of the mesencephalic nucleus and a loss of Nissl substance from the motor neurons of the trigeminal motor nucleus.
Full text
PDF


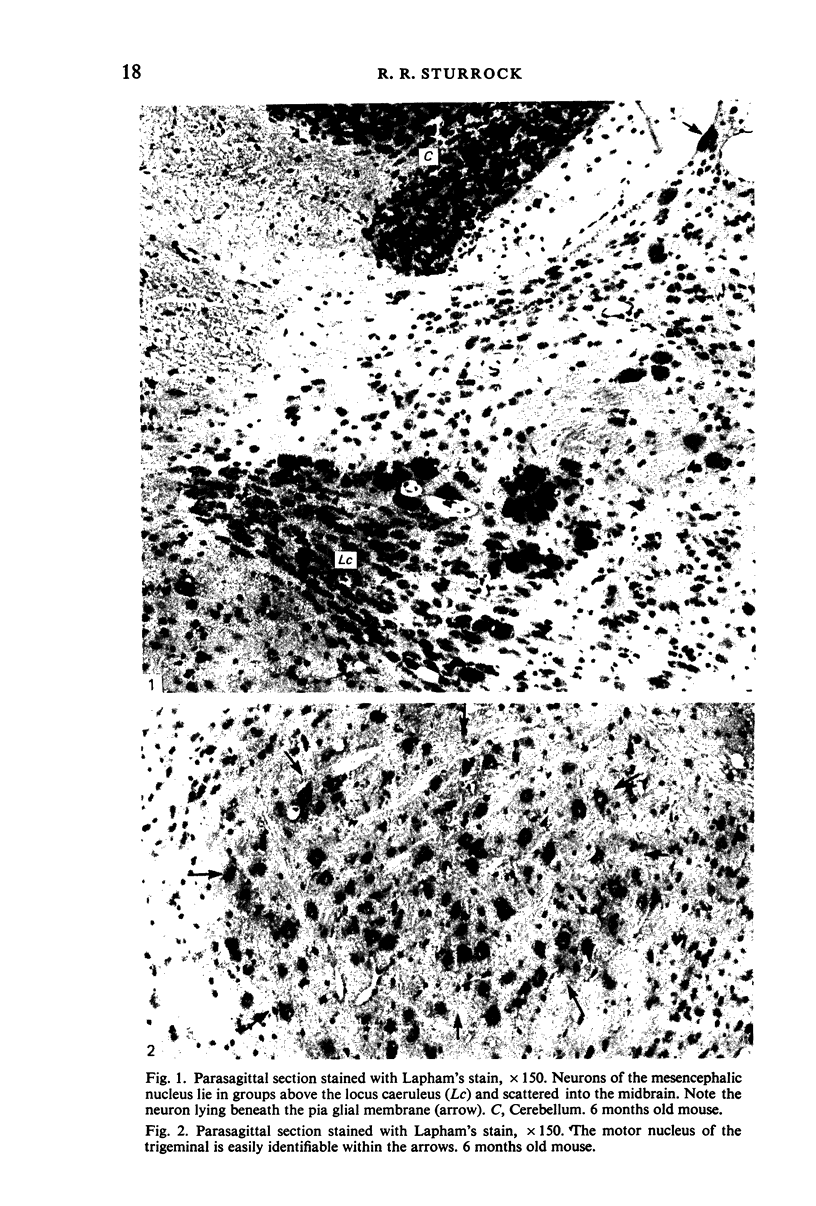
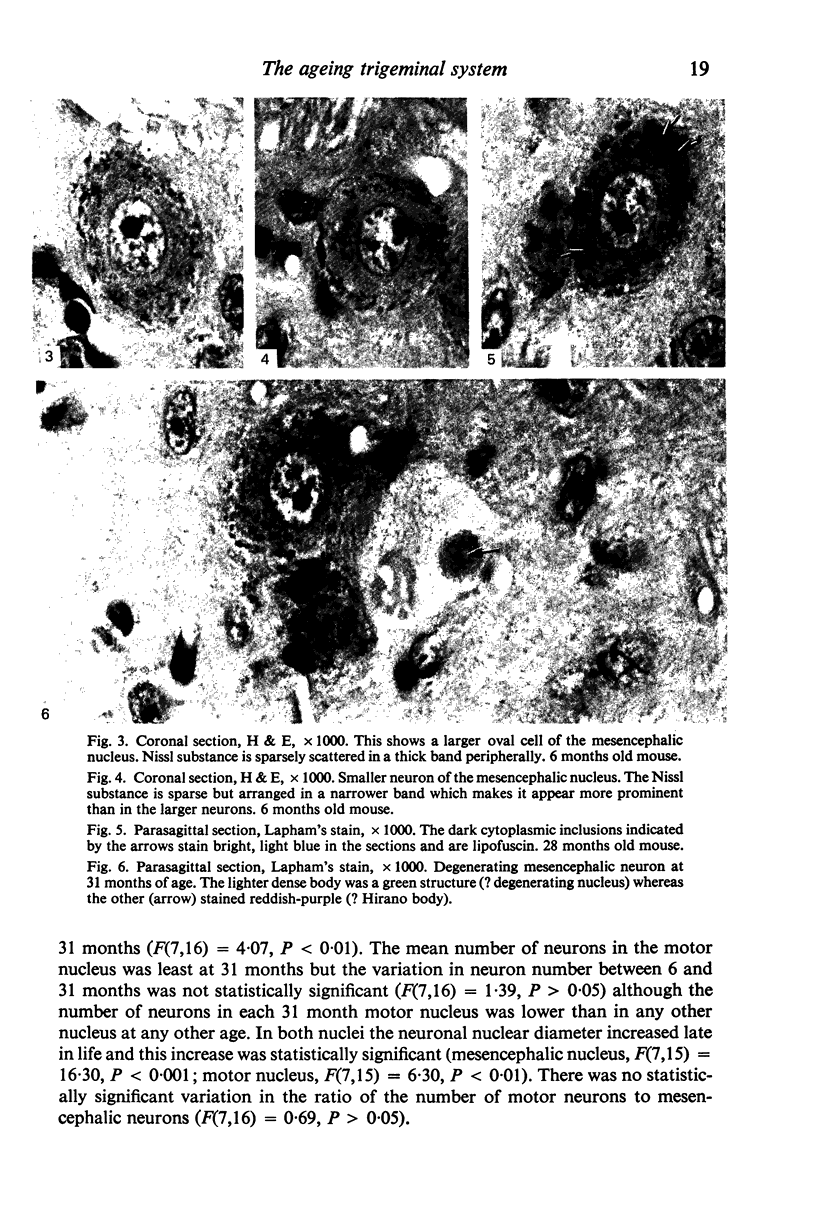
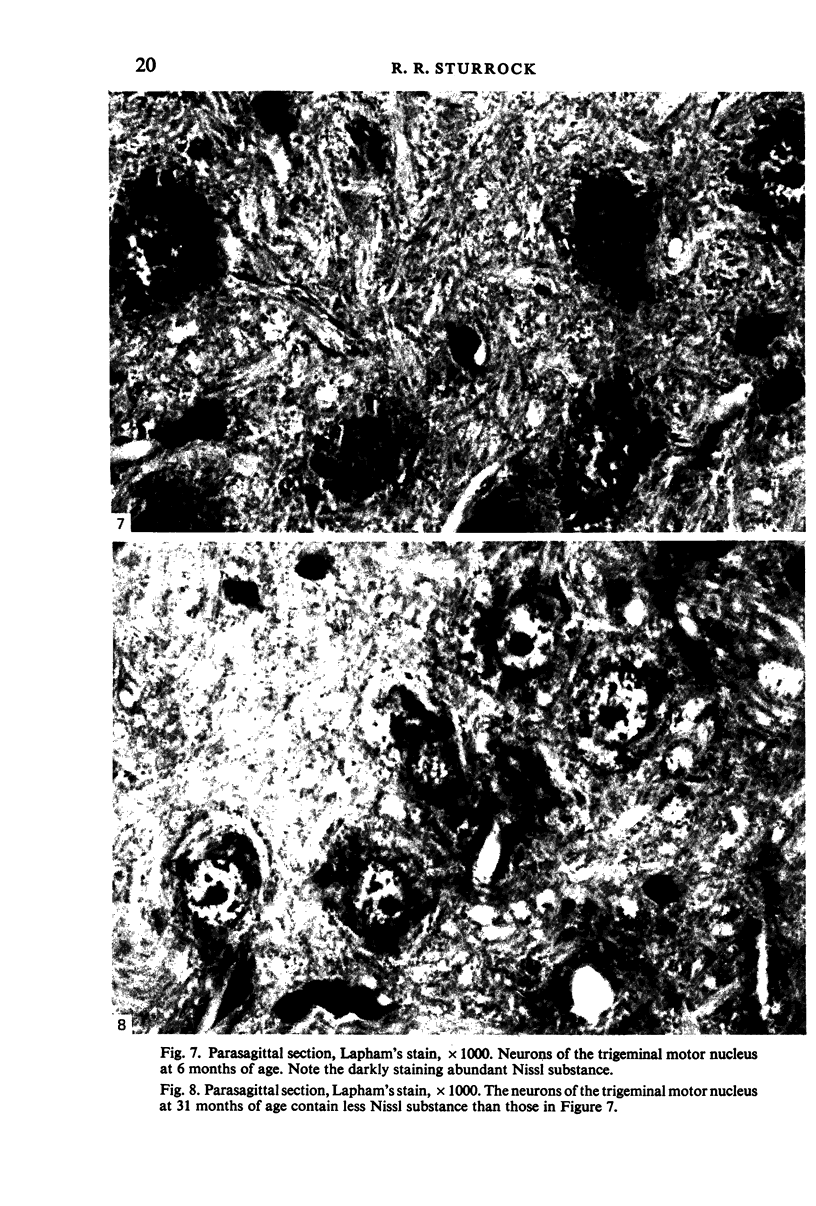
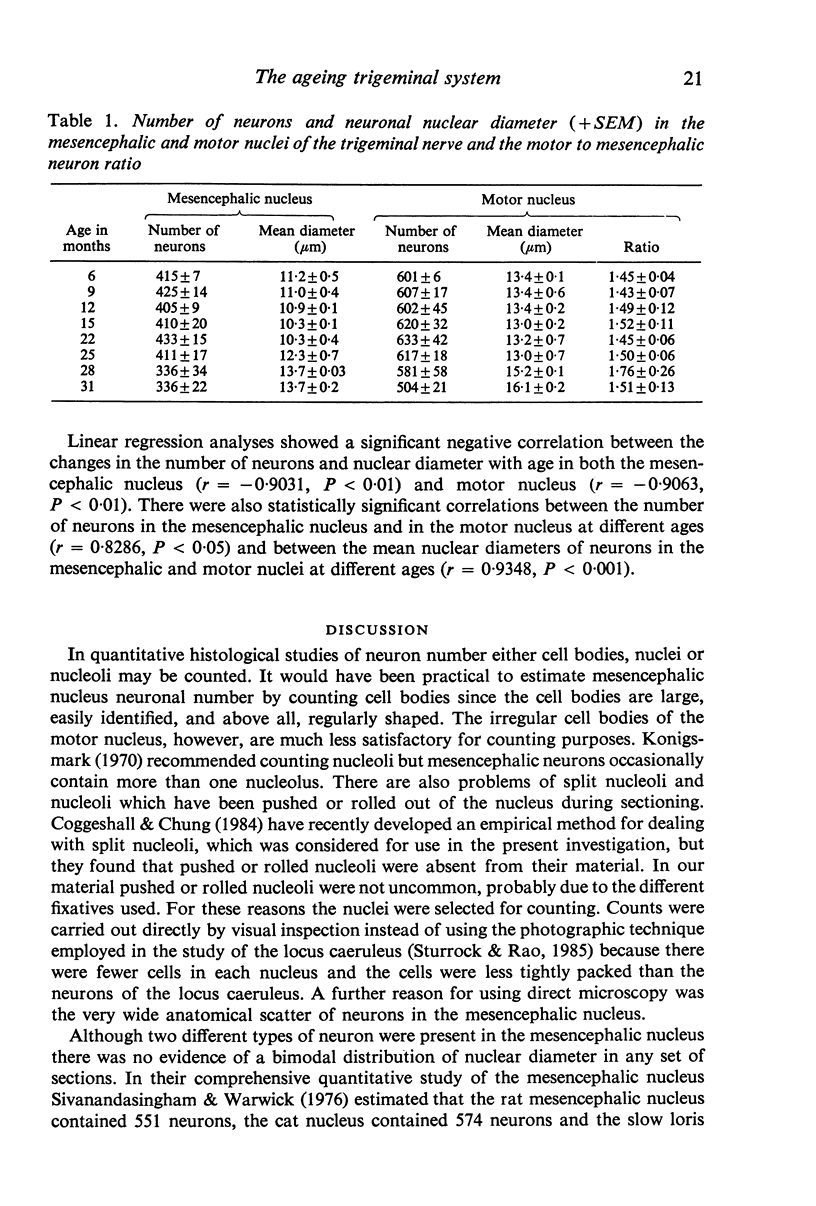




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger B., Hervé D., Dolphin A., Barthelemy C., Gay M., Tassin J. P. Genetically determined differences in noradrenergic input to the brain cortex: a histochemical and biochemical study in two inbred strains of mice. Neuroscience. 1979;4(7):877–888. doi: 10.1016/0306-4522(79)90172-6. [DOI] [PubMed] [Google Scholar]
- Brizzee K. R., Ordy J. M., Kaack B. Early appearance and regional differences in intraneuronal and extraneuronal lipofuscin accumulation with age in the brain of a nonhuman primate (Macaca mulatta). J Gerontol. 1974 Jul;29(4):366–381. doi: 10.1093/geronj/29.4.366. [DOI] [PubMed] [Google Scholar]
- CAMMERMEYER J. Cytological manifestations of aging in rabbit and chinchilla brains. J Gerontol. 1963 Jan;18:41–54. doi: 10.1093/geronj/18.1.41. [DOI] [PubMed] [Google Scholar]
