Abstract
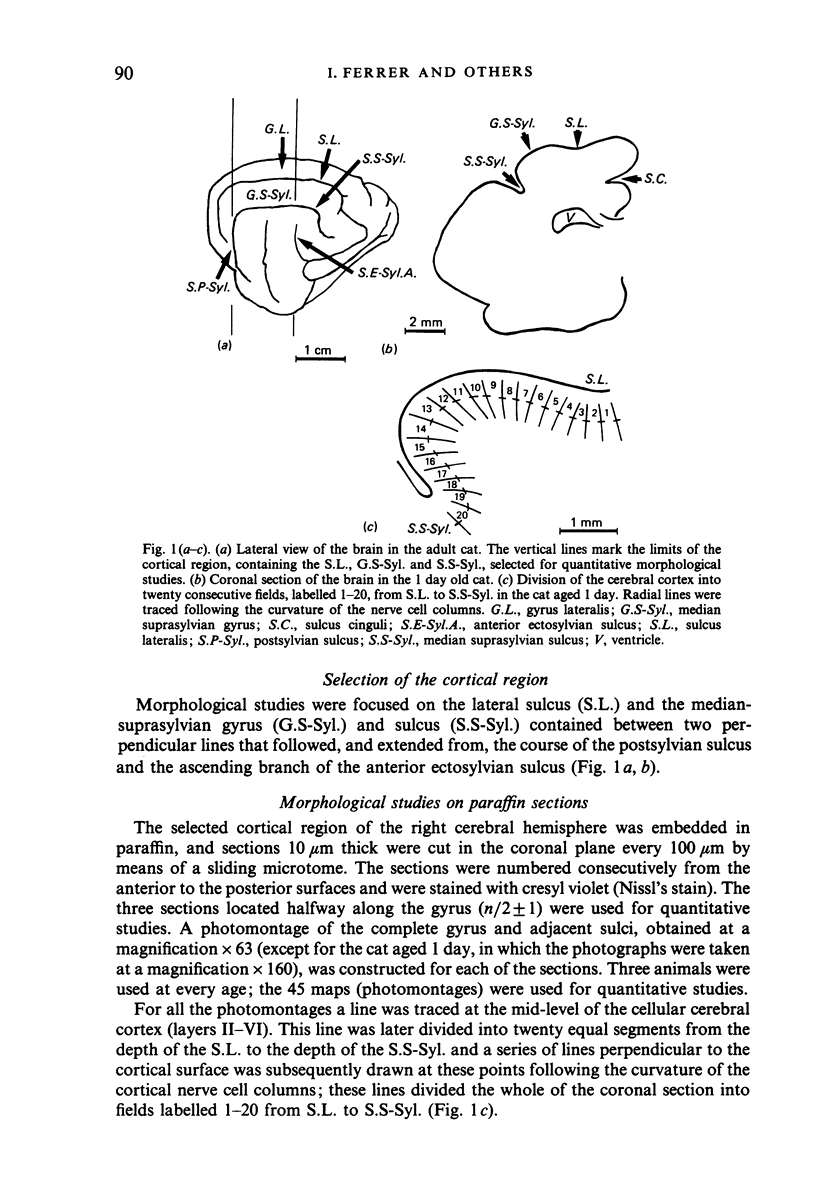
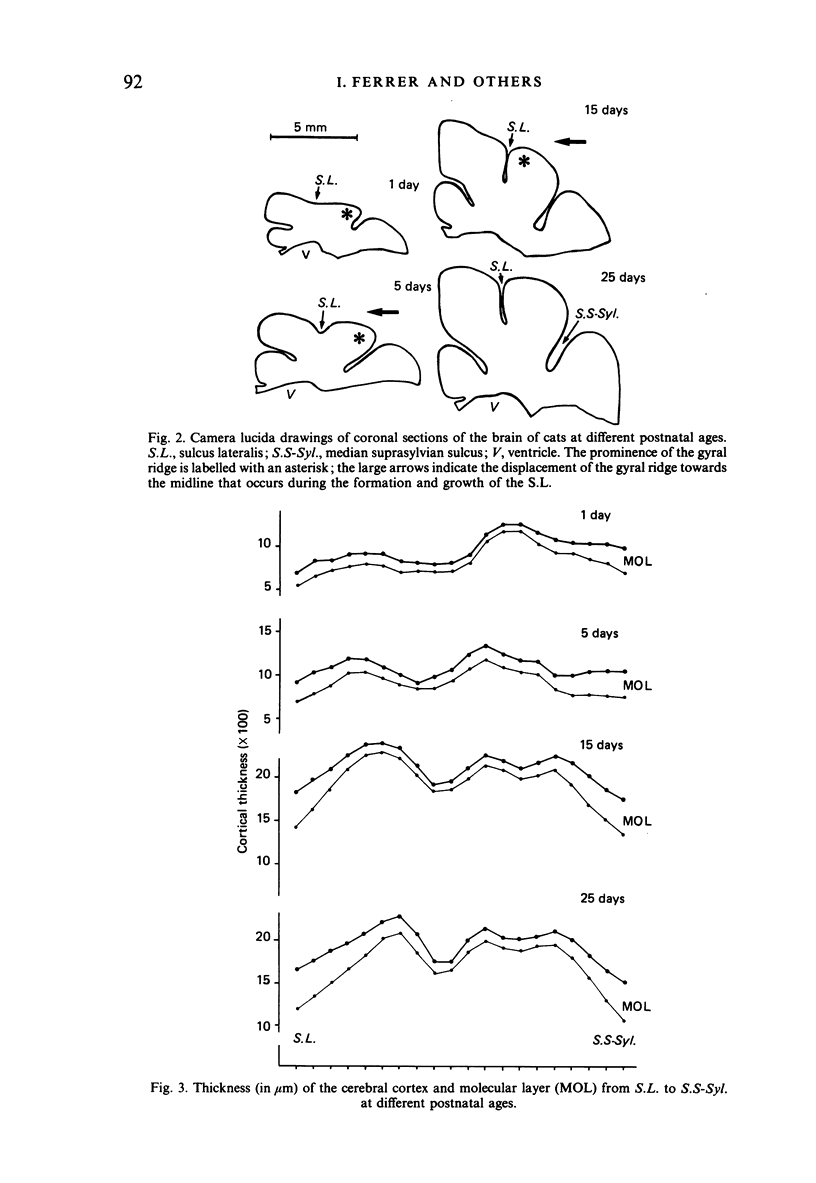
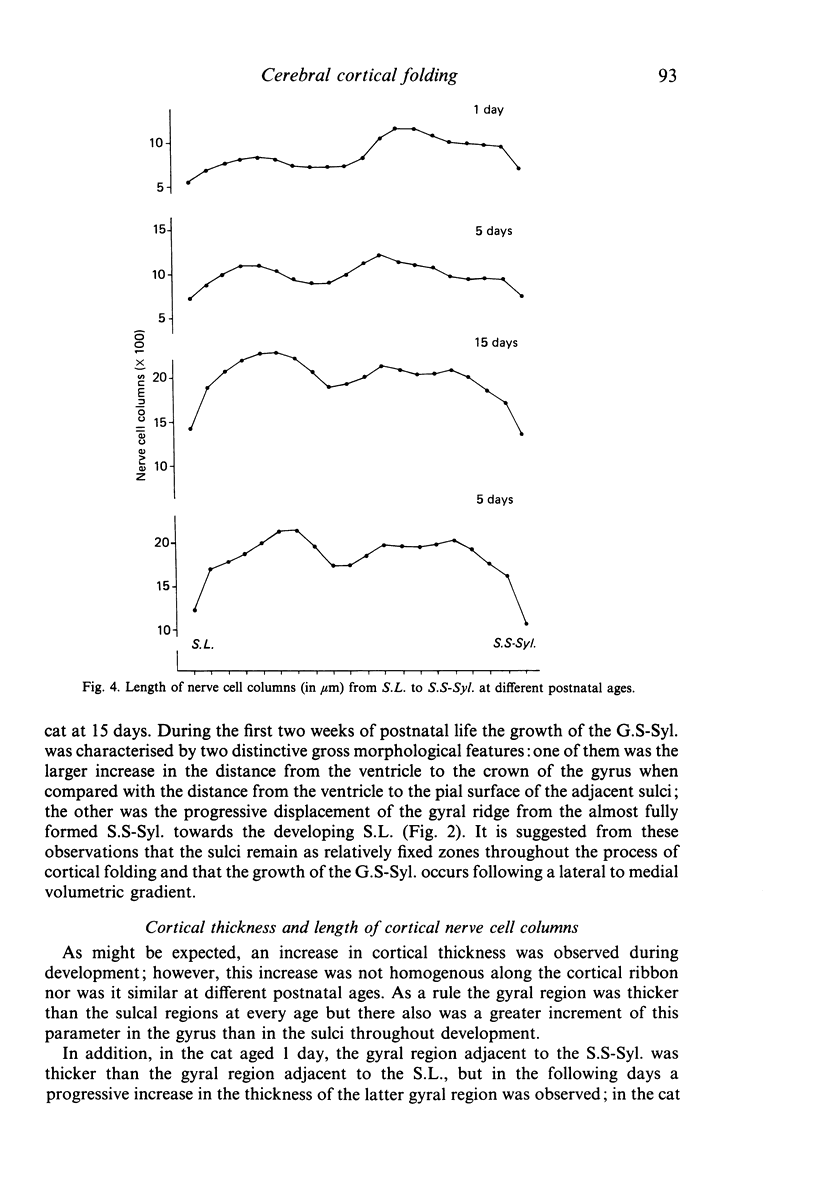
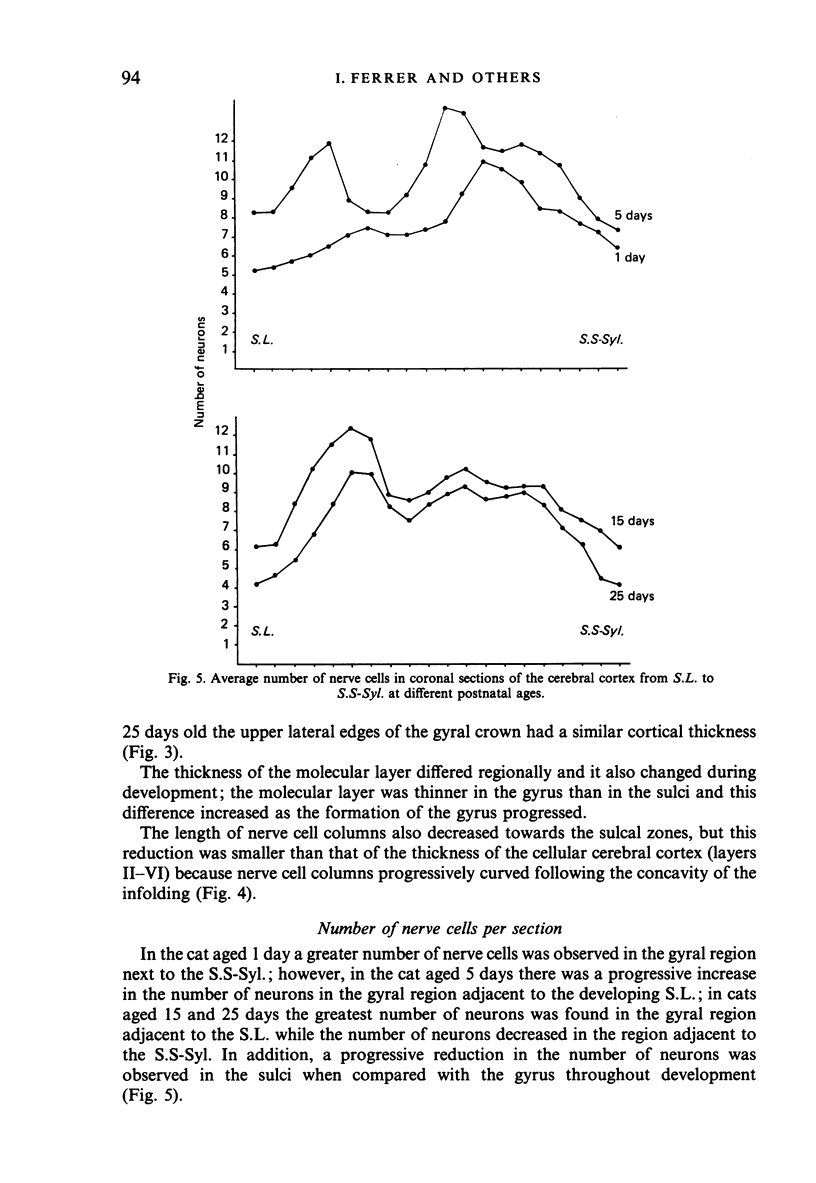
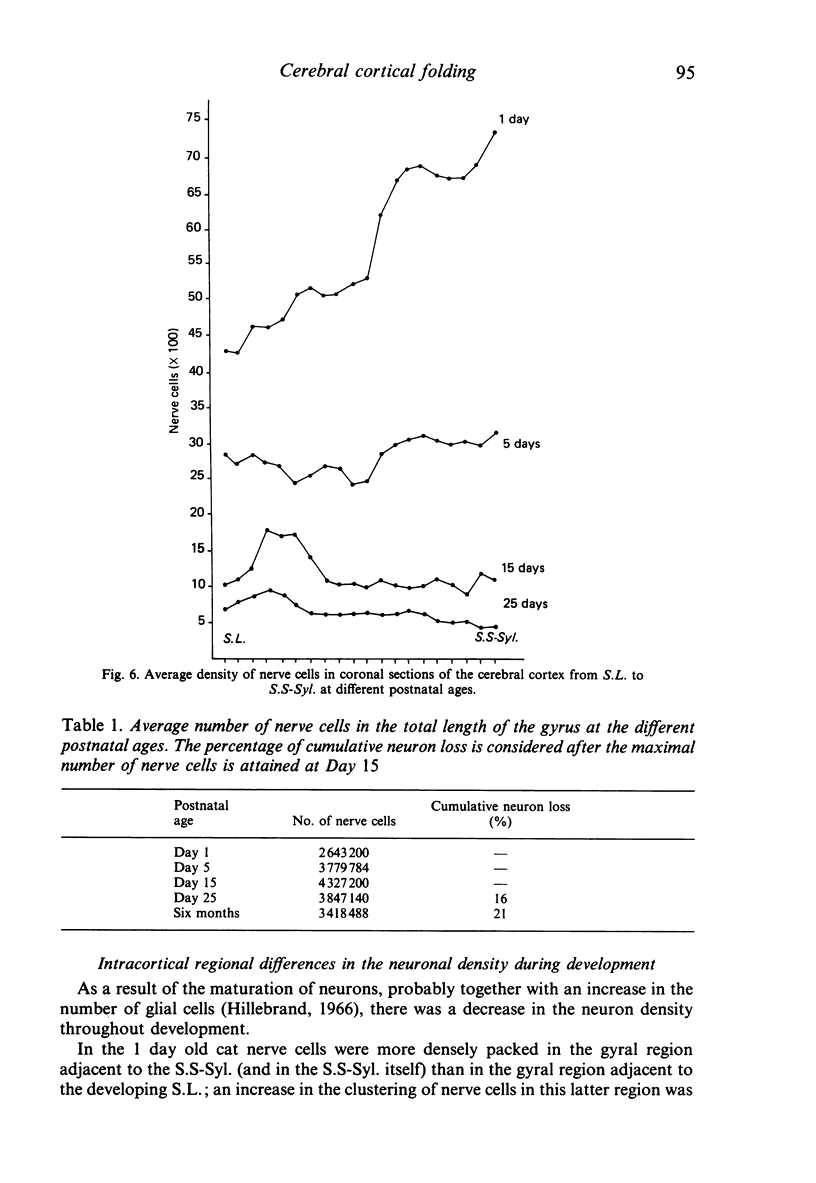
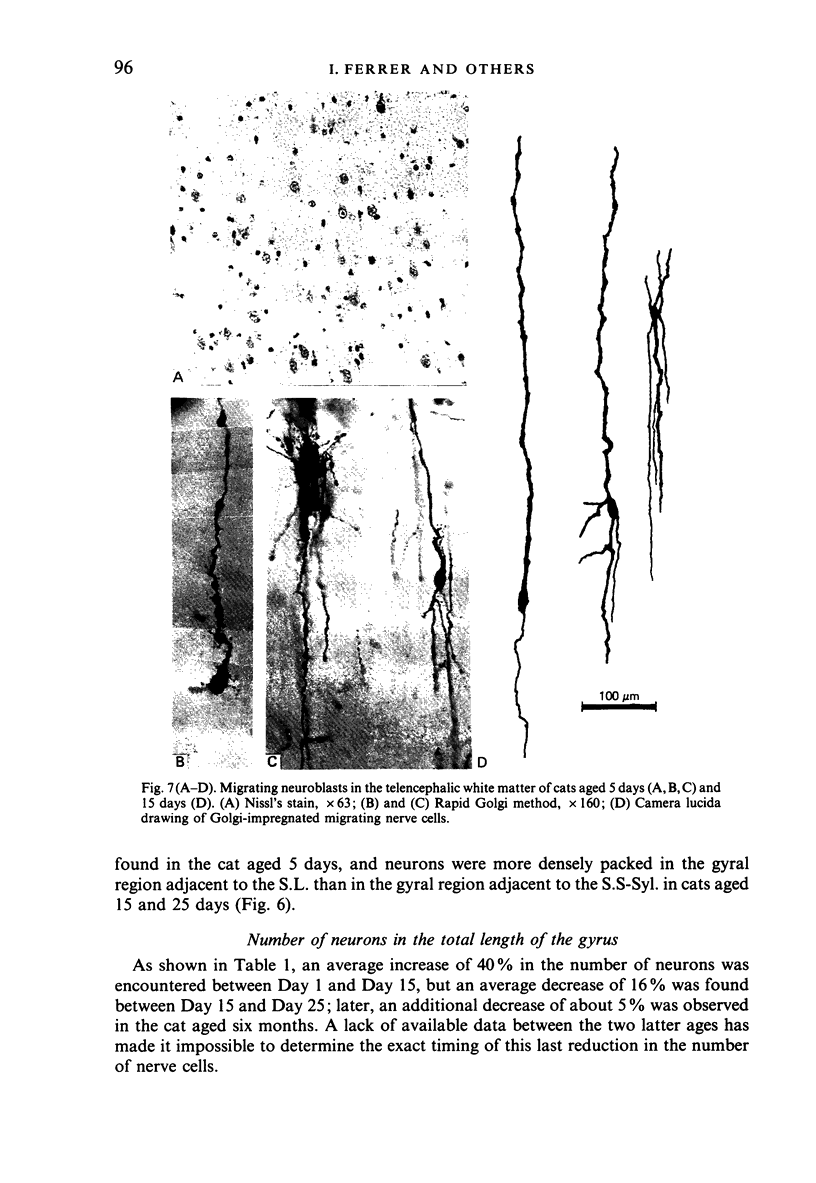
The postnatal development of these median-suprasylvian gyrus and adjoining sulci was studied in cats 1, 5, 15, 25 days and six months old. The median-suprasylvian gyrus (G.S.-Syl.) grows according to a lateral to medial intracortical gradient in which the adjoining sulci, sulcus lateralis (S.L.) and median-suprasylvian sulcus (S.S-Syl.), are considered to be fixed zones because of their relatively constant distance from the ventricular wall throughout the development. Thus the formation of the S.L. is a consequence of the increase in volume of the gyral region adjacent to this developing sulcus, whereas there is a smaller increase in volume of the gyral region adjacent to the almost fully formed, at birth, S.S-Syl. This increase in volume is associated with a regional increase in the number of nerve