Abstract
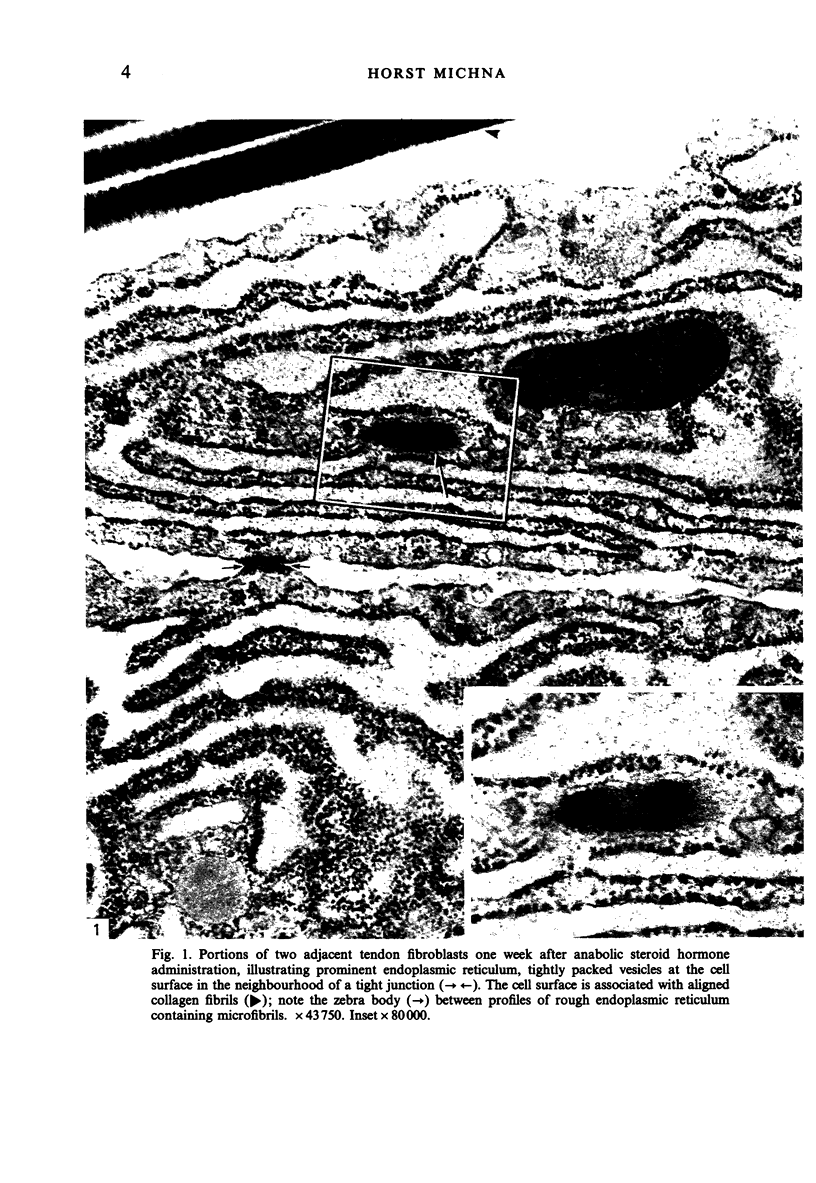
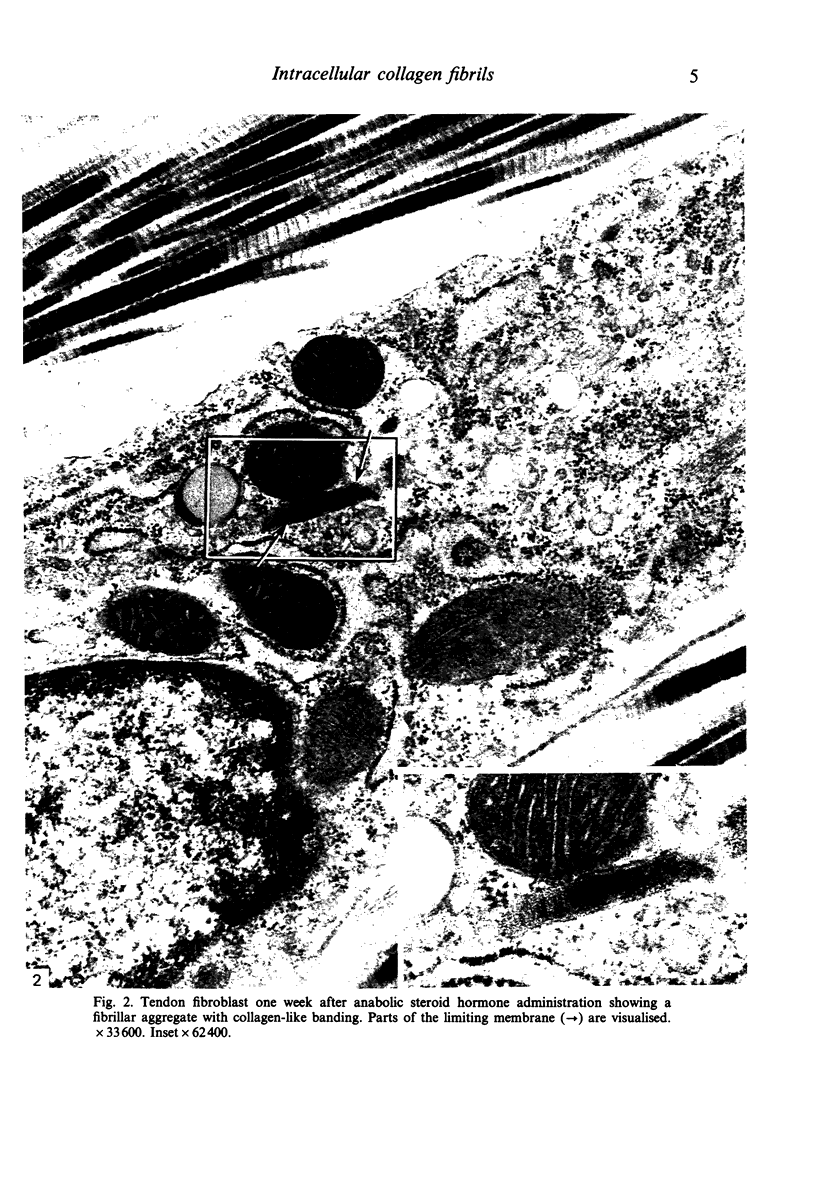
This study was designed to substantiate one or both of the two hypotheses for the explanation of intracellular collagen fibrils in collagen-producing cells. The more obvious is the phagocytosis of extracellular collagen fibrils by the cell and the other is a form of autophagocytosis of newly synthesised collagenous products. Information was collected on fibroblasts from murine tendons after exercise and simultaneously stimulating collagen synthesis by treatment with an anabolic steroid hormone. Moreover, in vivo and in vitro fibroblastic tumour cells which demonstrate enhanced protein synthesis were also treated with the anabolic steroid. The findings of intracellular collagen fibrils in tendon fibroblasts and the sarcoma cells after experimentally stimulating collagen synthesis are discussed in the light of the hypothesis that the findings may represent steps of autophagocytosis of newly synthesised collagenous products in the absence of a control mechanism to remove collagenous products which cannot be secreted.