- Coggeshall R. E., Chung K. The determination of an empirical correction factor to deal with the problem of nucleolar splitting in neuronal counts. J Neurosci Methods. 1984 Feb;10(2):149–155. doi: 10.1016/0165-0270(84)90069-4. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., Coleman P. D. Stability of neuron number in cortical barrels of aging mice. J Comp Neurol. 1982 Dec 1;212(2):158–172. doi: 10.1002/cne.902120206. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., McNelly N. A., Hinds J. W. Aging in the rat olfactory system: relative stability of piriform cortex contrasts with changes in olfactory bulb and olfactory epithelium. J Comp Neurol. 1985 May 22;235(4):519–528. doi: 10.1002/cne.902350409. [DOI] [PubMed] [Google Scholar]
- Dacey D. M. Axon morphology of mesencephalic trigeminal neurons in a snake, Thamnophis sirtalis. J Comp Neurol. 1982 Jan 20;204(3):268–279. doi: 10.1002/cne.902040306. [DOI] [PubMed] [Google Scholar]
- Few A., Getty R. Occurrence of lipofuscin as related to aging in the canine and porcine nervous system. J Gerontol. 1967 Jul;22(3):357–368. doi: 10.1093/geronj/22.3.357. [DOI] [PubMed] [Google Scholar]
- Glees P., Hasan M. Lipofuscin in neuronal aging and diseases. Norm Pathol Anat (Stuttg) 1976;32:1–68. [PubMed] [Google Scholar]