cells and with an increase in the density of neurons in the region adjacent to the S.L. as it fades in the region adjacent to the S.S-Syl. This process takes place from Day 1 until about Day 25 of postnatal life. An intralaminar displacement of nerve cells also occurs during the process of cortical folding:nerve cell columns converge towards the hilum in the gyral region, but the columns progressively curve following the concavity of the infolding in the sulcal zones; as a result, although the length of nerve cell columns tends to be preserved to some extent along the gyrus, the cerebral cortex is progressively thinner in the sulci than in the gyri and the molecular layer is progressively thicker in the former than in the latter. This process also occurs following a lateral to medial gradient in the G.S.-Syl. The present observations may suggest that cortical folding is largely dependent on intracortical mechanical forces but the regular distribution of the sulci, together with the orderly spatio-temporal pattern of gyral growth, points to the conclusion that this process may be controlled by extracortical signals.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferrer I. A Golgi analysis of unlayered polymicrogyria. Acta Neuropathol. 1984;65(1):69–76. doi: 10.1007/BF00689830. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Fábregues I., Condom E. A Golgi study of the sixth layer of the cerebral cortex. III. Neuronal changes during normal and abnormal cortical folding. J Anat. 1987 Jun;152:71–82. [PMC free article] [PubMed] [Google Scholar]
- Finlay B. L., Slattery M. Local differences in the amount of early cell death in neocortex predict adult local specializations. Science. 1983 Mar 18;219(4590):1349–1351. doi: 10.1126/science.6828866. [DOI] [PubMed] [Google Scholar]
- Friedman B., Price J. L. Age-dependent cell death in the olfactory cortex: lack of transneuronal degeneration in neonates. J Comp Neurol. 1986 Apr 1;246(1):20–31. doi: 10.1002/cne.902460103. [DOI] [PubMed] [Google Scholar]
