Abstract
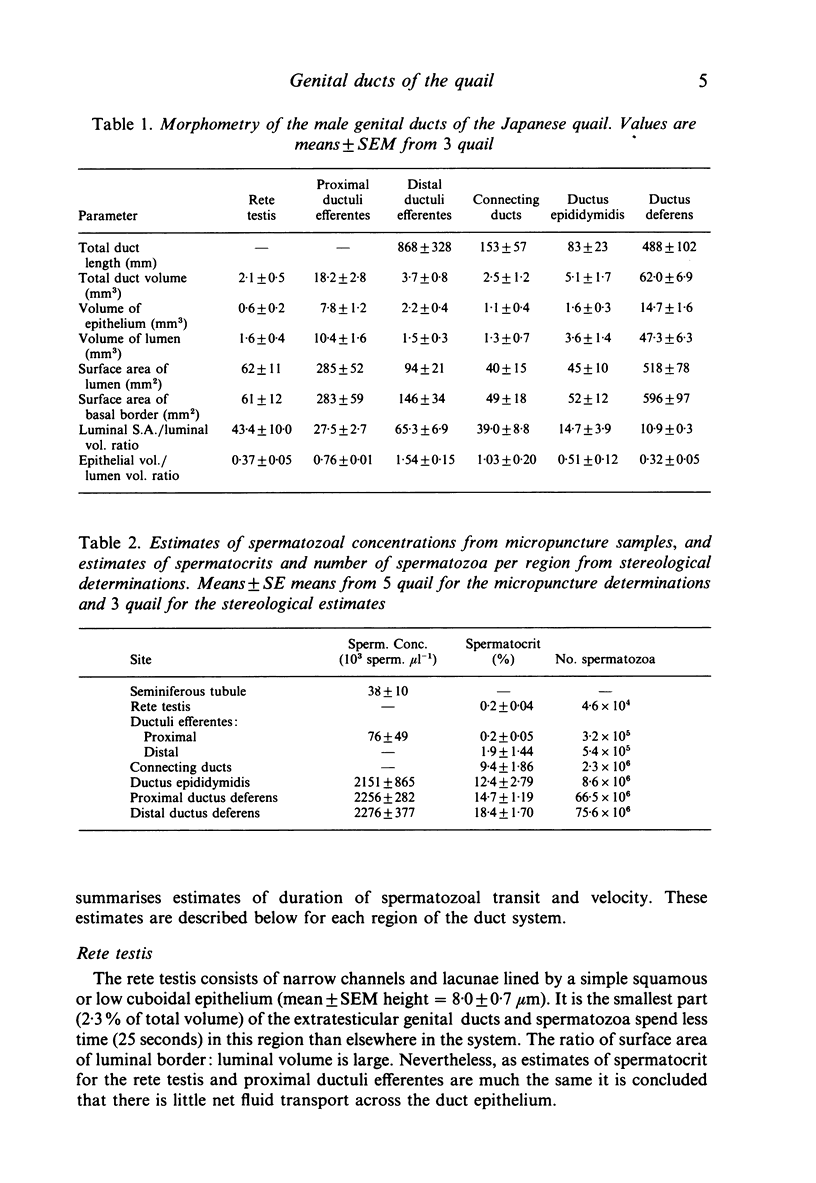
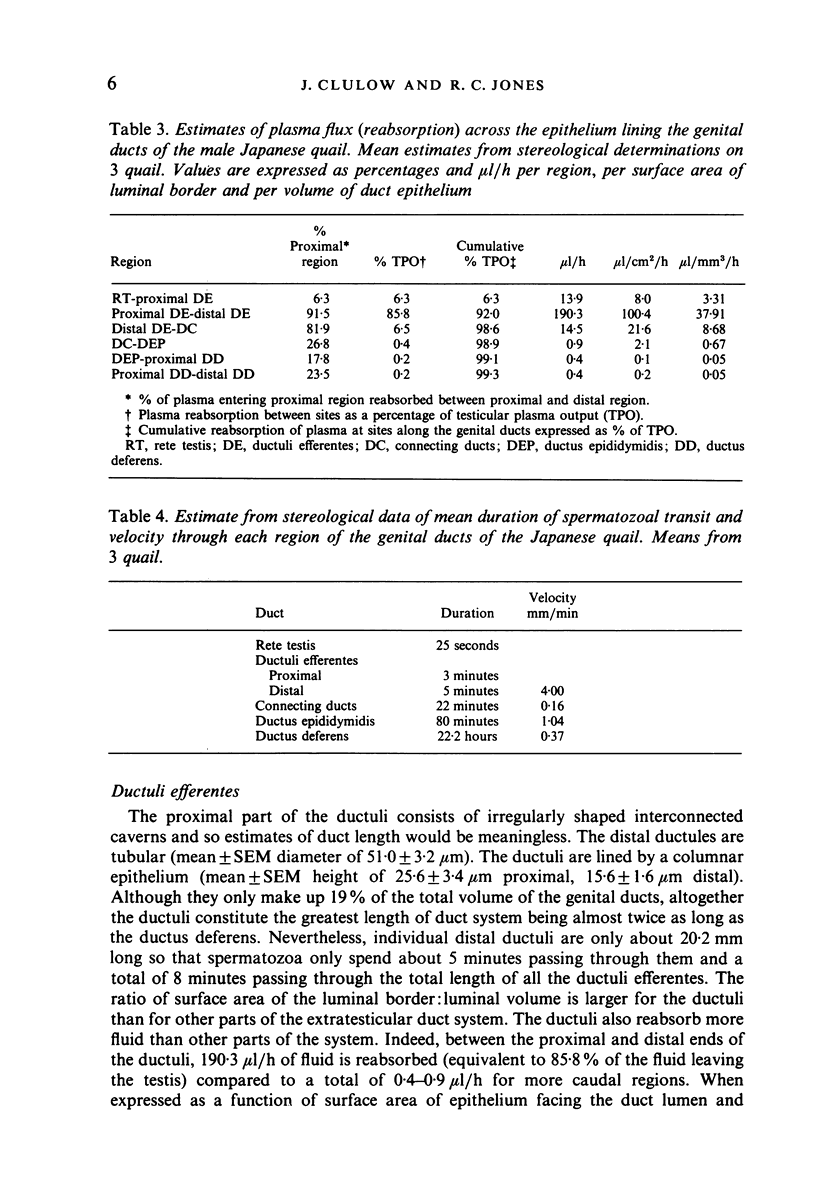
Stereological studies of the spermatic ducts of the quail were carried out for comparison between different parts of the system and those of other species, and to provide a basis for future physiological studies. Duct length, surface areas and volumes of various components of the ducts were determined. Values were subsequently used to calculate net fluxes of fluid across the duct epithelium, spermatozoal velocity and the distribution of spermatozoa throughout the system. It was concluded that the extratesticular spermatic ducts are divided into 2 main parts: (1) the ductuli efferentes where spermatozoa spend a brief period (8 minutes) and which are adapted for considerable net fluid reabsorption (100 microliters/cm2/h), and (2) the connecting ducts, ductus epididymidis and ductus deferens where spermatozoa spend a longer period (24 hours) and which are involved in little net fluid transport (0.14-2.1 microliter/cm2/h). Most spermatozoa (92.3%) are located in the ductus deferens. The velocity of spermatozoal transport is much the same through the quail spermatic ducts (0.37 mm/min) as through the mammalian epididymis, the difference between classes in the duration of spermatozoal transport being due to differences in the distance that they travel. In a comparison between estimates of spermatozoal concentration using stereological methods and direct counts of spermatozoa in samples collected using micropuncture procedures it was concluded that both methods gave similar results.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aire T. A., Ayeni J. S., Olowo-Okorun M. O. The structure of the excurrent ducts of the testis of the guinea-fowl (Numida meleagris). J Anat. 1979 Oct;129(Pt 3):633–643. [PMC free article] [PubMed] [Google Scholar]
