Abstract
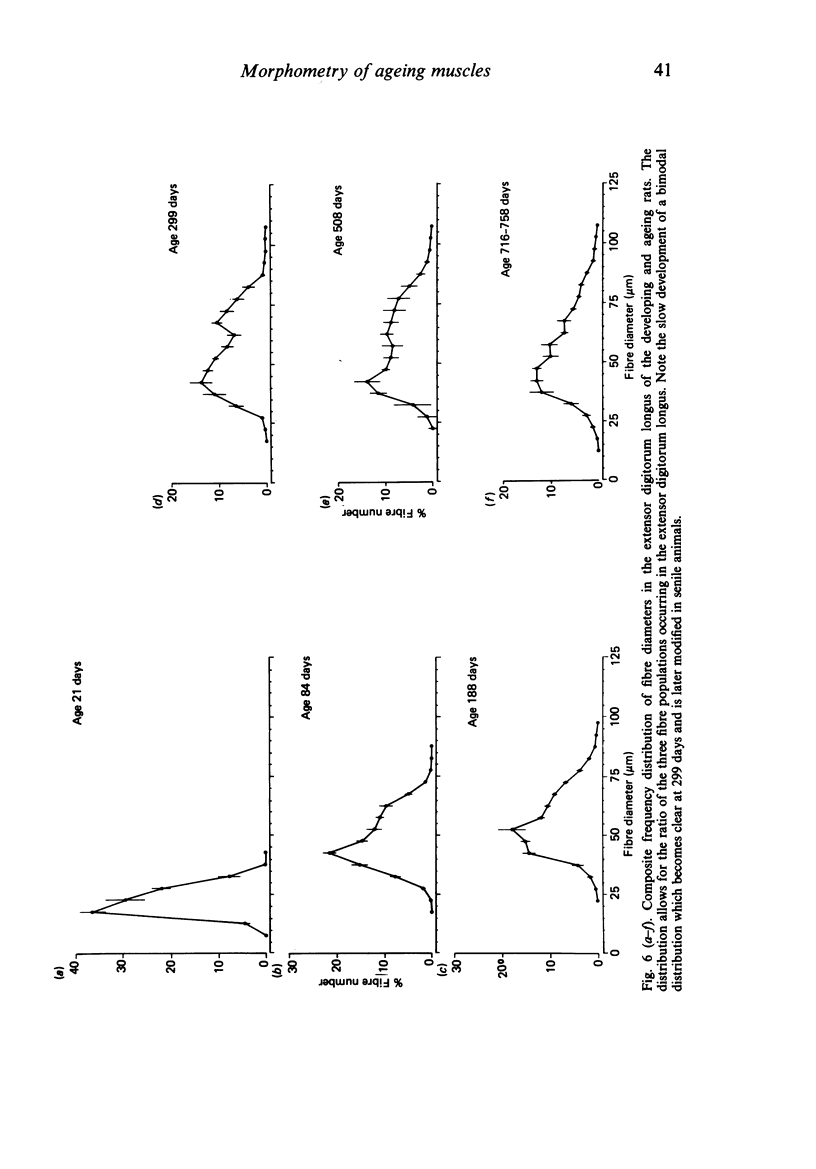
Morphometrical and histochemical features of muscle undergo continuous changes with age. Two representative muscles were studied to determine the nature of these changes, the extensor digitorum longus and the soleus. Muscle fibre type ratios were found to change with age so that there were more oxidative types. In the extensor digitorum longus the cross sectional area occupied by fast oxidative glycolytic fibres increased, while in the soleus fast oxidative glycolytic fibres apparently underwent conversion into slow oxidative fibres. Muscle fibre diameter increased dramatically during early growth but later in senile animals there was evidence of both atrophy and splitting. In the extensor digitorum longus the uneven growth of the two dominant fibre types gave rise to a bimodal fibre diameter distribution. The soleus, which is composed of predominantly one fibre type, did not show bimodality. Senile muscles had a characteristic wide distribution of fibre diameters with ill defined peaks. Total fibre number in the extensor digitorum longus decreased in early life while total fibre number in the soleus remained unchanged.