Full text
PDF



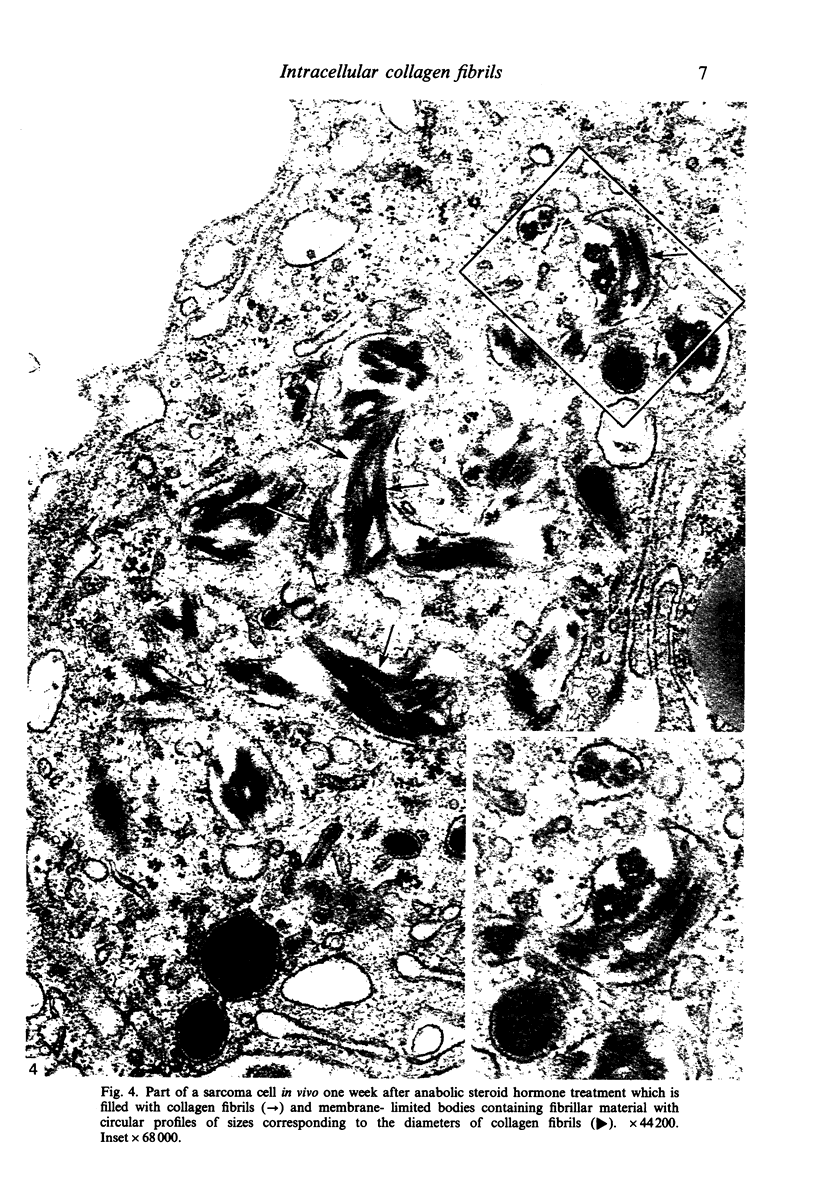





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra S. R., Broderick P. A. Desmoid fibroblastoma. Intracytoplasmic collagenosynthesis in a peculiar fibroblastic tumor: light and ultrastructural study of a case. Hum Pathol. 1973 Sep;4(3):419–429. doi: 10.1016/s0046-8177(73)80102-9. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S., Cowan M. J., McDonald J. A., Crystal R. G. Degradation of newly synthesized collagen. J Biol Chem. 1978 Jun 25;253(12):4356–4363. [PubMed] [Google Scholar]
- Bienkowski R. S., Engels C. J. Measurement of intracellular collagen degradation. Anal Biochem. 1981 Sep 15;116(2):414–424. doi: 10.1016/0003-2697(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Birk D. E., Trelstad R. L. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol. 1984 Dec;99(6):2024–2033. doi: 10.1083/jcb.99.6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk D. E., Trelstad R. L. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986 Jul;103(1):231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes D., Anton E. Lysosomes in uterine involution: intracytoplasmic degradation of myofilaments and collagen. J Gerontol. 1969 Jan;24(1):55–69. doi: 10.1093/geronj/24.1.55. [DOI] [PubMed] [Google Scholar]
- Cate A. R., Syrbu S. A relationship between alkaline phosphatase activity and the phagocytosis and degradation of collagen by the fibroblast. J Anat. 1974 Apr;117(Pt 2):351–359. [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. M., Greenlee T. K., Jr The development of human digital tendons. J Anat. 1975 Nov;120(Pt 2):253–274. [PMC free article] [PubMed] [Google Scholar]
- Cullen J. C. Intracellular collagen in experimental arthritis in rats. J Bone Joint Surg Br. 1972 May;54(2):351–359. [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dyer R. F., Peppler R. D. Intracellular collagen in the nonpregnant and IUD-containing rat uterus. Anat Rec. 1977 Feb;187(2):241–247. doi: 10.1002/ar.1091870209. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., DROLET B. P., McCARTHY R. E., GOULET K. A., DOKOS J. M., FILLER D. A. Isolation and serial propagation of malignant and normal cells in semi-defined media. Origins of CCRF cell lines. Cancer Res. 1960 Jul;20:930–939. [PubMed] [Google Scholar]
- Faure J. P., Graf B., de Kozak Y., Pouliquen Y. Etude microscopique d'une réaction inflammatoire de la cornée du lapin. II. Les altérations du collagène: dissociation oedémateuse, collagénolyse, fibrillogénèse. Arch Ophtalmol Rev Gen Ophtalmol. 1970 Feb;30(2):149–160. [PubMed] [Google Scholar]
- Fernandez-Madrid F., Noonan S., Riddle J., Karvonen R., Sasaki D. Intracellular processing of procollagen induced by the action of colchicine. J Anat. 1980 Mar;130(Pt 2):229–241. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Madrid F., Noonan S., Riddle J. The "spindle-shaped" body in fibroblasts: intracellular collagen fibrils. J Anat. 1981 Mar;132(Pt 2):157–166. [PMC free article] [PubMed] [Google Scholar]