- Aire T. A. Micro-stereological study of the avian epididymal region. J Anat. 1979 Dec;129(Pt 4):703–706. [PMC free article] [PubMed] [Google Scholar]
- Aire T. A. Surface morphology of the ducts of the epididymal region of the drake (Anas platyrhynchos) as revealed by scanning and transmission electron microscopy. J Anat. 1982 Oct;135(Pt 3):513–520. [PMC free article] [PubMed] [Google Scholar]
- Aire T. A. The ductuli efferentes of the epididymal region of birds. J Anat. 1980 Jun;130(Pt 4):707–723. [PMC free article] [PubMed] [Google Scholar]
- Aire T. A. The epididymal region of the Japanese quail (Coturnix coturnix japonica). Acta Anat (Basel) 1979;103(3):305–312. [PubMed] [Google Scholar]
- Amir D., Braun-Eilon B., Schindler H. Passage and disappearance of labelled spermatozoa in the genital tract of the male Japanese quail in segregation or cohabitation. Ann Biol Anim Biochim Biophys. 1973;13(3):321–328. doi: 10.1051/rnd:19730302. [DOI] [PubMed] [Google Scholar]
- Budras K. D., Meier U. The epididymis and its development in ratite birds (ostrich, emu, rhea). Anat Embryol (Berl) 1981;162(3):281–299. doi: 10.1007/BF00299973. [DOI] [PubMed] [Google Scholar]
- Budras K. D., Sauer T. Morphology of the epididymis of the cock (Gallus domesticus) and its effect upon the steroid sex hormone synthesis. I. Ontogenesis, morphology and distribution of the epididymis. Anat Embryol (Berl) 1975 Dec 23;148(2):175–196. doi: 10.1007/BF00315268. [DOI] [PubMed] [Google Scholar]
- Clulow J., Jones R. C. Production, transport, maturation, storage and survival of spermatozoa in the male Japanese quail, Coturnix coturnix. J Reprod Fertil. 1982 Mar;64(2):259–266. doi: 10.1530/jrf.0.0640259. [DOI] [PubMed] [Google Scholar]
- Djakiew D., Jones R. C. Sperm maturation, fluid transport, and secretion and absorption of protein in the epididymis of the echidna, Tachyglossus aculeatus. J Reprod Fertil. 1983 Jul;68(2):445–456. doi: 10.1530/jrf.0.0680445. [DOI] [PubMed] [Google Scholar]