Full text
PDF


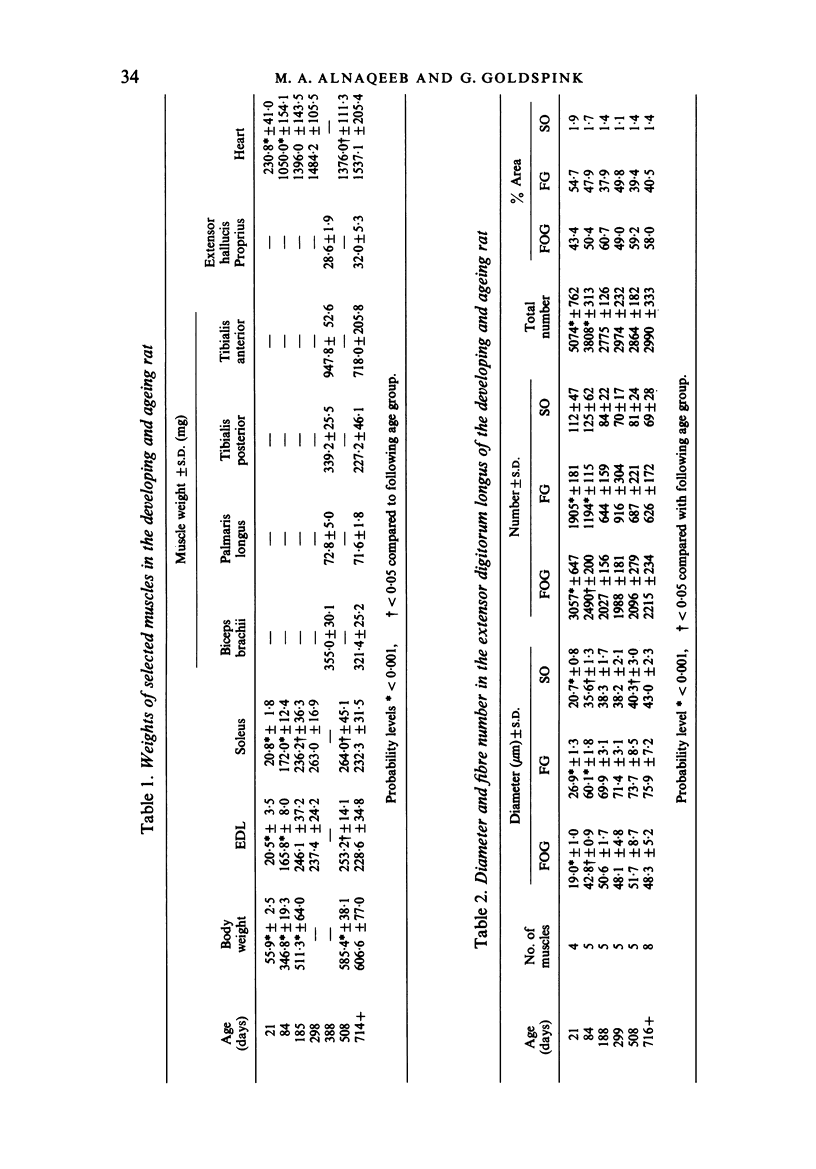
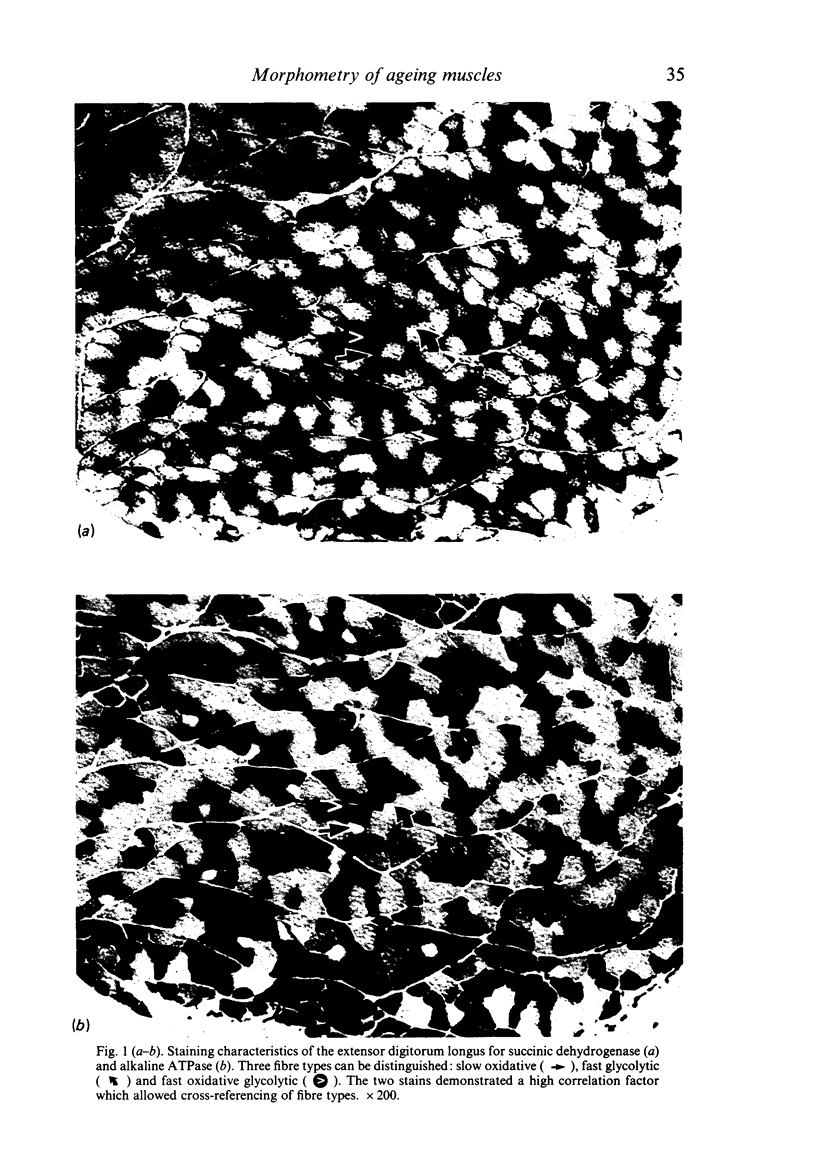
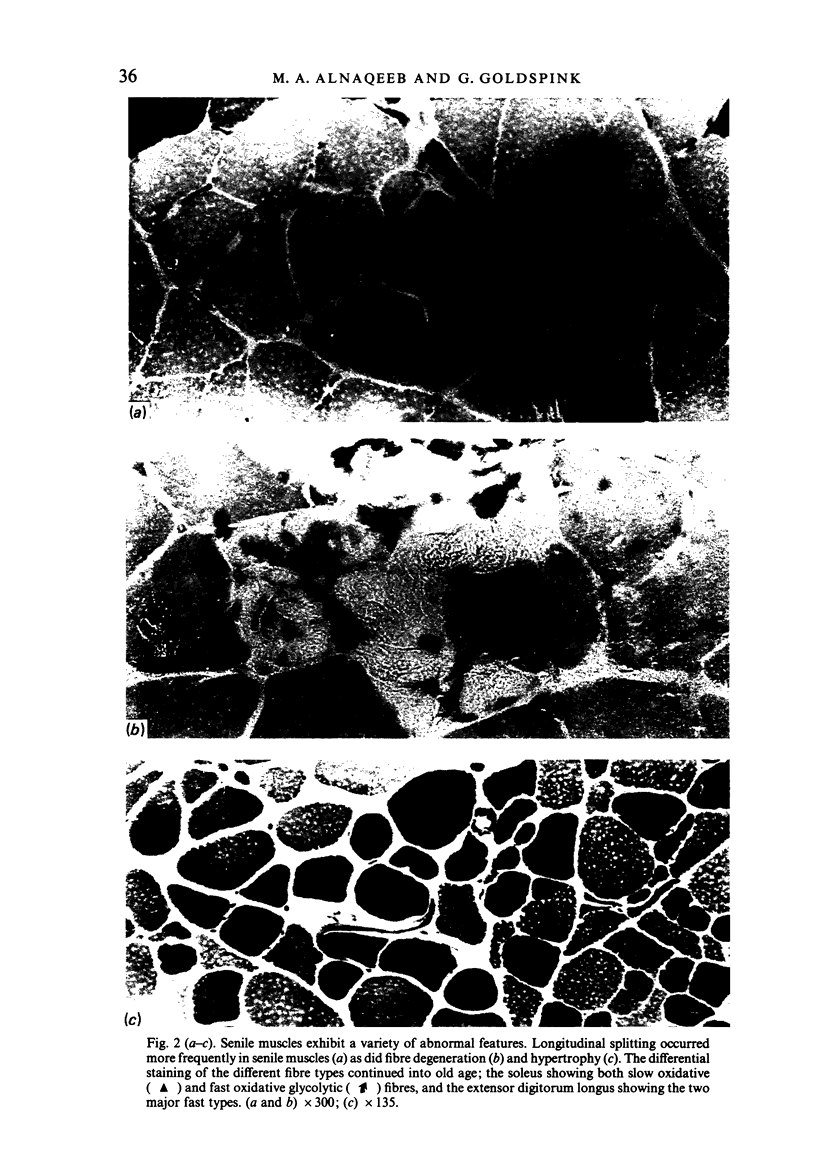
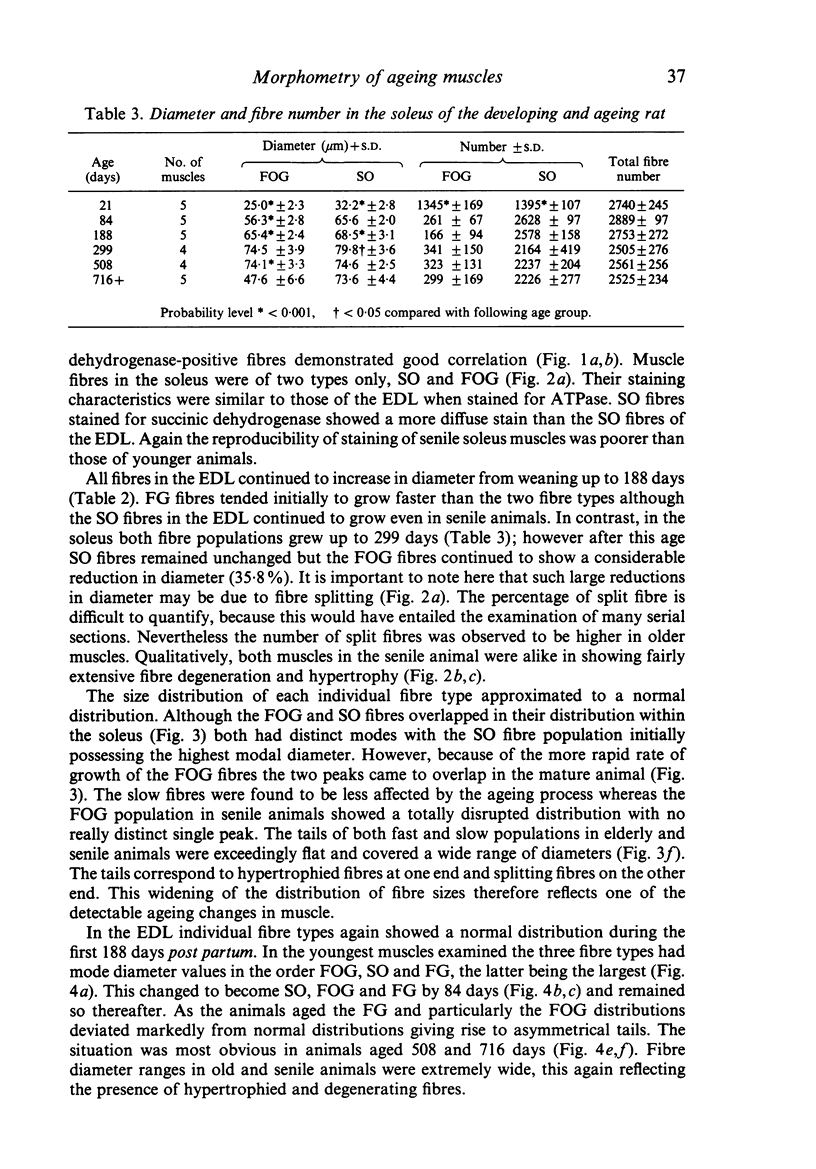
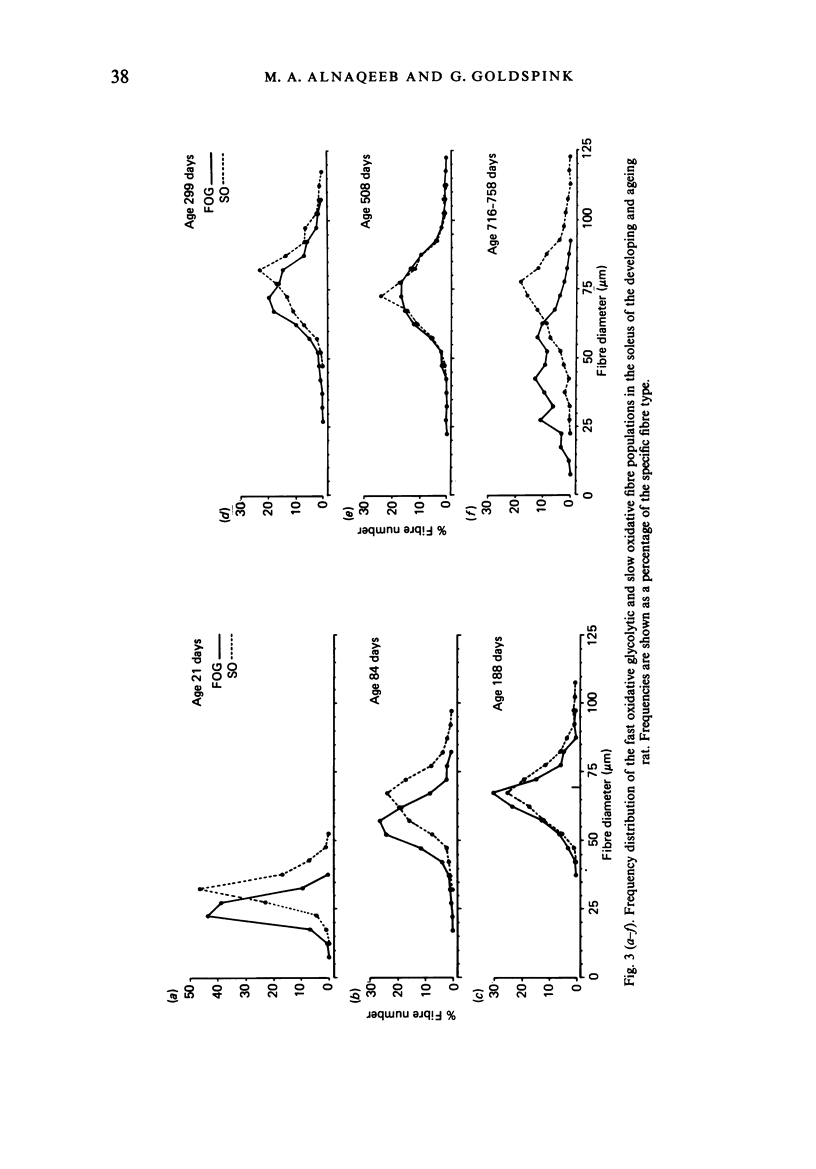
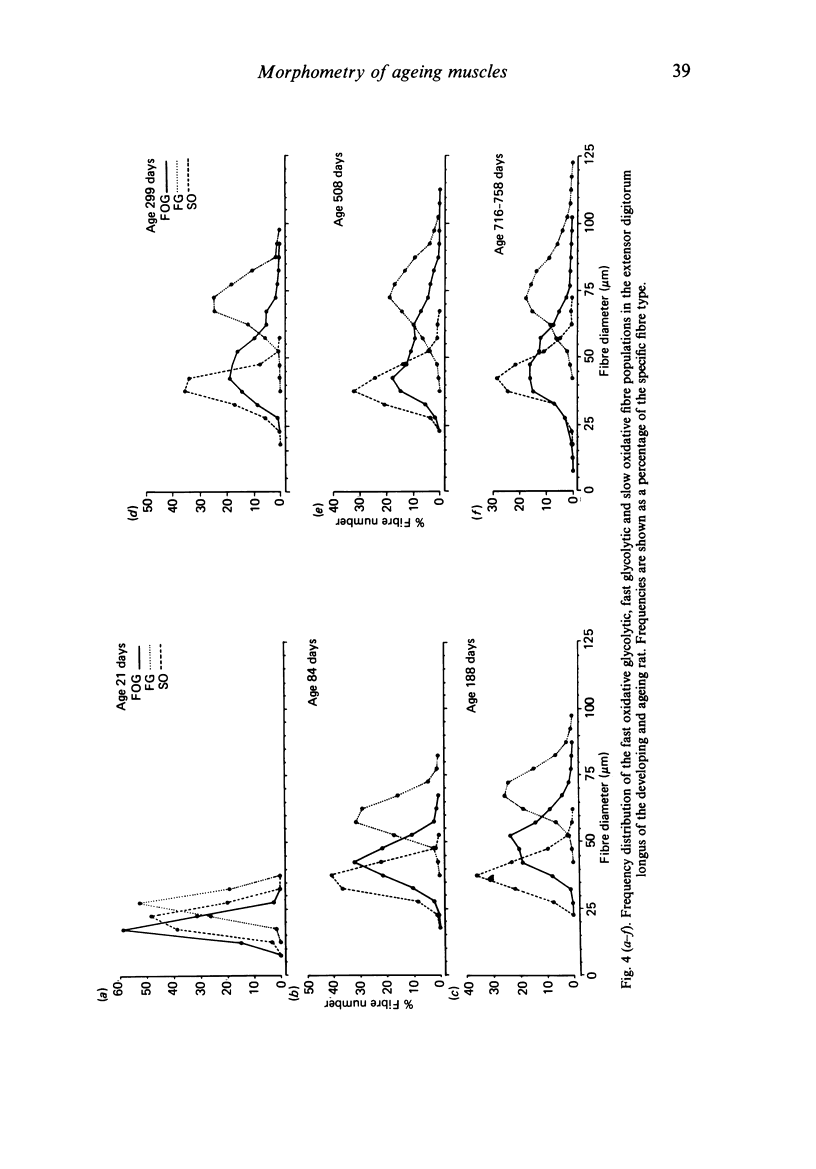
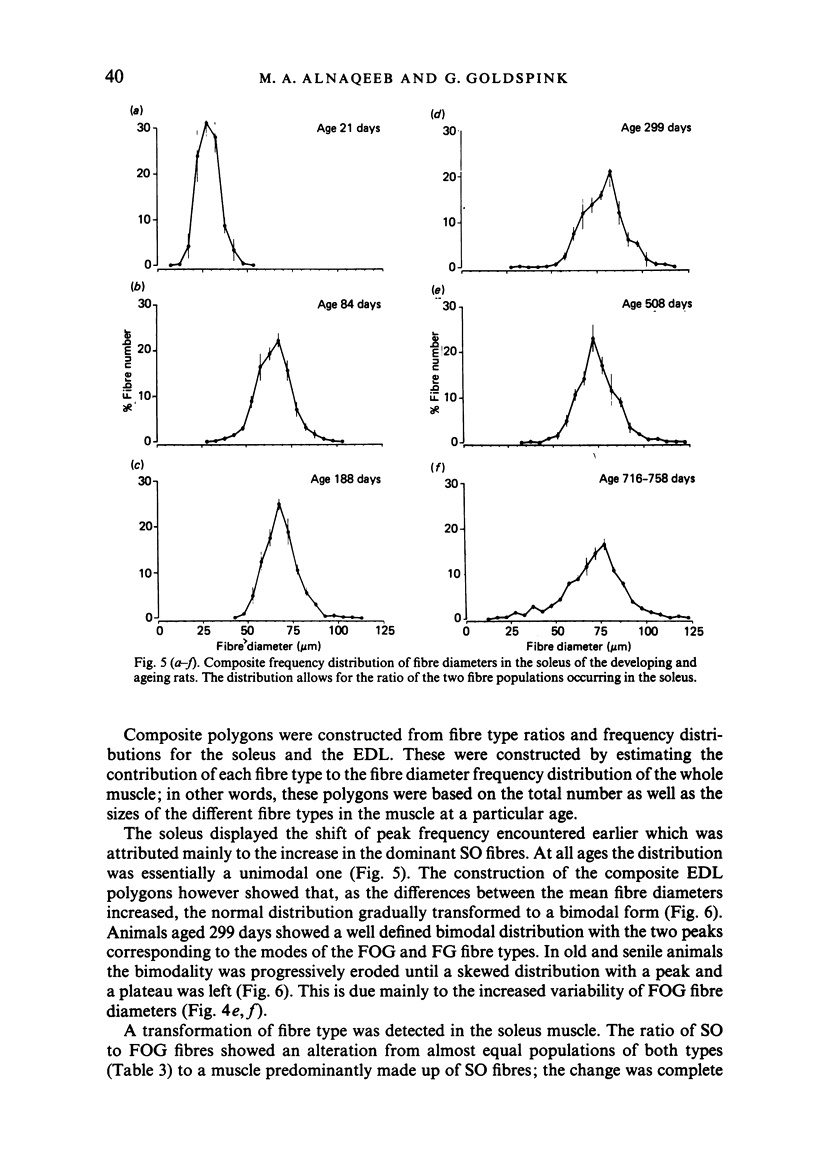




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaqeeb M. A., Al Zaid N. S., Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat. 1984 Dec;139(Pt 4):677–689. [PMC free article] [PubMed] [Google Scholar]
- Ashmore C. R., Addis P. B., Doerr L. Development of muscle fibers in the fetal pig. J Anim Sci. 1973 Jun;36(6):1088–1093. doi: 10.2527/jas1973.3661088x. [DOI] [PubMed] [Google Scholar]