- Hinds J. W., McNelly N. A. Aging in the rat olfactory bulb: quantitative changes in mitral cell organelles and somato-dendritic synapses. J Comp Neurol. 1979 Apr 15;184(4):811–820. doi: 10.1002/cne.901840412. [DOI] [PubMed] [Google Scholar]
- Hinds J. W., McNelly N. A. Aging of the rat olfactory bulb: growth and atrophy of constituent layers and changes in size and number of mitral cells. J Comp Neurol. 1977 Feb 1;72(3):345–367. doi: 10.1002/cne.901710304. [DOI] [PubMed] [Google Scholar]
- Hinrichsen C. F., Larramendi L. M. Features of trigeminal mesencephalic nucleus structure and organization. I. Light microscopy. Am J Anat. 1969 Dec;126(4):497–505. doi: 10.1002/aja.1001260408. [DOI] [PubMed] [Google Scholar]
- Keithley E. M., Feldman M. L. Spiral ganglion cell counts in an age-graded series of rat cochleas. J Comp Neurol. 1979 Dec 1;188(3):429–442. doi: 10.1002/cne.901880306. [DOI] [PubMed] [Google Scholar]
- LAPHAM L. W., JOHNSTONE M. A., BRUNDJAR K. H. A NEW PARAFFIN METHOD FOR THE COMBINED STAINING OF MYELIN AND GLIAL FIBERS. J Neuropathol Exp Neurol. 1964 Jan;23:156–160. [PubMed] [Google Scholar]