- Hess R. A., Thurston R. J., Biellier H. V. Morphology of the epididymal region and ductus deferens of the turkey (Meleagris gallopavo). J Anat. 1976 Nov;122(Pt 2):241–252. [PMC free article] [PubMed] [Google Scholar]
- Hess R. A., Thurston R. J. Ultrastructure of epithelial cells in the epididymal region of the turkey (Meleagris gallopavo). J Anat. 1977 Dec;124(Pt 3):765–778. [PMC free article] [PubMed] [Google Scholar]
- Howarth B., Jr Fertilizing ability of cock spermatozoa from the testis epididymis and vas deferens following intramagnal insemination. Biol Reprod. 1983 Apr;28(3):586–590. doi: 10.1095/biolreprod28.3.586. [DOI] [PubMed] [Google Scholar]
- LAKE P. E. The male reproductive tract of the fowl. J Anat. 1957 Jan;91(1):116–129. [PMC free article] [PubMed] [Google Scholar]
- Robb G. W., Amann R. P., Killian G. J. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil. 1978 Sep;54(1):103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Teshima F., Heller C. G. Duration of transit of spermatozoa through the human male ductular system. Fertil Steril. 1970 May;21(5):390–396. [PubMed] [Google Scholar]
- Tingari M. D. On the structure of the epididymal region and ductus deferens of the domestic fowl (Gallus domesticus). J Anat. 1971 Sep;109(Pt 3):423–435. [PMC free article] [PubMed] [Google Scholar]
- Tingari M. D. The fine structure of the epithelial lining of the ex-current duct system of the testis of the domestic fowl (Gallus domesticus). Q J Exp Physiol Cogn Med Sci. 1972 Jul;57(3):271–295. doi: 10.1113/expphysiol.1972.sp002162. [DOI] [PubMed] [Google Scholar]
- Waites G. M., Gladwell R. T. Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiol Rev. 1982 Apr;62(2):624–671. doi: 10.1152/physrev.1982.62.2.624. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]