- Ashmore C. R., Robinson D. W., Rattray P., Doerr L. Biphasic development of muscle fibers in the fetal lamb. Exp Neurol. 1972 Nov;37(2):241–255. doi: 10.1016/0014-4886(72)90071-4. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Williamson E., Kaiser K. K. The behavior of four fiber types in developing and reinnervated muscle. Arch Neurol. 1971 Oct;25(4):360–366. doi: 10.1001/archneur.1971.00490040086010. [DOI] [PubMed] [Google Scholar]
- CHIAKULAS J. J., PAULY J. E. A STUDY OF POSTNATAL GROWTH OF SKELETAL MUSCLE IN THE RAT. Anat Rec. 1965 May;152:55–61. doi: 10.1002/ar.1091520107. [DOI] [PubMed] [Google Scholar]
- CRITCHLEY M. Neurological changes in the aged. Res Publ Assoc Res Nerv Ment Dis. 1956;35:198–223. [PubMed] [Google Scholar]
- Caccia M. R., Harris J. B., Johnson M. A. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve. 1979 May-Jun;2(3):202–212. doi: 10.1002/mus.880020308. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Cross-innervated mammalian skeletal muscle: histochemical, physiological and biochemical observations. J Physiol. 1967 Dec;193(3):481–496.3. doi: 10.1113/jphysiol.1967.sp008373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg B. R., Mobley B. A. Size changes in single muscle fibers during fixation and embedding. Tissue Cell. 1975;7(2):383–387. doi: 10.1016/0040-8166(75)90013-0. [DOI] [PubMed] [Google Scholar]
- GOLDSPINK G. Fixation of muscle. Nature. 1961 Dec 30;192:1305–1306. doi: 10.1038/1921305a0. [DOI] [PubMed] [Google Scholar]
- Goldspink G., Gelder S., Clapison L., Overfield P. Pre- and post-rigor fixation of muscle. J Anat. 1973 Jan;114(Pt 1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Goldspink