- Garant P. R., Cho M. I. Cytoplasmic polarization of periodontal ligament fibroblasts. Implications for cell migration and collagen secretion. J Periodontal Res. 1979 Mar;14(2):95–106. doi: 10.1111/j.1600-0765.1979.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Fell H. B., Dingle J. T. Endocytosis of sugars in embryonic skeletal tissues in organ culture. II. Effect of sucrose on cellular fine structure. J Cell Sci. 1969 Jan;4(1):105–131. doi: 10.1242/jcs.4.1.105. [DOI] [PubMed] [Google Scholar]
- Gona A. G. Light and electron microscopic study on thyroxine-induced in vitro resorption of the tadpole tail fin. Z Zellforsch Mikrosk Anat. 1969;95(4):483–494. doi: 10.1007/BF00335142. [DOI] [PubMed] [Google Scholar]
- Groniowski J., Walski M. Electron microscopic observations on chronic aggressive hepatitis: participation of hepatocytes in liver fibrosis. Pathol Eur. 1975;10(1):37–50. [PubMed] [Google Scholar]
- HOLMBERG B. On the permeability to lissamine green and other dyes in the course of cell injury and cell death. Exp Cell Res. 1961 Jan;22:406–414. doi: 10.1016/0014-4827(61)90118-5. [DOI] [PubMed] [Google Scholar]
- Levine A. M., Reddick R., Triche T. Intracellular collagen fibrils in human sarcomas. Lab Invest. 1978 Dec;39(6):531–540. [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. The fine structure of connective tissue. I. The fibroblast. Exp Mol Pathol. 1962 Dec;1:509–534. doi: 10.1016/0014-4800(62)90040-0. [DOI] [PubMed] [Google Scholar]
- Marchi F., Leblond C. P. Collagen biogenesis and assembly into fibrils as shown by ultrastructural and 3H-proline radioautographic studies on the fibroblasts of the rat food pad. Am J Anat. 1983 Oct;168(2):167–197. doi: 10.1002/aja.1001680206. [DOI] [PubMed] [Google Scholar]
- Michna H. A peculiar myofibrillar pattern in the murine muscle-tendon junction. Cell Tissue Res. 1983;233(1):227–231. doi: 10.1007/BF00222246. [DOI] [PubMed] [Google Scholar]
- Michna H. Organisation of collagen fibrils in tendon: changes induced by an anabolic steroid. I. Functional and ultrastructural studies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(1):75–86. doi: 10.1007/BF02889952. [DOI] [PubMed] [Google Scholar]
- Michna H. Organisation of collagen fibrils in tendon: changes induced by an anabolic steroid. II. A morphometric and stereologic analysis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(1):87–98. doi: 10.1007/BF02889953. [DOI] [PubMed] [Google Scholar]
- Parakkal P. F. Involvement of macrophages in collagen resorption. J Cell Biol. 1969 Apr;41(1):345–354. doi: 10.1083/jcb.41.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakkal P. F. Role of macrophages in collagen resorption during hair growth cycle. J Ultrastruct Res. 1969 Nov;29(3):210–217. doi: 10.1016/s0022-5320(69)90101-4. [DOI] [PubMed] [Google Scholar]
- Postacchini F., Accinni L., Natali P. G., Ippolito E., DeMartino C. Regeneration of rabbit calcaneal tendon: a morphological and immunochemical study. Cell Tissue Res. 1978 Dec 14;195(1):81–97. doi: 10.1007/BF00233678. [DOI] [PubMed] [Google Scholar]
- Postacchini F., Ippolito E., Puddu G., De Martino C. Intracellular collagen fibres in regenerating tendon. Ric Clin Lab. 1981 Oct-Dec;11(4):343–347. doi: 10.1007/BF02909033. [DOI] [PubMed] [Google Scholar]
- Pérez-Tamayo R. Collagen resorption in carrageenin granulomas. II. Ultrastructure of collagen resorption. Lab Invest. 1970 Feb;22(2):142–159. [PubMed] [Google Scholar]