- Fujita S., Shimada M., Nakamura T. H3-thymidine autoradiographic studies on the cell proliferation and differentiation in the external and the internal granular layers of the mouse cerebellum. J Comp Neurol. 1966 Oct;128(2):191–208. doi: 10.1002/cne.901280206. [DOI] [PubMed] [Google Scholar]
- Heumann D., Leuba G. Neuronal death in the development and aging of the cerebral cortex of the mouse. Neuropathol Appl Neurobiol. 1983 Jul-Aug;9(4):297–311. doi: 10.1111/j.1365-2990.1983.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Hicks S. P., D'Amato C. J. Cell migrations to the isocortex in the rat. Anat Rec. 1968 Mar;160(3):619–634. doi: 10.1002/ar.1091600311. [DOI] [PubMed] [Google Scholar]
- Hillebrand H. Quantitative Untersuchungen über postnatale Veränderungen der Glia im Corpus callosum der Katze. Z Zellforsch Mikrosk Anat. 1966;73(2):303–312. [PubMed] [Google Scholar]
- KAELLEN B. DEGENERATION AND REGENERATION IN THE VERTEBRATE CENTRAL NERVOUS SYSTEM DURING EMBRYOGENESIS. Prog Brain Res. 1965;14:77–96. [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. II. Autoradiographic studies. Brain Res. 1971 May 21;28(3):421–441. [PubMed] [Google Scholar]