- Machado-Salas J., Scheibel M. E., Scheibel A. B. Neuronal changes in the aging mouse: spinal cord and lower brain stem. Exp Neurol. 1977 Mar;54(3):504–512. doi: 10.1016/0014-4886(77)90253-9. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Neary D., Yates P. O., Lincoln J., Snowden J. S., Stanworth P. Alterations in protein synthetic capability of nerve cells in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1981 Feb;44(2):97–102. doi: 10.1136/jnnp.44.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O. Lipoprotein pigments--their relationship to ageing in the human nervous system. I. The lipofuscin content of nerve cells. Brain. 1974 Sep;97(3):481–488. doi: 10.1093/brain/97.1.481. [DOI] [PubMed] [Google Scholar]
- Peters A., Feldman M. L., Vaughan D. W. The effect of aging on the neuronal population within area 17 of adult rat cerebral cortex. Neurobiol Aging. 1983 Winter;4(4):273–282. doi: 10.1016/0197-4580(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Sivanandasingham P., Warwick R. A comparative numerical study of the trigeminal mesencephalic nucleus. Acta Anat (Basel) 1976;96(3):449–458. doi: 10.1159/000144693. [DOI] [PubMed] [Google Scholar]
- Smolen A. J., Wright L. L., Cunningham T. J. Neuron numbers in the superior cervical sympathetic ganglion of the rat: a critical comparison of methods for cell counting. J Neurocytol. 1983 Oct;12(5):739–750. doi: 10.1007/BF01258148. [DOI] [PubMed] [Google Scholar]
- Strong R., Hicks P., Hsu L., Bartus R. T., Enna S. J. Age-related alterations in the rodent brain cholinergic system and behavior. Neurobiol Aging. 1980 Summer;1(1):59–63. doi: 10.1016/0197-4580(80)90025-1. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. Age related changes in cellularity, mitotic activity and pyknotic cell number in the mouse subependymal layer. J Anat. 1985 Aug;141:19–26. [PMC free article] [PubMed] [Google Scholar]
- Sturrock R. R., Rao K. A. A quantitative histological study of neuronal loss from the locus coeruleus of ageing mice. Neuropathol Appl Neurobiol. 1985 Jan-Feb;11(1):55–60. doi: 10.1111/j.1365-2990.1985.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Touret M., Valatx J. L., Jouvet M. The locus coeruleus: a quantitative and genetic study in mice. Brain Res. 1982 Nov 4;250(2):353–357. doi: 10.1016/0006-8993(82)90430-9. [DOI] [PubMed] [Google Scholar]
- WRIGHT E. A., SPINK J. M. A study of the loss of nerve cells in the central nervous system in relation to age. Gerontologia. 1959;3:277–287. doi: 10.1159/000210907. [DOI] [PubMed] [Google Scholar]