- Rentería V. G., Ferrans V. J. Intracellular collagen fibrils in cardiac valves of patients with the Hurler syndrome. Lab Invest. 1976 Mar;34(3):263–272. [PubMed] [Google Scholar]
- Rose G. G., Yajima T., Mahan C. J. Human gingival fibroblast cell lines in vitro. I. Electron microscopic studies of collagenolysis. J Periodontal Res. 1980 Jan;15(1):53–70. doi: 10.1111/j.1600-0765.1980.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Scherft J. P., Heersche J. N. Accumulation of collagen-containing vacuoles in osteoblasts after administration of colchicine. Cell Tissue Res. 1975;157(3):353–365. doi: 10.1007/BF00225526. [DOI] [PubMed] [Google Scholar]
- Seifert K. Elektronenmikroskopische Untersuchungen am juvenilen Nasenrachenfibrom. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1971 Jul 30;198(2):215–228. [PubMed] [Google Scholar]
- Soames J. V., Davies R. M. Intracellular collagen fibrils in early gingivitis in the beagle dog. J Periodontal Res. 1977 Sep;12(5):378–386. doi: 10.1111/j.1600-0765.1977.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Staubesand J. Intracellualr collagen in smooth muscle: the fine structure of the artificially occluded rat artery and ureter, and of human varicose and arteriosclerotic vessels. Beitr Pathol. 1977 Oct;161(2):187–193. doi: 10.1016/s0005-8165(77)80097-8. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev Biol. 1979 Aug;71(2):228–242. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L. Vacuoles in the embryonic chick corneal epithelium, an epithelium which produces collagen. J Cell Biol. 1971 Mar;48(3):689–694. doi: 10.1083/jcb.48.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuku G., Gross J. Morphologic studies of connective tissue resorption in the tail fin of metamorphosing bullfrog tadpole. Dev Biol. 1965 Jun;11(3):352–370. doi: 10.1016/0012-1606(65)90044-8. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOELZ H. THE "SPINDLE-SHAPED BODY" IN FIBROBLASTS. J Cell Biol. 1964 Feb;20:333–337. doi: 10.1083/jcb.20.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. A., Meyer A. T. Intracellular collagen fibers. In human mesenchymal tumors and inflammatory states. Arch Pathol. 1967 Oct;84(4):354–362. [PubMed] [Google Scholar]
- Yajima T. Acid phosphatase activity and intracellular collagen degradation by fibroblasts in vitro. Cell Tissue Res. 1986;245(2):253–260. doi: 10.1007/BF00213929. [DOI] [PubMed] [Google Scholar]
- Yajima T. Ultrastructural and cytochemical studies on the remodelling of the tracheal cartilage. Arch Histol Jpn. 1976 May;39(2):79–97. doi: 10.1679/aohc1950.39.79. [DOI] [PubMed] [Google Scholar]
- Yee J. A. Response of periodontal ligament cells to orthodontic force: ultrastructural identification of proliferating fibroblasts. Anat Rec. 1979 Aug;194(4):603–614. doi: 10.1002/ar.1091940412. [DOI] [PubMed] [Google Scholar]
- Zorn T. M., Bevilacqua E. M., Abrahamsohn P. A. Collagen remodeling during decidualization in the mouse. Cell Tissue Res. 1986;244(2):443–448. doi: 10.1007/BF00219220. [DOI] [PubMed] [Google Scholar]
- ten Cate A. R. Morphological studies of fibrocytes in connective tissue undergoing rapid remodelling. J Anat. 1972 Sep;112(Pt 3):401–414. [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]