G. The proliferation of myofibrils during muscle fibre growth. J Cell Sci. 1970 Mar;6(2):593–603. doi: 10.1242/jcs.6.2.593. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Hanzlíková V. Fast and slow motor units in ageing. Gerontology. 1976;22(4):280–300. doi: 10.1159/000212144. [DOI] [PubMed] [Google Scholar]
- Hegarty P. V., Hooper A. C. Sarcomere length and fibre diameter distributions in four different mouse skeletal muscles. J Anat. 1971 Nov;110(Pt 2):249–257. [PMC free article] [PubMed] [Google Scholar]
- Ihemelandu E. C. Decrease in fibre numbers of dog pectineus muscle with age. J Anat. 1980 Jan;130(Pt 1):69–73. [PMC free article] [PubMed] [Google Scholar]
- Larsson L., Sjödin B., Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22--65 years. Acta Physiol Scand. 1978 May;103(1):31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Layman D. K., Hegarty P. V., Swan P. B. Comparison of morphological and biochemical parameters of growth in rat skeletal muscles. J Anat. 1980 Jan;130(Pt 1):159–171. [PMC free article] [PubMed] [Google Scholar]
- Lieberman D. A., Maxwell L. C., Faulkner J. A. Adaptation of guinea pig diaphragm muscle to aging and endurance training. Am J Physiol. 1972 Mar;222(3):556–560. doi: 10.1152/ajplegacy.1972.222.3.556. [DOI] [PubMed] [Google Scholar]
- Moore M. J., Rebeiz J. J., Holden M., Adams R. D. Biometric analyses of normal skeletal muscle. Acta Neuropathol. 1971;19(1):51–69. doi: 10.1007/BF00690954. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Ontell M., Dunn R. F. Neonatal muscle growth: a quantitative study. Am J Anat. 1978 Aug;152(4):539–555. doi: 10.1002/aja.1001520408. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pullen A. H. The distribution and relative sized of fibre types in the extensor digitorum longus and soleus muscles of the adult rat. J Anat. 1977 Apr;123(Pt 2):467–486. [PMC free article] [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Muscle fibre growth in five different muscles in both sexes of mice. J Anat. 1969 May;104(Pt 3):519–530. [PMC free article] [PubMed] [Google Scholar]
- SONG S. K., SHIMADA N., ANDERSON P. J. ORTHOGONAL DIAMETERS IN THE ANALYSIS OF MUSCLE FIBRE SIZE AND FORM. Nature. 1963 Dec 21;200:1220–1221. doi: 10.1038/2001220a0. [DOI] [PubMed] [Google Scholar]
- Schmitt H. P. Measurement of voluntary muscle fiber cross sections: a comparative study of different possible methods. Microsc Acta. 1976 Jan;77(5):427–440. [PubMed] [Google Scholar]
- Tauchi H., Yoshioka T., Kobayashi H. Age change of skeletal muscles of rats. Gerontologia. 1971;17(4):219–227. doi: 10.1159/000211826. [DOI] [PubMed] [Google Scholar]
- Tunell G. L., Hart M. N. Simultaneous determination of skeletal muscle fiber, types I, IIA, and IIB by histochemistry. Arch Neurol. 1977 Mar;34(3):171–173. doi: 10.1001/archneur.1977.00500150057011. [DOI] [PubMed] [Google Scholar]
- Watt P. W., Goldspink G., Ward P. S. Changes in fiber type composition in growing muscle as a result of dynamic exercise and static overload. Muscle Nerve. 1984 Jan;7(1):50–53. doi: 10.1002/mus.880070108. [DOI] [PubMed] [Google Scholar]