- Luskin M. B., Shatz C. J. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985 Dec 22;242(4):611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Prothero J. W., Sundsten J. W. Folding of the cerebral cortex in mammals. A scaling model. Brain Behav Evol. 1984;24(2-3):152–167. doi: 10.1159/000121313. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974 Feb 1;183(4123):425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Richman D. P., Stewart R. M., Hutchinson J. W., Caviness V. S., Jr Mechanical model of brain convolutional development. Science. 1975 Jul 4;189(4196):18–21. doi: 10.1126/science.1135626. [DOI] [PubMed] [Google Scholar]
- Smart I. H., McSherry G. M. Gyrus formation in the cerebral cortex in the ferret. I. Description of the external changes. J Anat. 1986 Jun;146:141–152. [PMC free article] [PubMed] [Google Scholar]
- Smart I. H., McSherry G. M. Gyrus formation in the cerebral cortex of the ferret. II. Description of the internal histological changes. J Anat. 1986 Aug;147:27–43. [PMC free article] [PubMed] [Google Scholar]
- Todd P. H. A geometric model for the cortical folding pattern of simple folded brains. J Theor Biol. 1982 Aug 7;97(3):529–538. doi: 10.1016/0022-5193(82)90380-0. [DOI] [PubMed] [Google Scholar]
- Valverde F., Facal-Valverde M. V. Transitory population of cells in the temporal cortex of kittens. Brain Res. 1987 Apr;429(2):283–288. doi: 10.1016/0165-3806(87)90108-8. [DOI] [PubMed] [Google Scholar]
- WELKER W. I., CAMPOS G. B. Physiological significance of sulci in somatic sensory cerebral cortex in mammals of the family procyonidae. J Comp Neurol. 1963 Feb;120:19–36. doi: 10.1002/cne.901200103. [DOI] [PubMed] [Google Scholar]
- Wahle P., Meyer G. Morphology and quantitative changes of transient NPY-ir neuronal populations during early postnatal development of the cat visual cortex. J Comp Neurol. 1987 Jul 8;261(2):165–192. doi: 10.1002/cne.902610202. [DOI] [PubMed] [Google Scholar]
- de León G. A. Observations on cerebral and cerebellar microgyria. Acta Neuropathol. 1972;20(4):278–287. doi: 10.1007/BF00691746. [DOI] [PubMed] [Google Scholar]



